Abstract
Sol-gel derived Nafion®/silica hybrid membranes were investigated as a potential polymer electrolyte for fuel cell applications. Membrane proton conductivity and water content were measured as a function of temperature, water vapor activity, and silica content. The hybrid membranes have a higher water content at 25 and 120°C, but not at 150 and 170°C. Despite the higher water content, the proton conductivities in the hybrid membranes are lower than, or equal to, that in unmodified Nafion membranes under all conditions investigated. The proton conductivity of the hybrid membrane decreases with increasing silica content under all conditions. © 2001 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
In recent years, there has been intense research interest in the development of proton electrolyte membrane (PEM) fuel cells for transportation, distributed power, and portable power applications. A hydrated perfluorosulfonic acid membrane, such as Nafion, is typically used as the polymer electrolyte in low-temperature hydrogen/oxygen fuel cells because of its excellent chemical, mechanical, and thermal stability, as well as its relatively high proton conductivity of ca. 0.08 S/cm.1 2 3 Nafion and other perfluorinated ionomer membranes are already commercially available, and there is an extensive literature reporting physical, chemical, and operational data from these materials.
The perfluorinated ionomer membrane has some disadvantages for PEM fuel cell applications. First, the proton conductivity of a Nafion membrane depends strongly upon the relative humidity because of the hydrophilic nature of the sulfonic acid groups attached to the polymer backbone and the need for water to hydrate ionic clusters.4 5 6 When a Nafion membrane is dehydrated at temperatures exceeding the boiling point of water, the water content in the membrane diminishes significantly, thus lowering the conductivity. Consequently, Nafion is regarded as inappropriate for fuel cell applications above 100°C, whereas higher temperature operation is attracting increasing interest in the development of direct methanol fuel cells (DMFC) and reformate/air fuel cells.7 8 9 Nafion also has a relatively high rate of methanol crossover, which exerts a largely negative impact on the performance of DMFCs. Finally, the mechanical strength needed for self-supporting thin films, especially less than 50 μm thick, is lacking in the perfluorinated ionomer membranes.
Mauritz and co-workers have reported a novel Nafion/silica hybrid membrane, which is made by carrying out the acid-catalyzed sol-gel reaction of tetraethoxysilane (TEOS) to form silica inside a Nafion membrane.10
11
12
13
14
15 On exposure of a Nafion membrane to a solution containing water, alcohol, and hydrolyzed alkoxides, these polar molecules will preferentially migrate to the polar clusters inside the membrane. Subsequent hydrolysis and polymerization of sorbed alkoxides are confined to these clusters as if they are nanometer in scale reaction vessels. Small-angle X-ray scattering measurements revealed that the polar/nonpolar nanophase-separated morphological template persists despite the invasion by silica. Mauritz et al.12
13 also reported the possibility of using Nafion/silica hybrid membrane in PEM fuel cell applications. Anticipated advantages include a higher water uptake, but lower methanol uptake, and greater mechanical strength as compared to an unmodified Nafion membrane. In addition, porous  is known as a proton conductor,16 which may help to improve the conductivity at elevated temperatures. These features, if realized, could overcome some of the above-mentioned disadvantages of an unmodified Nafion membrane in fuel cell use.
is known as a proton conductor,16 which may help to improve the conductivity at elevated temperatures. These features, if realized, could overcome some of the above-mentioned disadvantages of an unmodified Nafion membrane in fuel cell use.
The equilibrium water uptake measurements of Deng et al.13 found that inserting silica enhanced the hydrophilicity of clusters in Nafion. This effect was attributed to the presence of  groups, as revealed by Fourier transform infrared (FTIR) spectroscopy,14 to which
groups, as revealed by Fourier transform infrared (FTIR) spectroscopy,14 to which  molecules can hydrogen bond. A differential scanning calorimetry (DSC) study, reported in Ref. 13 suggests that the physically sorbed water should be driven off at a higher temperature, around 170°C, as compared to
molecules can hydrogen bond. A differential scanning calorimetry (DSC) study, reported in Ref. 13 suggests that the physically sorbed water should be driven off at a higher temperature, around 170°C, as compared to  in
in  bonds. The report also suggests, based on dielectric relaxation measurements,13
15 an intercluster proton hopping mechanism along the large hydrative silica, which may lead to enhanced proton conductivity. Consequently, these experimental findings suggest that proton conductivity in a Nafion/silica hybrid membrane will be higher than in unmodified Nafion, especially at higher temperatures when the water content is low.
bonds. The report also suggests, based on dielectric relaxation measurements,13
15 an intercluster proton hopping mechanism along the large hydrative silica, which may lead to enhanced proton conductivity. Consequently, these experimental findings suggest that proton conductivity in a Nafion/silica hybrid membrane will be higher than in unmodified Nafion, especially at higher temperatures when the water content is low.
Lee et al.17 and Adjemian et al.18 reported that a Nafion/silica hybrid membrane with low silica content, around 6 wt %, gave better electrochemical performance than an unmodified Nafion membrane in fuel cells operated above 100°C. However, the reason for the better electrochemical performance was not elucidated.
The purpose of this paper is to report measurements of proton conductivity in sol-gel derived Nafion/silica hybrid membranes and relate these values to their water content. Measurements were made at temperatures of 25, 120, 150, and 170°C, and as a function of water vapor activity and silica content.
Experimental
Preparation of Nafion/silica hybrid membranes
All membranes utilized in this work were Nafion 117 (DuPont, acid form). Prior to modification, the membranes were purified as follows. First, they were boiled in deionized water for 2 h, and then immersed in 6 wt %  at 90°C for 8 h. Next, they were cleaned by immersion in 6 wt %
at 90°C for 8 h. Next, they were cleaned by immersion in 6 wt %  and 5 wt %
and 5 wt %  at 90°C for 10 min each. Finally, they were boiled again for 2 h, and then immersed in deionized water until used. Samples were dried at 110°C under vacuum to determine the initial, dry
at 90°C for 10 min each. Finally, they were boiled again for 2 h, and then immersed in deionized water until used. Samples were dried at 110°C under vacuum to determine the initial, dry  form weight before the sol-gel reaction.
form weight before the sol-gel reaction.
