Abstract
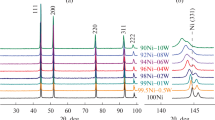
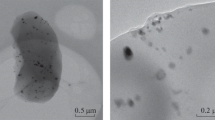
Concept of complex processing of chlorinated hydrocarbons, involving catalytic decomposition of 1,2-dichloroethane on Ni–M alloys to obtain a carbon nanomaterial (CNM) showing high performance in adsorption treatment of water to remove 1,2-dichlorobenzene, was presented. A series of finely dispersed Ni–Pd (5 wt %) and Ni–Mo (5 wt %) alloys were synthesized and studied. The samples were studied as catalysts in decomposition of C2H4Cl2 vapor at 600°С to obtain a carbon nanomaterial. The addition of 5 wt % second metal leads to an increase in the yield of the carbon nanomaterial from 20.1 to 25.4 (Ni–Pd) and 31.8 gCNM g–1cat (Ni–Mo). Analysis by electron microscopy and Raman spectroscopy shows that the carbon product consists of nanofibers of segmented structure, constituted by a poorly ordered graphite phase. The specific surface area of the carbon nanomaterial is 230–280 m2 g–1. The CNM/Ni, CNM/Ni–Pd, and CNM/Ni–Mo samples obtained were tested as adsorbents for water treatment to remove dissolved 1,2-dichlorobenzene (с0 = 73–880 μM) in the batch mode. The 1,2-dichlorobenzene adsorption isotherms were constructed. The degree of filling of the carbon nanomaterial surface with the adsorbate at equilibrium is 43–47%, exceeding by a factor of more than 2 the utilization efficiency of AG-2000 activated carbon (SBET = 1230 m2 g–1).






Similar content being viewed by others
REFERENCES
Muganlinskii, F.F., Treger, Yu.A., and Lyushin, M.M., Khimiya i tekhnologiya galogenorganicheskikh soedinenii (Chemistry and Technology of Halogenated Organic Compounds), Moscow: Khimiya, 1991.
Flid, M.R. and Treger, Yu.A., Vinilkhlorid: khimiya i tekhnologiya (Vinyl Chloride: Chemistry and Technology), Moscow: Kalvis, 2008, book 1.
Zhou, Y., Tigane, T., Li, X., Truu, M., Truu, J., and Mander, U., Water Res., 2013, vol. 47, no. 1, pp. 102–110. https://doi.org/10.1016/j.watres.2012.09.030
Yufit, S.S., Yady vokrug nas: Vyzov chelovechsetvu (Poisons around Us: a Challenge for Mankind), Moscow: Klassik Stil’, 2002.
Stockholm Convention on Persistent Organic Pollutants, ratified by Federal Law no. 164-FZ of June 27, 2011.
Yalkowsky, S.H. and Yan, H., Handbook of Aqueous Solubility Data, CRC, 2003, pp. 205–206.
Pelech, R., Milchert, E., and Wrobel, R., J. Hazard. Mater., 2006, vol. 137, no. 3, pp. 1479–1487. https://doi.org/10.1016/j.jhazmat.2006.04.023
Kirsanov, M.P. and Shishkin, V.V., Foods Raw Mater., 2016, vol. 4, pp. 148–153. https://doi.org/10.21179/2308-4057-2016-1-148-153
Mishakov, I.V., Chesnokov, V.V., Buyanov, R.A., and Chuvilin, A.L., React. Kinet. Catal. Lett., 2002, vol. 76, no. 2, pp. 361–367. https://doi.org/10.1023/A:1016504532177
Bauman, Yu.I., Mishakov, I.V., Vedyagin, A.A., and Dmitriev, S.V., Catal. Ind., 2012, vol. 4, no. 4, pp. 261–266. https://doi.org/10.1134/S2070050412040034
Mishakov, I.V., Vedyagin, A.A., Bauman, Y.I., Shubin, Y.V., and Buyanov, R.A., in Carbon Nanofibers: Synthesis,Applications, and Performance, Nova Science, 2018, pp. 77–181.
Bauman, Y.I., Mishakov, I.V., Rudneva, Y.V., Plyusnin, P.E., Shubin, Y.V., Korneev, D.V., and Vedyagin, A.A., Ind. Eng. Chem. Res., 2019, vol. 58, no. 2, pp. 685–694. https://doi.org/10.1021/acs.iecr.8b02186
Bauman, Y.I., Rudneva, Y.V., Mishakov, I.V., Plyusnin, P.E., Shubin, Y.V., Korneev, D.V., Stoyanovskii, V.O., Vedyagin, A.A., and Buyanov, R.A., Heliyon, 2019, vol. 5, ID e02428. https://doi.org/10.1016/j.heliyon.2019.e02428
Rudnev, A.V., Lysakova, A.S., Plyusnin, P.E., Bauman, Yu.I., Shubin, Yu.V., Mishakov, I.V., Vedyagin, A.A., and Buyanov, R.A., Inorg. Mater., 2014, vol. 50, no. 6, pp. 566–571. https://doi.org/10.1134/S0020168514060156.
