Abstract
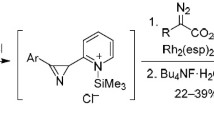
Dirhodium tetraacetate-catalyzed decomposition of diazo esters in the presence of 3-aryl-2H-azirines having no substituent in the 2-position gives rise to azirinium ylides which then undergo isomerization into 2-azabuta-1,3-diene derivatives or (in the presence of excess diazo ester) react with the corresponding rhodium carbenoid to form substituted 3,4-dihydro-2H-pyrroles. 2-Mono-and 2,2-disubstituted 3-phenyl-2H-azirines react with rhodium carbenoids generated from diazo esters to give azirinium ylides which are converted into the corresponding 2-azabuta-1,3-dienes.
Similar content being viewed by others
References
Padwa, A. and Hornbuckle, S.F., Chem. Rev., 1991, vol. 91, p. 263.
Khlebnikov, A.F., Novikov, M.S., and Kostikov, R.R., Adv. Heterocycl. Chem., 1996, vol. 65, p. 93.
Khlebnikov, A.F., Novikov, M.S., and Kostikov, R.R., Usp. Khim., 2005, vol. 74, p. 183.
Khlebnikov, A.F., Novikov, M.S., and Kostikov, R.R., Ros. Khim. Zh., 1999, vol. 43, p. 70.
Gilchrist, T.L., Aldrichim. Acta, 2001, vol. 34, p. 51.
Zwanenburg, B. and ten Holte, P., Top. Cur. Chem., 2001, vol. 216, p. 93.
Palacios, F., Ochoa de Retana, A.M., Martynez de Marigorta, E., and de los Santos, J.M., Eur. J. Org. Chem., 2001, p. 2401.
Rai, K.M.L. and Hassner, A., Advances in Strained and Interesting Organic Molecules, Halton, B., Ed., Stamford: JAI, 2000, vol. 8, p. 187.
Hassner, A., Currie, J.O., Steinfeld, A.S., and Atkinson, R.F., J. Am. Chem. Soc., 1973, vol. 95, p. 2982.
Khlebnikov, A.F., Novikov, M.S., and Amer, A.A., Tetrahedron Lett., 2002, vol. 43, p. 8523.
Khlebnikov, A.F., Novikov, M.S., and Amer, A.A., Izv. Ross. Akad. Nauk, Ser. Khim., 2004, p. 1049.
Nair, V., J. Org. Chem., 1968, vol. 33, p. 2121.
Nair, V., J. Org. Chem., 1968, vol. 33, p. 4316.
Komatsu, M., Nishikaz, N., Ohshiro, Y., and Agawa, T., J. Org. Chem., 1976, vol. 41, p. 3642.
Khlebnikov, A.F., Nikiforova, T.Yu., and Kostikov, R.R., Russ. J. Org. Chem., 1996, vol. 32, p. 715.
Tsuge, O., Ueno, K., Kanemasa, S., and Yorozu, K., Bull. Chem. Soc. Jpn., 1986, vol. 59, p. 1809.
Achiwa, K., Sugiyama, K., and Sekiya, M., Chem. Pharm. Bull., 1985, vol. 33, p. 1975.
Achiwa, K., Sugiyama, K., and Sekiya, M., Tetrahedron Lett., 1982, vol. 23, p. 2589.
Joucla, M., Gree, D., and Hamelin, J., Tetrahedron, 1973, vol. 29, p. 2315.
Schulthess, H. and Hansen, H.-J., Helv. Chim. Acta, 1981, vol. 64, p. 1322.
Fowler, F.W., Hassner, A., and Levy, L.A., J. Am. Chem. Soc., 1967, vol. 89, p. 2077.
Hortmann, G., Robertson, D.A., and Gillard, B.K., J. Org. Chem., 1972, vol. 37, p. 322.
Khlebnikov, A.F., Novikov, M.S., and Amer, A.A., Tetrahedron Lett., 2004, vol. 45, p. 6003.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.F. Khlebnikov, M.S. Novikov, A.A. Amer, R.R. Kostikov, J. Magull. D. Vidovic, 2006, published in Zhurnal Organicheskoi Khimii, 2006, Vol. 42, No. 4, pp. 533–544.
Rights and permissions
About this article
Cite this article
Khlebnikov, A.F., Novikov, M.S., Amer, A.A. et al. Azirinium ylides from alkoxycarbonylcarbenoids and 2H-azirines: Generation and transformations. Russ J Org Chem 42, 515–526 (2006). https://doi.org/10.1134/S1070428006040075
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070428006040075