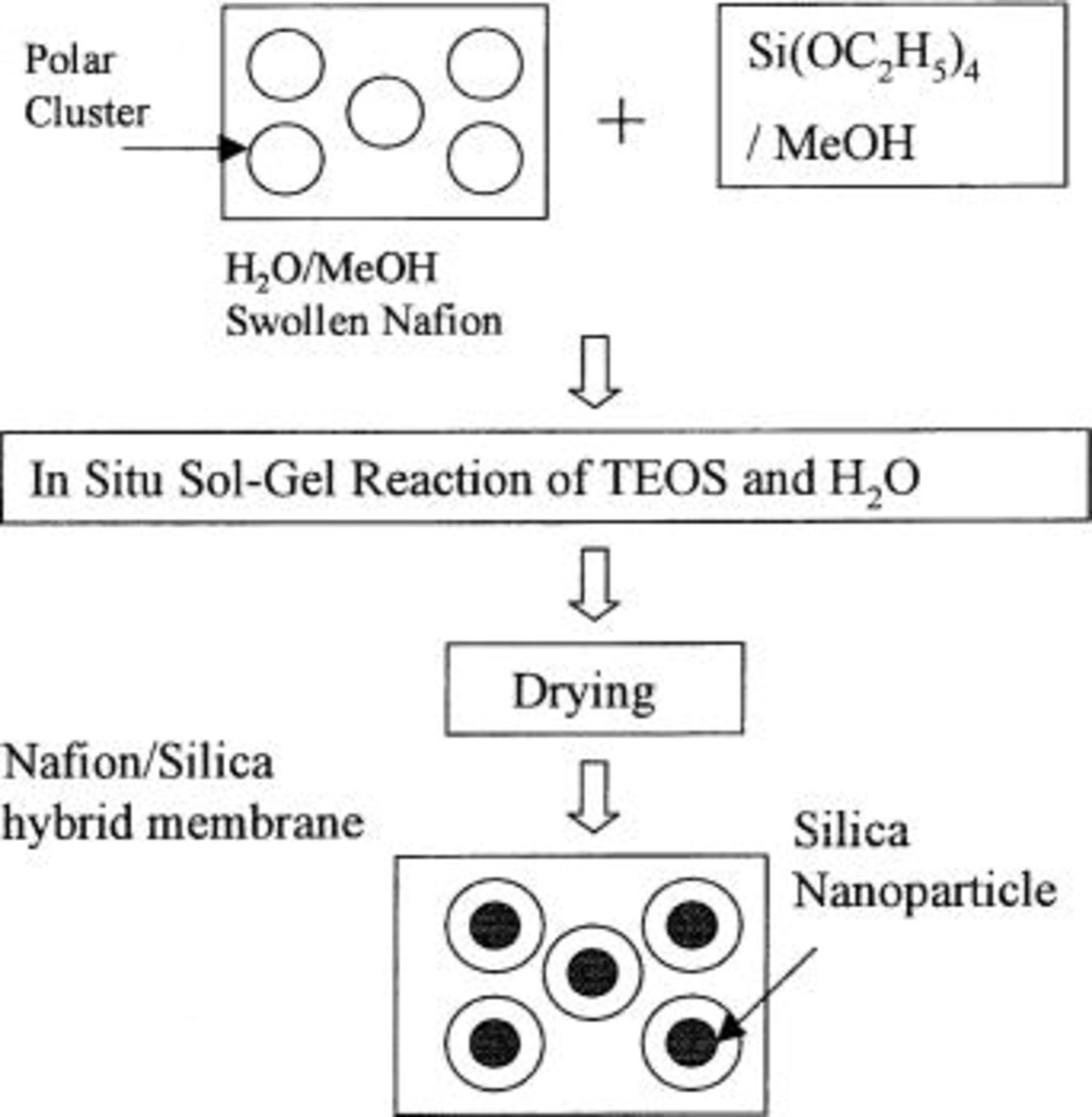
The samples of Nafion/silica hybrid membrane were made by the method reported by Mauritz et al.13 In Fig. 1 a schematic of the preparation of the Nafion/silica hybrid membrane is shown. Nafion 117 membrane samples were swollen overnight in stirred room temperature solutions of  Premixed TEOS/MeOH solutions were then introduced into the flask with the membrane samples so that the
Premixed TEOS/MeOH solutions were then introduced into the flask with the membrane samples so that the  ratio was 4:1 (mol/mol), then the flask was capped and stirred. After the prescribed time for the sol-gel reaction, the samples were removed from the flask, and then quickly (1-2 s) soaked in MeOH to wash away excess reactants adhering to the surface. The time between the addition of the TEOS/MeOH solution and removal of samples from the flask is referred to as the "reaction time." The silica content was dependent on the reaction time as well as on the membrane volume relative to the total volume of solution (e.g.,
ratio was 4:1 (mol/mol), then the flask was capped and stirred. After the prescribed time for the sol-gel reaction, the samples were removed from the flask, and then quickly (1-2 s) soaked in MeOH to wash away excess reactants adhering to the surface. The time between the addition of the TEOS/MeOH solution and removal of samples from the flask is referred to as the "reaction time." The silica content was dependent on the reaction time as well as on the membrane volume relative to the total volume of solution (e.g.,  Nafion 117 sample immersed in 560 mL solution for 1, 5, and 35 min results in a 4, 10, and 20 wt % silica hybrid membrane, respectively). Finally, samples were surface-blotted, then dried at 110°C under vacuum. In each case, the silica content was normalized relative to the initial weight of the unfilled dry
Nafion 117 sample immersed in 560 mL solution for 1, 5, and 35 min results in a 4, 10, and 20 wt % silica hybrid membrane, respectively). Finally, samples were surface-blotted, then dried at 110°C under vacuum. In each case, the silica content was normalized relative to the initial weight of the unfilled dry  form. The silica content was varied between approximately 3 and 26 wt %. Prior to performing conductivity measurements, all samples were boiled in water.
form. The silica content was varied between approximately 3 and 26 wt %. Prior to performing conductivity measurements, all samples were boiled in water.
Figure 1. Schematic showing preparation of Nafion/silica hybrid membrane.
Characterization of Nafion/silica hybrid membranes by microscopy
A scanning electron microscope (Hitachi, S-2700) was used to probe surface morphology of the hybrid membrane samples. All micrographs were taken at an acceleration voltage of 1 kV and at a magnification of 10,000 times. The X-ray energy-dispersive spectrometer attachment of the electron spin echo envelope modulation (ESEEM) (Horiba, EMAX-5770; spot probe set at 15 kV and 0.3 nA) was used to assess the distribution of silicon and sulfur across fields of view on surfaces and cross sections. The integrated intensity of the sulfur (S) peak in the X-ray spectrum quantifies the population of  groups. The S peak intensity for a selected region is taken as a reference for the polymer matrix so that the Si/S intensity ratio is a relative measure of silica content. In order to obtain a silica concentration profile across the cross section, each membrane sample was fractured smoothly after being immersed and cured in an epoxy resin. The Si/S intensity ratio was measured at five equally spaced points across the membrane thickness. The Si/S intensity ratio was also measured on the surface of the membrane samples. While the microstructure at the scale of clusters is beyond ESEEM resolution, the important aspect of the silica concentration profile can be explored.
groups. The S peak intensity for a selected region is taken as a reference for the polymer matrix so that the Si/S intensity ratio is a relative measure of silica content. In order to obtain a silica concentration profile across the cross section, each membrane sample was fractured smoothly after being immersed and cured in an epoxy resin. The Si/S intensity ratio was measured at five equally spaced points across the membrane thickness. The Si/S intensity ratio was also measured on the surface of the membrane samples. While the microstructure at the scale of clusters is beyond ESEEM resolution, the important aspect of the silica concentration profile can be explored.
