Abstract
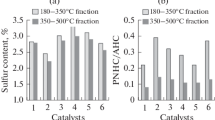
The conversion of oil shale (OS) in a hydrogen medium at a pressure of 6.0 MPa and a temperature of 400°C with the subsequent refining of the resulting products in the presence of NiMo sulfide catalysts supported on materials exhibiting different acidities has been studied. It has been found that the use of the catalysts leads to a significant decrease in the content of sulfur, nitrogen, aromatic hydrocarbons, and olefins in the liquid products obtained from the shale. It has been shown that the catalytic refining of OS hydroconversion products makes the fractional composition of synthetic oil lighter and increases the H/C ratio. The use of the catalysts leads to a decrease in the synthetic oil yield owing to the formation of hydrocarbon gas and coke deposits on the catalysts; in this case, the synthetic oil yield varies in the following order: catalyst-free (25.7 wt %) > NiMo/ASA–Al2O3 (22.0 wt %) > NiMo/Al2O3 (20.7 wt %) > NiMo/USY–Al2O3 (15.8 wt %).

Similar content being viewed by others
REFERENCES
J. E. Gwyn, Fuel Process. Technol. 70, 27 (2001).
J. R. Dyni, 2010 Survey of Energy Resources, Ed. by A. W. Clarke and J.A. Trinnaman (World Energy Council, London, 2010), p. 93.
J. G. Speight, Shale Oil Production Processes (Elsevier, Amsterdam, 2012).
Yu. A. Strizhakova and T. V. Usova, Solid Fuel Chem. 42, 197 (2008).
A. L. Zaidentsal’, J. H. Soone, and R. T. Muoni, Solid Fuel Chem. 42, 74 (2008).
A. V. Anisimov, S. V. Kardashev, A. V. Akopyan, et al., Solid Fuel Chem. 49, 324 (2015).
S. V. Kardashev, A. L. Maksimov, A. V. Tarakanova, et al., Solid Fuel Chem. 50, 232 (2016).
S. B. Romadenkina and V. A. Reshetov, Solid Fuel Chem. 50, 130 (2016).
M. O. Kazakov, P. P. Dik, O. V. Klimov, S. V. Cherepanova, Yu. A. Chesalov, A. S. Noskov, Russ. J. Appl. Chem. 89, 254 (2016).
M. O. Kazakov, P. P. Dik, O. V. Klimov, et al., Solid Fuel Chem. 52, 26 (2018).
M. O. Kazakov, O. V. Klimov, P. P. Dik, et al., Pet. Chem. 57, 1169 (2017).
P. P. Dik, O. V. Klimov, G. I. Koryakina, et al., Catal. Today 220–222, 124 (2014).
S. Chen, T. Li, G. Cao, and M. Guan, US Patent No. 6 399 530 (1998).
ACKNOWLEDGMENTS
The authors thank V.N. Rybokonenko (Boreskov Institute of Catalysis, Siberian Branch, Russian Academy of Sciences) for assistance in the studies.
Funding
This work was supported by the Russian Science Foundation (project no. 15-13-00057) and performed under a state task of the Russian Federation to Boreskov Institute of Catalysis of the Siberian Branch of the Russian Academy of Sciences (budget project no. AAAA-A17-117041710077-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Dik, P.P., Kazakov, M.O., Saiko, A.V. et al. Conversion of Oil Shale Hydroconversion Products in the Presence of Supported Nickel–Molybdenum Sulfide Catalysts. Pet. Chem. 60, 744–750 (2020). https://doi.org/10.1134/S096554412007004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S096554412007004X