Abstract
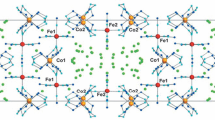
Transition-metal hydrogen maleates of composition M(C4H3O4)2 · 4H2O, where M = Mn, Fe, Co, and Ni, and their solid solutions were synthesized and characterized by X-ray crystallography, IR spectroscopy, mass spectrometry, and thermal analysis. X-ray crystallography and IR spectroscopy showed that both intramolecular and intermolecular H-bonds exist in these compounds. The generation of continuous substitutional solid solutions with cation substitution in these compounds was studied. The thermolysis mechanism was studied for both transition-metal hydrogen maleates and their solid solutions. The composite produced by thermolysis is stable up to 1200°C in flowing helium.
Similar content being viewed by others
References
Rama K. Barman, Rajesh Chkrabarty, and Birinchi K. Das, Polyhedron 21, 1189 (2002).
N. P. Porolo, Z. G. Aliev, G. I. Dzhardimalieva, et al., Izv. Akad. Nauk, Ser. Khim., No. 2, 375 (1997).
A. Sequire, H. Rajagopal, M. P. Gupta, et al., Acta Crystallogr., Sect. C 48, 1192 (1992).
A. D. Pomogailo, G. I. Dzhardimalieva, A. S. Rozenberg, and D. N. Muraviev, J. Nanoparticle Res., No. 5, 497 (2003).
M. P. Gupta, H. J. Geise, and A. T. H. Leustra, Acta Crystallogr., Sect. C 40, 1152 (1984).
V. A. Logvinenko, N. F. Yudanov, G. N. Chekhova, et al., Khim. Interesah Ustoich. Razvit. 8, 171 (2000).
V. Logvinenko, L. Yudanova, N. Yudanov, and G. Chekhova, J. Thermal Anal. Calorim. 74, 395 (2003).
A. G. Kudashov, O. G. Abrosimov, R. G. Gorbachev, et al., Fullerence, Nanotubes Carbon Nanostruct. 12(1), 93 (2004).
R. P ibil, Kompleksony v chimicheske analyse (Prague, 1953; Moscow, 1960).
Yu. Yu. Lur’e, The Analytical Chemistry Handbook (Khimiya, Moscow, 1989) [in Russian].
Analytikum. Methoden der analytischen Chemie und ihre theoretischen Grundlagen, Ed. By R. Hartig (Deutchland Verlad fuer Grundstoffindustries, 1971; Mir, Moscow, 1975).
D. Hadzi, Chimia 26,(1), 7 (1972).
M. P. Gupta and A. P. Mahata, Indian J. Phys. 49, 74 (1975).
J. Cornak, J. Chomic, Ch. Kappenstein, and F. Robert, J. Chem. Soc., Dalton Trans., 2981 (1997).
H. M. F. Cardwell, J. D. Dunitz, and L. E. Orgel, J. Chem. Soc. 764(12), 3740 (1953).
Author information
Authors and Affiliations
Additional information
Original Russian Text © L.I. Yudanova, V.A. Logvinenko, L.A. Sheludyakova, N.F. Yudanov, G. N. Chekhova, N.I. Alferova, V.I. Alekseev, P.P. Semyannikov, V.I. Lisoivan, 2008, published in Zhurnal Neorganicheskoi Khimii, 2008, Vol. 53, No. 9, pp. 1559–1565.
Rights and permissions
About this article
Cite this article
Yudanova, L.I., Logvinenko, V.A., Sheludyakova, L.A. et al. Transition-metal bimaleates and their solid solutions: Synthesis, structural, and thermoanalytical study. Russ. J. Inorg. Chem. 53, 1459–1464 (2008). https://doi.org/10.1134/S0036023608090180
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023608090180