Measurement of conductivity and water content at 25°C
Conductivity measurements were based on a two-electrode configuration along the long axis of membrane samples that were cut into strips of approximately 0.5 cm wide and 3 cm long. The samples were equilibrated with water vapor by suspending them above a large volume of aqueous LiCl solution in a closed container. The composition of the LiCl solution then determined the water vapor activity. The conductivity was measured while the sample was in the equilibration chamber using ac impedance measured between 1 Hz and 10 kHz by using a Solartron 1260 frequency response analyzer with a Solartron 1287 potentiostat. The resistance value associated with the membrane conductance was determined from the high frequency intercept of the impedance with the real axis. The conductivity was calculated using the equation
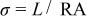
where σ, L, R, and A denote the membrane conductivity, the distance between the two electrodes, the measured resistance of the membrane, and the cross-sectional area of the membrane perpendicular to current flow, respectively. The thickness of each membrane was measured at 35% RH and room temperature before equilibrating the samples for the conductivity measurements.
Following the conductivity measurements, the samples were removed from the equilibrium bottle and weighed quickly, then dried at 110°C under vacuum, and weighed again. The water content of the membrane samples was calculated from the weight loss on drying the sample, normalized to the dry weight.
Measurement of water content at 120, 150, and 170°C
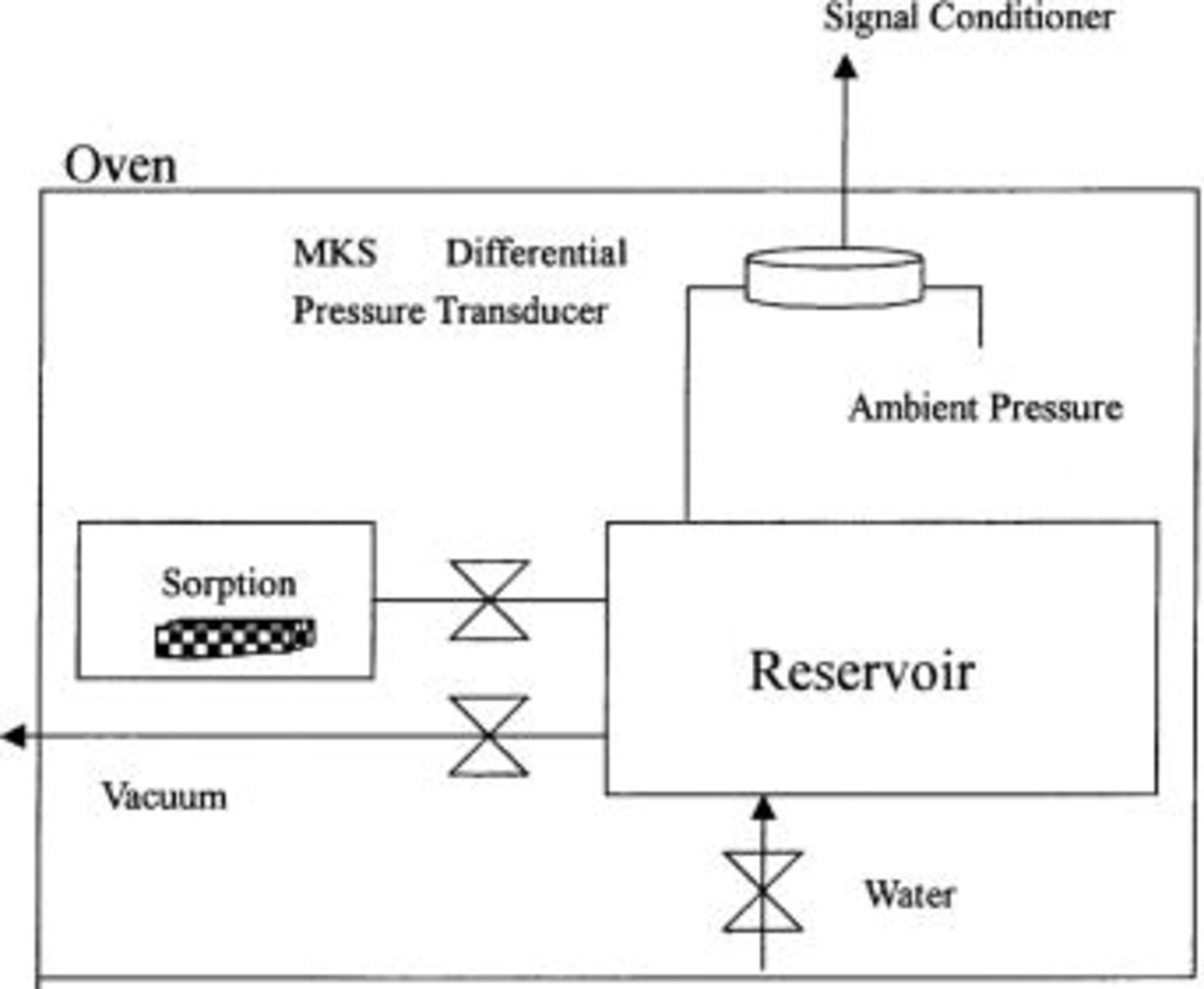
Water content determinations at 120, 150, and 170°C were made using a vapor sorption technique.19 20 The schematic of the barometric sorption cell is shown in Fig. 2. Two chambers, reservoir (310 cm3) and sorption (19 cm3), are connected by a plug valve. Individual membrane samples were placed in the sorption chamber. After the system was evacuated, the valve between the reservoir and the sorption chamber was closed. The reservoir was then filled with water vapor to the desired pressure, and the valve reopened. The pressure drop, initially due to the system volume change and then due to sorption, was recorded with a differential pressure transducer (MKS Instruments, Inc.). The change in equilibrium pressure, due to the water sorbed by the membrane sample, was used with the ideal gas law and total chamber volume to estimate the water content in the membrane. The water uptake was normalized to the dry weight of the polymer sample.
