Abstract
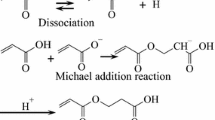
The effect of microwave heating (MWH) on the isothermal kinetic of crosslinking-polymerization of acrylic acid (CPAA) was investigated. The kinetic curves of CPAA were determined in the temperature range from 303 to 328 K. By applying the model-fitting method it was revealed that the isothermal kinetics of CPAA was described by the first order chemical reaction kinetics model under the MWH and by the second order chemical reaction rate model for the conventionally heated (CH) process. The values of the reaction rate constants of CPAA are about 40 times higher for the microwave heated system than for the conventional heating. The kinetic parameters (activation energy (E a) and pre-exponential factor (lnA)) of the CPAA are significantly lower than the corresponding values for CH process. It was found that the increase in the reaction rate of CPAA for MWH was not a consequence of overheating neither the hot spots in the reaction system. Based on model of selective energy transfer between the reacting molecules and the heating bath a novel explanation of the established effects of MWH on the kinetics of CPAA is given. A quantized nature and value of activation energy was confirmed. The decrease in the value of activation energy of CPAA under microwave heating is explained by the increased value of energy of ground vibration level of resonant oscillator in the AA molecule (v = 1417 cm−1) caused by the absorption of non-thermal energy of MW field.
Similar content being viewed by others
References
H. M. Kingston and S. J. Haswall, Microwave Enhanced Chemistry (American Chemical Society, Washington, 1997).
A. Loupy, Microwave in Organic Synthesis (Wiley-VCH, Weinheim, 2002).
D. Bogdal and A. Prociak, Microwave-Enhanced Polymer Chemistry and Technology (Blackwell, Oxford, UK, 2007).
D. Baghurst and D. Mingos, J. Chem. Soc. Chem. Commun. 9, 674 (1992).
X. Zhang, D. Hayward, and D. Mingos, Chem. Commun. 9, 975 (1999).
K. D. Raner, C. R. Strauss, F. Vyskoc, and L. Mokbel, J. Org. Chem. 58, 950 (1993).
A. Hoz, A. Diaz-Ortiz, and A. Moreno, Chem. Soc. Rev. 34, 164 (2005).
J. Berlan, P. Giboran, S. Lefeuvre, and C. Marcheno, Tetrahedron Lett. 32, 2363 (1998).
D. Lewis, J. Summers, T. Ward, and J. McCrath, J. Polym. Sci. A: Polym. Chem. 30, 1647 (1992).
K. Rybakov and V. Semenov, Phys. Rev. B 49, 64 (1994).
C. Strauss and R. Trainor, Aust. J. Chem. 48, 1665 (1995).
G. Binner, A. Hassine, and T. Cross, J. Mater. Sci. 30, 5389 (1995).
D. Stuerga and P. Gaillard, J. Microwave Power Electromagn. Energy 31, 101 (1996).
J. Booske, R. F. Cooper, and S. A. Freeman, Mat. Res. Innovat. 1, 77 (1997).
C. Shibata, T. Kashima, and K. Ohuchi, Jpn. J. Appl. Phys. 35, 316 (1996).
L. Perreux and A. Loupy, Tetrahedron 57, 9199 (2000).
C. Blanco and S. Auerbach, J. Phys. Chem. B 107, 2490 (2003).
V. Conner and G. Tumpsett, J. Phys. Chem. B 111, 2110 (2008).
B. Adnadjevi and J. Jovanović, J. Appl. Polym. Sci. 107, 3579 (2008).
M. E. Brown, D. Dollimore, and A. K. Galway, in Comprehensive Chemical Kinetics, Vol. 22: Reaction in the Solid State, Ed. by C. H. Bamford (Elsevier, Oxford, 1980).
H. Friedman, J. Polym. Sci. C 6, 183 (1964).
H. Tobita, and A. E. Hamilec, Macromolecules 22, 3098 (1988).
S. Vyazovkin and A. Lesnikovich, Thermochim. Acta 165, 273 (1990).
S. Vyazovkin and C. Wight, Ann. Rev. Phys. Chem. 48, 125 (1997).
R. Larsson, J. Mol. Catal. 55, 70 (1989).
A. Pourjavadi, Sh. Barzegar, and F. Zeidebi, React. Funct. Polym. 67, 644 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adnadjević, B., Jovanović, J. & Potkonjak, B. A novel approach to the explanation the effect of microwave heating on isothermal kinetic of crosslinking polymerization of acrylic acid. Russ. J. Phys. Chem. 87, 2115–2120 (2013). https://doi.org/10.1134/S0036024413130025
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024413130025