Abstract
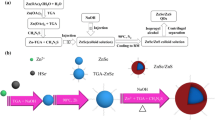
Zinc-blende CdSe cores were used to produce CdSe/ZnS core-shell quantum dots with green emission (λem = 520 nm). The shell growth was realized at low temperature using stable reagents as ZnS precursors. The optimal conditions for shell growth were determined. It was shown that the reaction time and concentration of ZnS precursors in initial solution strongly affects the optical properties of the resulted coreshell quantum dots. The CdSe/ZnS quantum dots were transferred to aqueous solution by ligand exchange with mercaptopropionic acid, and denaturated bovine serum albumin was added to improve chemical stability of CdSe/ZnS solution. As a result, highly luminescent water-soluble CdSe/ZnS quantum dots (quantum yield 36%) which can be used as biolabels in immunoassay were obtained.
Similar content being viewed by others
References
J. L. Sung, Ch. Bonghwan, J. Taiha, and K. Sh. Seung, “Synthesis and Characterization of Zinc-Blende CdSe-Based Core/Shell Nanocrystals and Their Luminescence in Water,” J. Phys. Chem. 112(6), 1744–1747 (2008).
G. Xiaohu, C. W. Warren, and Sh. N. Chan, “Quantum-Dot Nanocrystals for Ultrasensitive Biological Labeling and Multicolor Optical Encoding,” J. Biomed. Opt. 7(4), 532–537 (2002).
J. Drbohlavova, V. Adam, R. Kizek, and J. Hubalek, “Quantum Dots—Characterization, Preparation and Usage in Biological Systems,” Int. J. Mol. Sci. 10(2), 656–673 (2009).
R. Gill, M. Zayats, and I. Willner, “Semiconductor Quantum Dots for Bioanalysis,” Angew. Chem. Int. Ed. 47(40), 7602–7625 (2008).
M. M. Dzagli, V. Canpean, M. Iosin, M. A. Mohou, and S. Astilean, “Study of the Interaction between CdSe/ZnS Core-Shell Quantum Dots and Bovine Serum Albumin by Spectroscopic Techniques,” J. Photochem. Photobiol. A: Chem. 215(1), 118–122 (2010).
P. Reiss, M. Protière, and L. Liang, “Core/Shell Semiconductor Nanocrystals,” Small 5(2), 154–168 (2009).
D. V. Talapin, A. L. Rogach, A. Kornowski, M. Haase, and H. Weller, “Highly Luminescent Monodisperse CdSe and CdSe/ZnS Nanocrystals Synthesized in a Hexadecylamine-Trioctylphosphine Oxide-Trioctylphospine Mixture,” Nano Lett. 1(4), 207–211 (2001).
M. Protiere and P. Reiss, “Facile Synthesis of Monodisperse ZnS Capped CdS Nanocrystals Exhibiting Efficient Blue Emission,” Nanoscale Res. Lett. 1, 62–67 (2006).
X. Xia, Z. Liu, G. Du, Y. Li, and M. Ma, “Structural Evolution and Photoluminescence of Zinc-Blende CdSe-Blende CdSe/ZnS Nanocrystals,” J. Phys. Chem. 114(32), 13414–13420 (2010).
J. J. Li, Y. A. Wang, W. Guo, J. C. Keay, T. D. Mishima, M. B. Johnson, and X. Peng, “Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive ion Layer Adsorption and Reaction,” J. Am. Chem. Soc. 125(41), 12567–12575 (2003).
D. V. Talapin and I. Mekis, S. Götzinger, A. Kornowski, O. Benson, and H. Weller, “CdSe/CdS/ZnS and CdSe/ZnSe/ZnS Core-Shell-Shell Nanocrystals,” J. Phys. Chem. B. 108(49), 18826–18831 (2004).
R. Xie, U. Kolb, J. Li, T. Basche, and A. Mews, “Synthesis and Characterization of Highly Luminescent CdSe-Core CdS/Zn0.5Cd0.5S/ZnS Multishell Nanocrystals,” J. Am. Chem. Soc. 127, 7480–7488 (2005).
M. A. Hines and P. Guyot-Sionnest, “Synthesis and Characterization of Strongly Luminescing ZnS-Capped CdSe Nanocrystals,” J. Phys. Chem. 100(2), 468–471 (1996).
J. Jasieniak, C. Bullen, J. van Embden, and P. Mulvaney, “Phosphine-Free Synthesis of CdSe Nanocrystals,” J. Phys. Chem. B 109(44), 20665–20668 (2005).
Q. Wang, Yu. Kuo, Yu. Wang, G. Shin, C. Ruengrugli- kit, and Q. Huang, “Luminescent Properties of Water-Soluble Denatured Bovine Serum Albumin-Coated CdTe Quantum Dots,” J. Phys. Chem. B 110, 16860–16866 (2006).
M. B. Mohamed, D. Tonti, A. Al-Salman, A. Chemseddine, and M. Chergui, “Synthesis of High Quality Zinc Blende CdSe Nanocrystals,” J. Phys. Chem. B 109(21), 10533–10537 (2005).
B. Fritzinger, R. K. Capek, K. Lambert, J. C. Martins, and Z. Hens, “Utilizing Self-Exchange to Address the Binding of Carboxylic Acid Ligands to CdSe Quantum Dots,” J. Am. Chem. Soc. 132, 10195–10201 (2010).
R. K. Capek, I. Moreels, K. Lambert, D. De Muynck, Q. Zhao, A. van Tomme, F. Vanhaecke, and Z. Hens, “Optical Properties of Zincblende Cadmium Selenide Quantum Dots,” J. Phys. Chem. 114(14), 6371–6376 (2010).
W. W. Yu, E. Chang, R. Drezek, and V. L. Colvin, “Water-Soluble Quantum Dots for Biomedical Applications,” Biochem. Biophys. Res. Commun. 348, 781–786 (2006).
J. Aldana, Y. A. Wang, and X. Peng, “Photochemical Instability of CdSe Nanocrystals Coated by Hydrophilic Thiols,” J. Am. Chem. Soc. 123(36), 8844–8850 (2001).
J. Aldana, N. Lavelle, Yu. Wang, and X. Peng, “Size-Dependent Dissociation pH of Thiolate Ligands from Cadmium Chalcogenide Nanocrystals,” J. Am. Chem. Soc. 127(8), 2496–2504 (2005).
D. M. Willard, L. L. Carillo, J. Jung, and A. van Orden, “CdSe-ZnS Quantum Dots as Resonance Energy Transfer Donors in a Model Protein-Protein Binding Assay,” Nano Lett. 1(9), 469–474 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Speranskaya, V.V. Goftman, I.Yu. Goryacheva, 2013, published in Rossiiskie Nanotekhnologii, 2013, Vol. 8, Nos. 1–2.
Rights and permissions
About this article
Cite this article
Speranskaya, E.S., Goftman, V.V. & Goryacheva, I.Y. Preparation of water soluble zinc-blende CdSe/ZnS quantum dots. Nanotechnol Russia 8, 129–135 (2013). https://doi.org/10.1134/S1995078013010163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995078013010163