Figure 2. Schematic of the barometric sorption cell.
Conductivity measurement at 120, 150, and 170°C
Conductivity measurements at temperatures above 100°C were made with a four-probe apparatus constructed at Case Western Reserve University.21 AC current was applied to the ends of long, thin  samples. Two platinum probe wires spaced 1 cm apart near the center of the sample were used to measure the voltage drop along the long axis of the sample. The apparatus was enclosed within a sealed stainless steel vessel, which was placed inside an oven. Following heating to the prescribed temperature, thus dehydrating the membrane, liquid water was injected into this vessel through a septum to yield the desired water vapor activity. An accurate water vapor activity is expected, as there is no significant water vapor leakage from the pressurized vessel. The calculation of conductivity was done in the same manner as reported above, however, with the four-electrode apparatus it is not necessary to find the high frequency intercept of the real axis, as the resistance measured is essentially constant at all frequencies.
samples. Two platinum probe wires spaced 1 cm apart near the center of the sample were used to measure the voltage drop along the long axis of the sample. The apparatus was enclosed within a sealed stainless steel vessel, which was placed inside an oven. Following heating to the prescribed temperature, thus dehydrating the membrane, liquid water was injected into this vessel through a septum to yield the desired water vapor activity. An accurate water vapor activity is expected, as there is no significant water vapor leakage from the pressurized vessel. The calculation of conductivity was done in the same manner as reported above, however, with the four-electrode apparatus it is not necessary to find the high frequency intercept of the real axis, as the resistance measured is essentially constant at all frequencies.