Peng, X., Li, Y., Luan, Z., Di, Z., Wang, H., Tian, B., and Jia, Z., Chem. Phys. Lett., 2003, vol. 376, nos. 1–2, pp. 154–158. https://doi.org/10.1016/S0009-2614(03)00960-6
Klyuchnikov, N.G., Rukovodstvo po neorganicheskomu sintezu (Guide to Inorganic Synthesis), Moscow: Khimiya, 1965.
Li, X. and Chen, G.-H., Mater. Lett., 2009, vol. 63, no. 11, pp. 930–932. https://doi.org/10.1016/j.matlet.2009.01.042
Negrea, P., Sidea, F., Negrea, A., Lupa, L., Ciopec, M., and Muntean, C., Bul. Sti. Univ. Politeh. Timisoara, 2008, vol. 53, nos. 1–2, pp. 144–146.
Kazakova, M.A., Kuznetsov, V.L., Bokova-Sirosh, S.N., Krasnikov, D.V., Golubtsov, G.V., Romanenko, A.I., Prosvirin, I.P., Ishchenko, A.V., Orekhov, A.S., Chuvilin, A.L., and Obraztsova, E.D., Phys. Status Solidi B, 2018, vol. 255, p. 1700260. https://doi.org/10.1002/pssb.201700260
Bayat, N., Rezaei, M., and Meshkani, F., Int. J. Hydrogen Energy, 2016, vol. 41, pp. 5494–5503. https://doi.org/10.1016/j.ijhydene.2016.01.134
Grabke, H.J., Spiegel, M., and Zahs, A., Mater. Res., 2004, vol. 7, pp. 89–95. https://doi.org/10.1590/S1516-14392004000100013
Chambers, A. and Baker, R.T.K., J. Phys. Chem. B, 1997, vol. 101, pp. 1621–1630. https://doi.org/10.1021/jp963031i
Nemanich, R.J. and Solin, S.A., Phys. Rev. B, 1979, vol. 20, pp. 392–401. https://doi.org/10.1103/PhysRevB.20.392
Tuinstra, F. and Koenig, J.L., J. Chem. Phys., 1970, vol. 53, pp. 1126–1130. https://doi.org/10.1063/1.1674108
Ferrari, A.C. and Robertson, J., Phys. Rev. B, 2000, vol. 61, pp. 14095–14107. https://doi.org/10.1103/PhysRevB.61.14095
Derylo-Marczewska, A., Marczewski, A.W., Winter, Sz., and Sternik, D., Appl. Surf. Sci., 2010, vol. 256, no. 17, pp. 5164–5170. https://doi.org/10.1016/j.apsusc.2009.12.085
Oliveira, L.C.A., Rios, R.V.R.A., Fabris, J.D., Garg, V., Sapag, K., and Lago, R.M., Carbon, 2002, vol. 40, no. 12, pp. 2177–2183. https://doi.org/10.1016/S0008-6223(02)00076-3
Derylo-Marczewska, A., Buczek, B., and Swiatkowski, A., Appl. Surf. Sci., 2011, vol. 257, pp. 9466–9472. https://doi.org/10.1016/j.apsusc.2011.06.036
Kaneko, Y., Abe, M., and Ogino, K., Colloids Surf., 1989, vol. 37, pp. 211–222. https://doi.org/10.1016/0166-6622(89)80120-9
Giles, C.H., MacEwan, T.H., Nakhwa, S.N., and Smith, D., J. Chem. Soc., 1960, vol. 111, pp. 3973–3993. https://doi.org/10.1016/j.ijhydene.2016.01.134
Sule, M.N., Templeton, M.R., and Bond, T., Environ. Technol., 2015, vol. 37, no. 11, pp. 1382–1389. https://doi.org/10.1080/09593330.2015.1116610
Netskina, O.V., Komova, O.V., Tayban, E.S., Oderova, G.V., Mukha, S.A., Kuvshinov, G.G., and Simagina, V.I., Appl. Catal. A, 2013, vol. 467, pp. 386–393. https://doi.org/10.1016/j.apcata.2013.07.046
ACKNOWLEDGMENTS
The authors are grateful to A.S. Smorygina for the spectrophotometric study of the 1,2-dichlorobenzene adsorption, to D.V. Korneev for the study of the carbon material samples by transmission electron microscopy, and to M.Yu. Tashlanov for technical support of the synthesis of the carbon nanomaterial.
Funding
The study was performed within the framework of government assignment no. 075-00268-20-02 (identifier: 0718-2020-0040).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 12, pp. 1779–1789, December, 2020 https://doi.org/10.31857/S0044461820120099
Rights and permissions
About this article
Cite this article
Bauman, Y.I., Netskina, O.V., Mukha, S.A. et al. Adsorption of 1,2-Dichlorobenzene on a Carbon Nanomaterial Prepared by Decomposition of 1,2-Dichloroethane on Nickel Alloys. Russ J Appl Chem 93, 1873–1882 (2020). https://doi.org/10.1134/S1070427220120095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220120095