Abstract
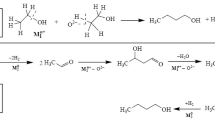
A study of the kinetics of ethanol conversion in the presence of Zr-containing zeolites BEA doped with palladium particles has revealed the order of formation of the main reaction products. It has been shown that the primary processes are ethanol dehydrogenation to acetaldehyde on Pd sites and ethanol dehydration to diethyl ether on the acid sites of the catalyst. After that, acetaldehyde undergoes the aldol–croton condensation reaction to form crotonal, which is hydrogenated to butanol on the metal sites. Butanol, in turn, is dehydrated into butenes, which undergo hydrogenation to butane. The presence of hydrogen in the gas phase leads to the displacement of ethanol from the metal surface and prevents the formation of surface carbonates and acetates. It has been found that hydrogen significantly accelerates ethanol dehydration owing to a decrease in the activation energy, which can be attributed to hydrogen spillover to the zeolite. The addition of water inhibits all acid-catalyzed reactions owing to competitive adsorption on acid sites and thereby decreases the butanol yield and the ethanol conversion.








Similar content being viewed by others
REFERENCES
J. Sun and Y. Wang, ACS Catal. 4, 1078 (2014).
A. Mohsenzadeh, A. Zamani, and M. J. Taherzadeh, ChemBioEng Rev. 4, 75 (2017).
M. Iwamoto, Catal. Today 242, 243 (2015).
O. A. Ponomareva, P. A. Shaposhnik, P. A. Kots, et al., Pet. Chem. 58, 1023 (2018).
J. T. Kozlowski and R. J. Davis, ACS Catal. 3, 1588 (2013).
V. V. Markovnikov and P. V. Zubov, Zh. Russ. Fiz.-Khim. Ob-va 2 (1), 128 (1889).
M. Guerbet, C.R. Acad. Sci. 128, 511 (1899).
C. R. Ho, S. Shylesh, and A. T. Bell, ACS Catal. 6, 939 (2016).
I. C. Marcu, N. Tanchoux, F. Fajula, and D. Tichit, Catal. Lett. 143, 23 (2013).
A. V. Chistyakov, P. A. Zharova, M. V. Tsodikov, et al., Kinet. Catal. 57, 803 (2016).
S. A. Nikolaev, A. V. Chistyakov, P. A. Zharova, et al., Pet. Chem. 56, 730 (2016).
S. Hanspal, Z. D. Young, J. T. Prillaman, and R. J. Davis, J. Catal. 352, 182 (2017).
J. S. Bates and R. Gounder, J. Catal. 365, 213 (2018).
P. A. Kots, V. L. Sushkevich, O. A. Tyablikov, and I. I. Ivanova, Microporous Mesoporous Mater. 243, 186 (2017).
P. A. Kots, A. V. Zabilska, E. V. Khramov, et al., Inorg. Chem. 57), 11 978 (2018).
V. L. Sushkevich, P. A. Kots, Y. G. Kolyagin, et al., J. Phys. Chem. C 123, 5540 (2019).
R. G. Greenler, J. Chem. Phys. 37, 2094 (1962).
A. Yee, S. J. Morrison, and H. Idriss, J. Catal. 186, 279 (1999).
H. J. Kim and C. Song, Energy Fuels 28, 6788 (2014).
S. Roy, K. Bakhmutsky, E. Mahmoud, Ret al., ACS Catal. 3, 573.
F. Roessner and U. Roland, J. Mol. Catal., A 112, 401 (1996).
D. Varisli T. Dogu, and G. Dogu, Chem. Eng. Sci. 62, 5349 (2007).
A. H. Yonli, I. Gener, and S. Mignard, Microporous Mesoporous Mater. 132, 37 (2010).
ACKNOWLEDGMENTS
The electron microscopy studies were conducted using the equipment of the Center for collective use of Crystallography and Photonics Federal Research Center of the Russian Academy of Sciences under the state task to Crystallography and Photonics Federal Research Center.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-33-01011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Kots, P.A., Zabilska, A.V., Grigor’ev, Y.V. et al. Ethanol to Butanol Conversion over Bifunctional Zeotype Catalysts Containing Palladium and Zirconium. Pet. Chem. 59, 925–934 (2019). https://doi.org/10.1134/S0965544119080097
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544119080097