Results and Discussion
Characterization of Nafion/silica hybrid membranes by microscopy
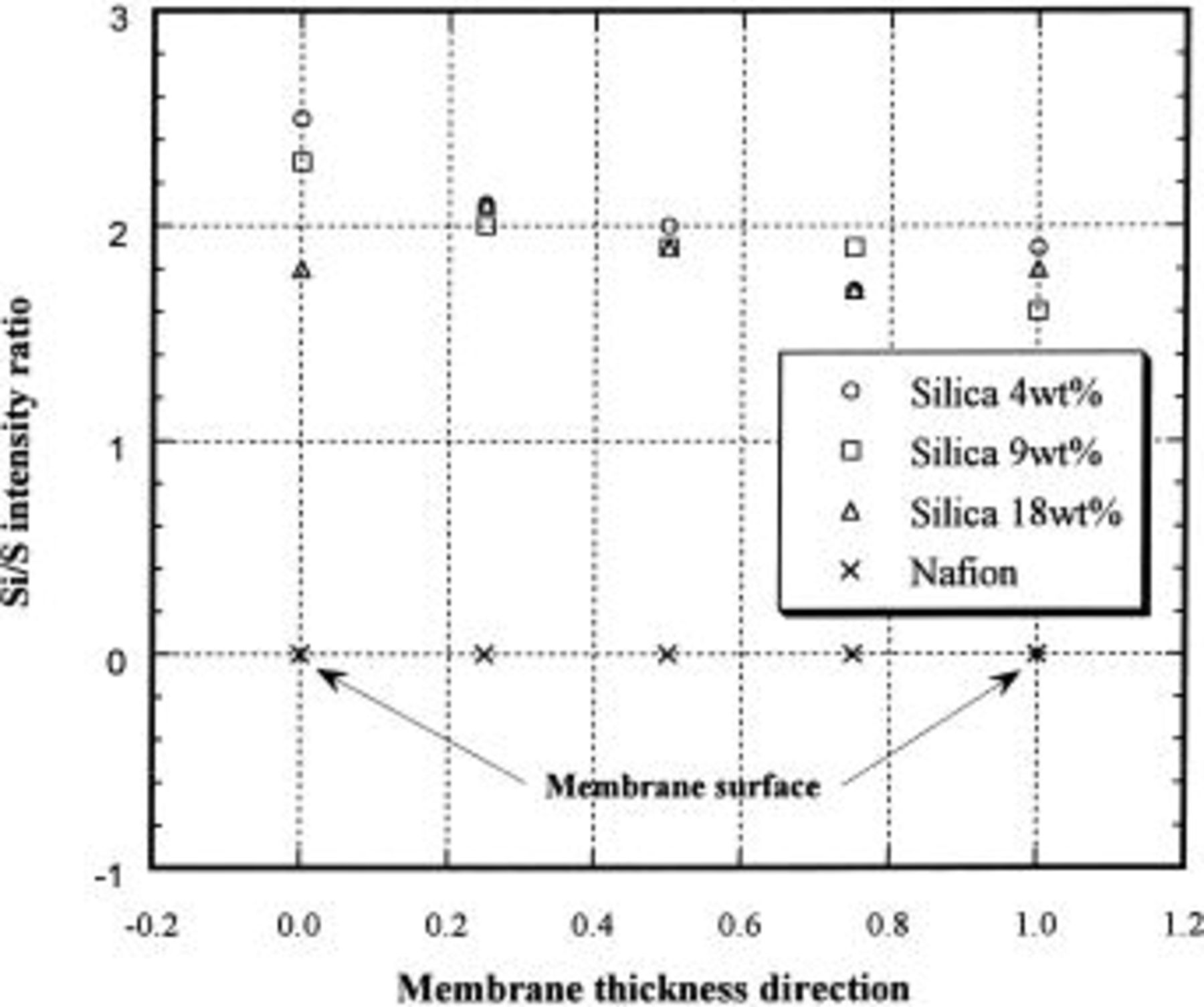
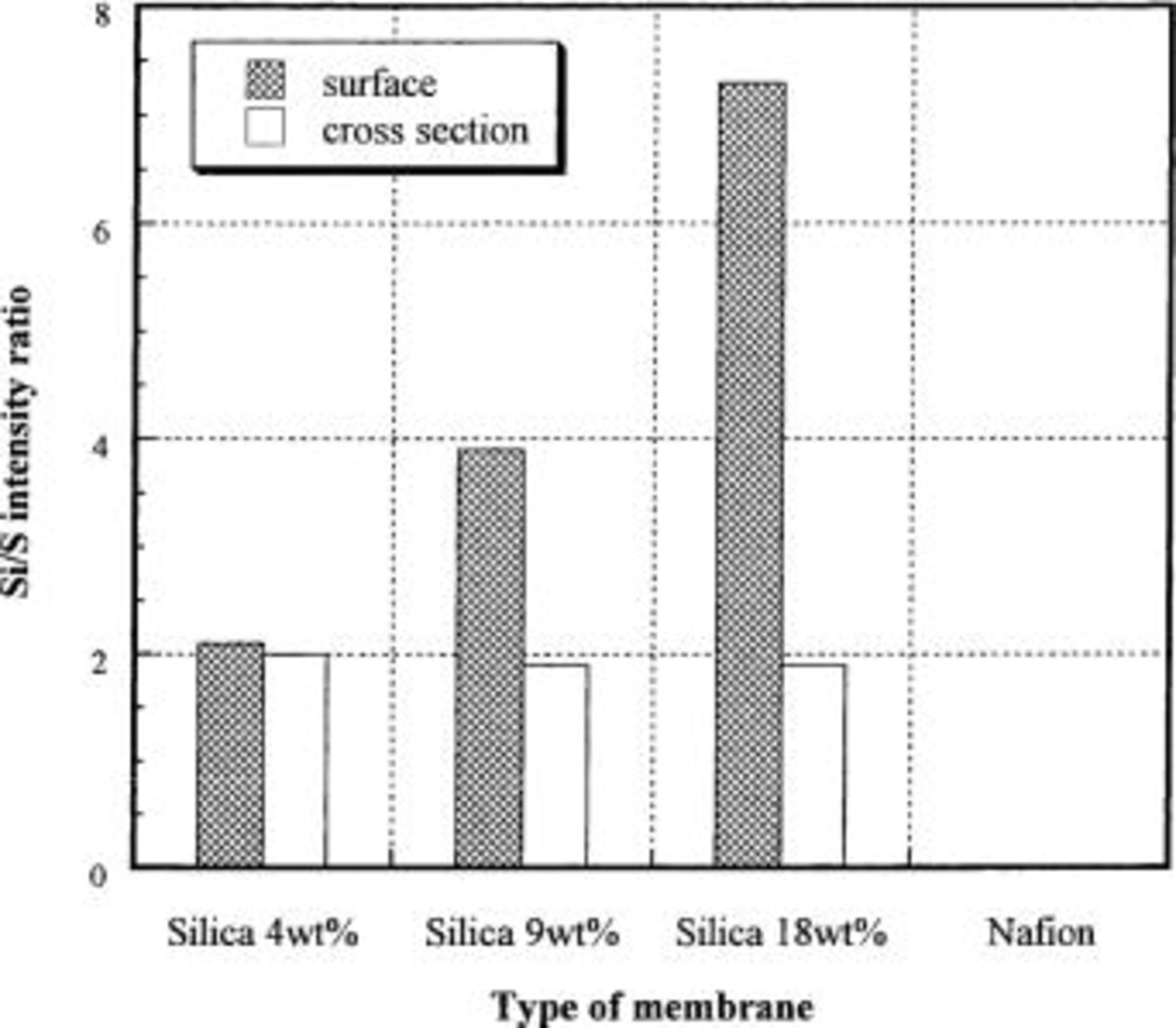
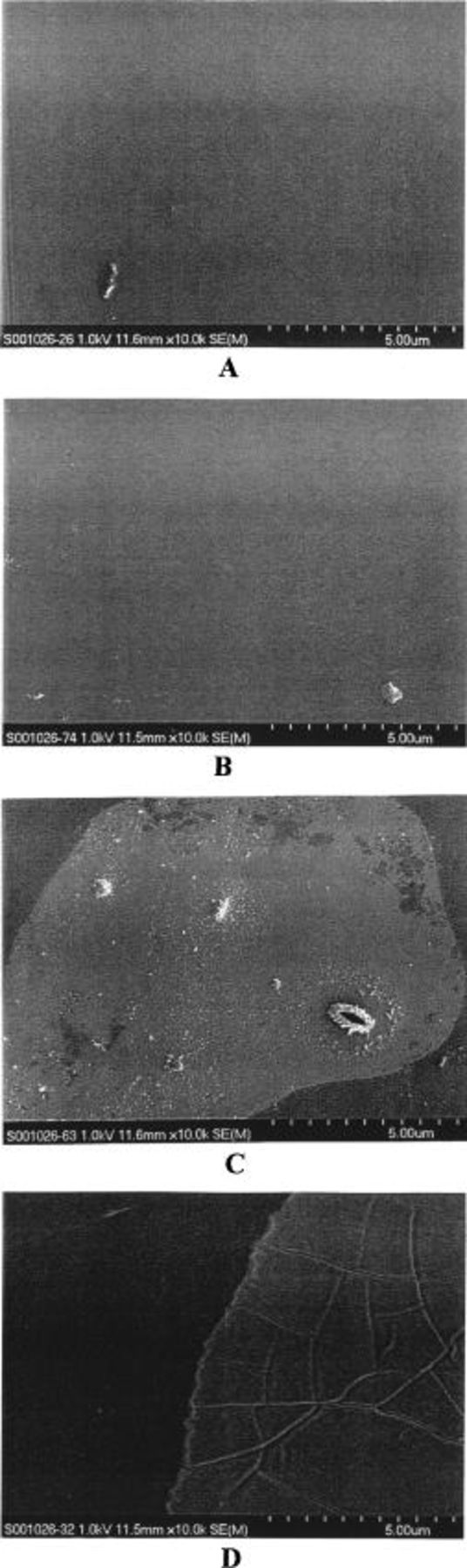
In Fig. 3 the Si/S X-ray intensity ratio (across the membrane thickness) for the Nafion/silica hybrid membranes is shown. All of the hybrid membranes have a uniform intensity ratio across the membrane thickness. In addition, there is no significant difference in intensity ratio in Fig. 3 between the hybrid membranes with different silica contents as determined by weight. In Fig. 4 the Si/S X-ray intensity ratio on the surface and in the cross section of hybrid membranes is reported. The 4 wt % silica hybrid membrane has the same intensity ratio on the surface and in the cross section. In contrast, the 9 and 18 wt % silica hybrid membranes had a higher intensity ratio on the surface than that in the cross section. The intensity ratio observed on the membrane surface clearly increases with increasing silica content. This result implies that the hybrid membranes with high silica content have a thin silica layer attached on the membrane surface, which was not detected by the analysis along the membrane cross section. The existence of the thin silica layer was confirmed by scanning electron microscopy (SEM) micrographs on the surface of hybrid membranes, as shown in Fig. 5. An 18 wt % the silica hybrid membrane has a fractured surface morphology. In comparison, a 4 wt % silica hybrid membrane has a smooth surface, much like the unmodified Nafion membrane.
Figure 3. Si/S X-ray intensity ratio across the membrane thickness for Nafion/silica hybrid membranes.
Figure 4. Si/S X-ray intensity ratio on surfaces and cross sections of Nafion/silica hybrid membranes.
Figure 5. SEMs of the surface of various membrane samples (10,000 ×): (a) unmodified Nafion membrane, (b) 4 wt % silica hybrid membrane, (c) 9 wt % silica hybrid membrane, (d) 18 wt % silica hybrid membrane.
This analysis suggests that with low silica content, the polymer/silica phase is uniform and homogeneous. However, the higher silica content systems may actually consist of two phases, one with silica in intimate contact with the polymer, and the other located on the surface and silica-rich. According to Ref. 11, the sol-gel reaction is confined mainly to polar clusters at short reaction times, but upon further reaction, the silica phase begins to percolate and becomes knit together through the ionomer matrix. The presence of two separate phases will complicate the interpretation of the adsorption and conductivity data shown below. Nevertheless, these data are reported here for completeness.
Water content in Nafion/silica hybrid membranes
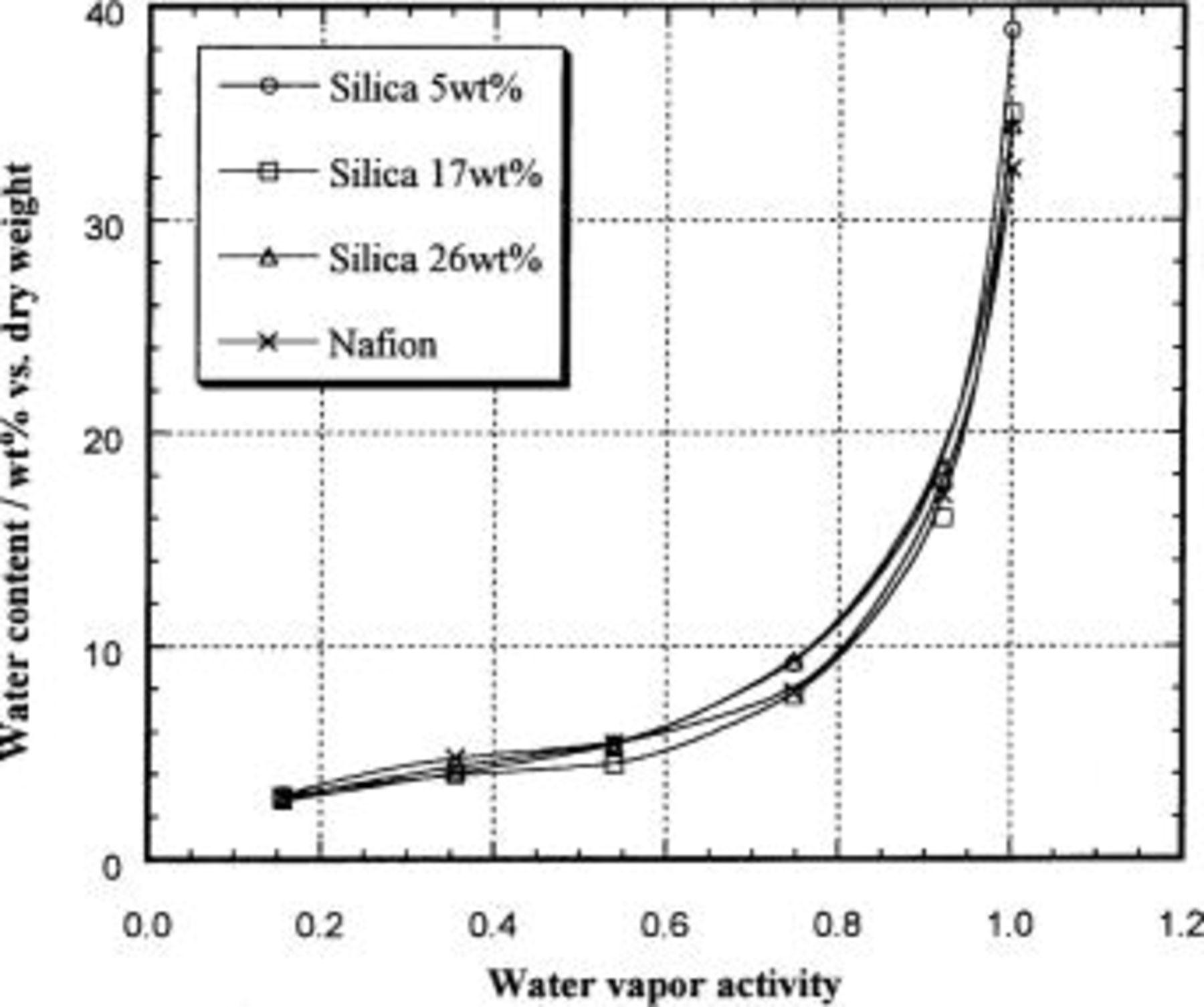
The water contents measured in Nafion/silica hybrid membranes as well as in unmodified Nafion are reported in Table I, and for a temperature of 25°C, are shown in Fig. 6 as a function of the water vapor activity. In agreement with previous reports,4 5 6 the water content in unmodified Nafion strongly depends upon the water vapor activity, and similar results were obtained for the hybrid membranes. All of the hybrid membranes have higher water content as compared to unmodified Nafion under saturated conditions. This result agrees with the water uptake study from the liquid phase, which revealed that the incorporated silica has a hygroscopic effect at room temperature.13 The highest water uptake, around 40 wt %, is obtained in a 5 wt % silica hybrid membrane. However, at water vapor activities of less than 1.0, the water content in all of the hybrid membranes is essentially the same as that of unmodified Nafion.
Table I.
| Water content wt % (based on dry membrane weight) measured on Nafion and Nafion/silica hybrid membranes at various temperatures and water vapor activities. | ||||||
|---|---|---|---|---|---|---|
| Temperature(°C) | Silica wt %Water vapor activity | 0wt % | 4-5wt % | 10-12wt % | 16-17wt % | 21-26wt % |
| 25 | 0.16 | 3.0 | 2.9 | - | 3.0 | 2.8 |
| 0.36 | 4.8 | 4.4 | - | 4.0 | 4.1 | |
| 0.54 | 5.5 | 5.0 | - | 4.5 | 5.4 | |
| 0.75 | 7.9 | 9.2 | - | 7.7 | 9.3 | |
| 0.92 | 17.1 | 17.7 | - | 16.0 | 18.4 | |
| 1.0 | 32.4 | 38.9 | - | 35.0 | 34.4 | |
| 120 | 0.02 | 1.4 | 1.0 | 0.9 | - | 1.0 |
| 0.17-0.22 | 3.5 | 4.8 | 4.7 | - | 4.7 | |
| 0.27-0.30 | 4.5 | 6.2 | 6.1 | - | 6.3 | |
| 0.34-0.38 | 5.6 | 8.1 | 7.5 | - | 8.1 | |
| 150 | 0.03 | 2.1 | 2.1 | 1.8 | - | 1.8 |
| 0.11 | 3.9 | 4.0 | 3.4 | - | 3.7 | |
| 0.18-0.19 | 5.6 | 6.1 | 5.2 | - | 5.6 | |
| 170 | 0.018-0.021 | 1.9 | 1.9 | 1.8 | - | 1.9 |
| 0.044-0.047 | 2.8 | 2.8 | 2.6 | - | 2.6 | |
| 0.10-0.12 | 4.7 | 4.8 | 4.0 | - | 4.2 | |
Figure 6. Water content in Nafion/silica hybrid membranes at 25°C as a function of water vapor activity.
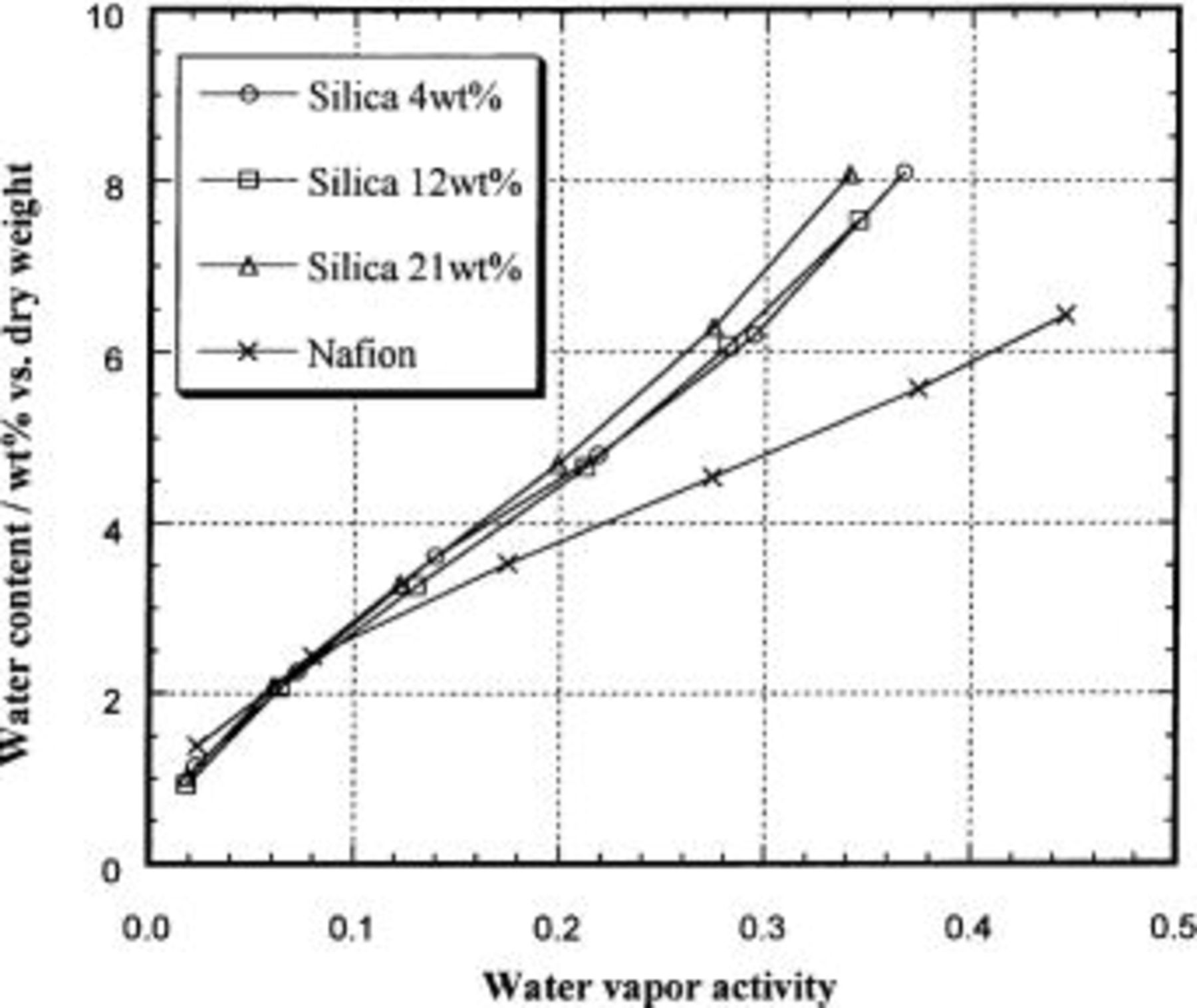
The water content vs. water vapor activity sorption isotherm for Nafion/silica hybrid membranes at 120°C is shown in Fig. 7. The water uptake by unmodified Nafion increases rapidly at low activities, but becomes linear at activities greater than 0.1. This behavior is consistent with the dual-mode sorption mechanism consisting of Henry's law sorption and Langmuir-type sorption.19 In contrast, the water uptake by the hybrid membranes appears to increase steadily as the activity is raised. The water content at 120°C of the hybrid membranes is always higher than that of unmodified Nafion above a water activity of 0.1. Furthermore, the water content in the hybrid membranes is not enhanced by increasing the silica content. This suggests that the number of ≡SiOH groups, which are thought to adsorb water, does not increase significantly with increasing silica content.
Figure 7. Water content in Nafion/silica hybrid membranes at 120°C as a function of water vapor activity.
The data reported in Table I suggest that the hygroscopic effect is not seen in any of the hybrid membranes at 150 or 170°C. Although the water content in a 4 wt % silica hybrid membrane appears slightly higher than that in unmodified Nafion above a water activity of 0.1, the difference is within experimental error. At 170°C, there is no difference in water content between the various membranes.
In differential scanning calorimetry (DSC) studies, Mauritz et al.13 showed that a broad endotherm for Nafion increases in Nafion/silica hybrid membranes. This result suggested that water molecules in the hybrid membranes are released at higher temperatures than those in an unmodified Nafion membrane, and strong hydrogen bonding sites in the form of ≡SiOH groups reside on the surface of incorporated silica. Hench et al.22 established for silica gel that the chemisorbed water (i.e.,  molecules bound to surface ≡SiOH groups) could be eliminated and surface ≡SiOH groups condensed starting around 170°C. The results reported here show that the water content in the Nafion/silica hybrid membrane is clearly higher than that in unmodified Nafion at 120°C and a water activity above 0.1, but this hygroscopic effect disappears at 150 and 170°C.
molecules bound to surface ≡SiOH groups) could be eliminated and surface ≡SiOH groups condensed starting around 170°C. The results reported here show that the water content in the Nafion/silica hybrid membrane is clearly higher than that in unmodified Nafion at 120°C and a water activity above 0.1, but this hygroscopic effect disappears at 150 and 170°C.
Proton conductivity of Nafion/silica hybrid membranes at 25°C
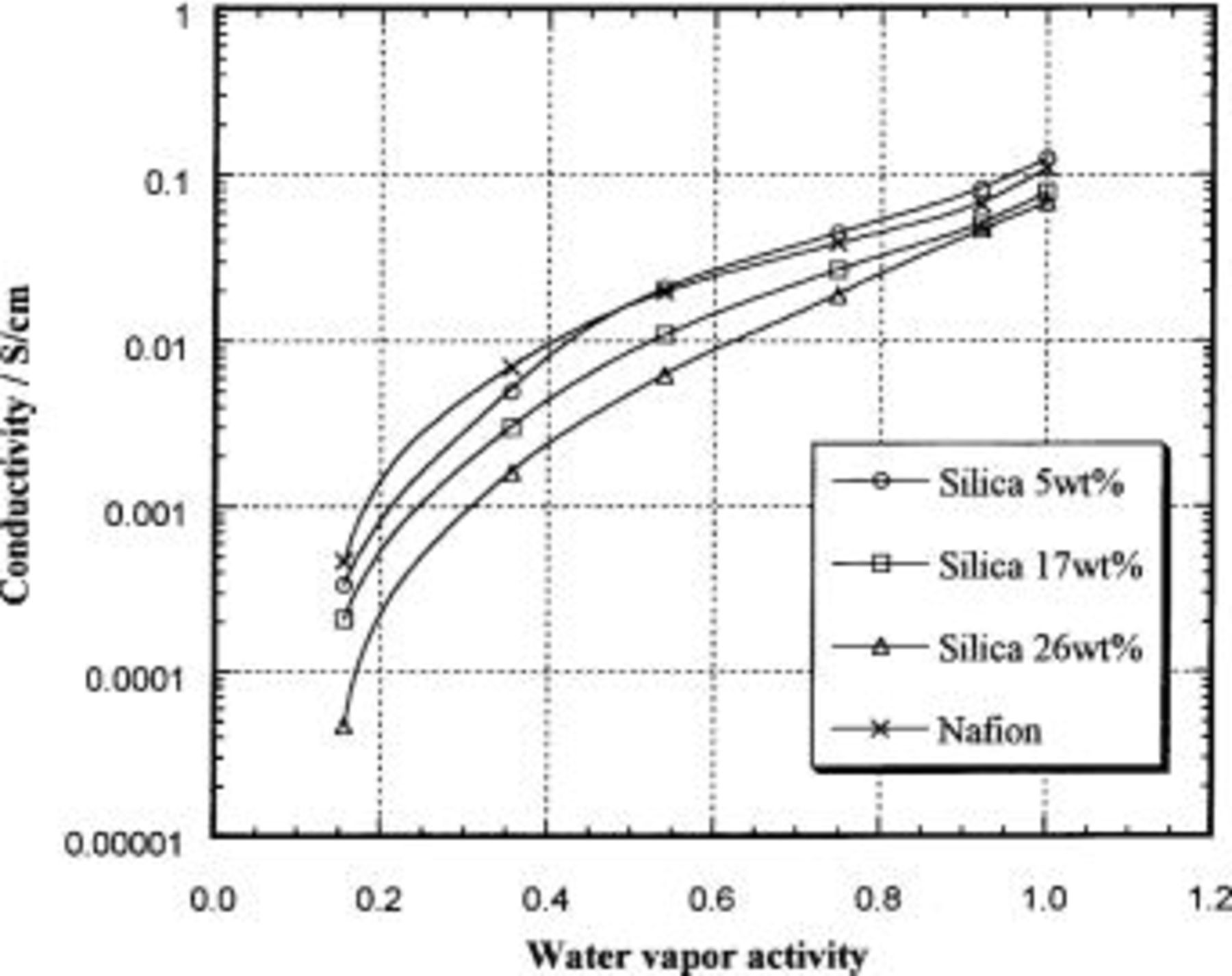
The conductivity of unmodified Nafion and the Nafion/silica hybrid membranes is reported in Table II and shown as a function of water vapor activity at 25°C in Fig. 8. The conductivity measured for unmodified Nafion agrees approximately with the data in Ref. 4 5 6. The conductivities of the hybrid membranes depend on the water vapor activity in a similar manner. The conductivity of a 5 wt % silica hybrid membrane appears to be slightly higher at high water vapor activity and slightly lower at low water vapor activity, but its value can be regarded as almost identical to that of the unmodified Nafion membrane. The conductivities of the 17 and 26 wt % silica hybrid membranes are definitely lower than that of unmodified Nafion at all water vapor activities. The conductivities were lower in spite of the fact that all of the hybrid membranes have a higher water content than unmodified Nafion at a water vapor activity of 1.0. Even at water vapor activities of 0.15 and 0.35, the conductivities of the hybrid membranes are lower than that of unmodified Nafion, although their water contents are similar.
Table II.
| Proton conductivity (S/cm) measured on Nafion and Nafion/silica hybrid membranes at various temperatures and water vapor activities. | ||||||
|---|---|---|---|---|---|---|
| Temperature(°C) | Silica wt %Water vapor activity | 0wt % | 4-5wt % | 10-12wt % | 16-17wt % | 21-26wt % |
| 25 | 0.16 | 0.0005 | 0.0003 | - | 0.0002 | 0.00005 |
| 0.36 | 0.0070 | 0.0050 | - | 0.0030 | 0.0016 | |
| 0.54 | 0.020 | 0.021 | - | 0.011 | 0.0063 | |
| 0.75 | 0.039 | 0.045 | - | 0.027 | 0.019 | |
| 0.92 | 0.067 | 0.083 | - | 0.051 | 0.047 | |
| 1.0 | 0.110 | 0.13 | - | 0.079 | 0.069 | |
| 120 | 0.29 | 0.026 | 0.025 | 0.018 | 0.016 | - |
| 0.58 | 0.117 | 0.106 | 0.082 | 0.061 | - | |
| 0.78 | 0.210 | 0.185 | 0.160 | 0.112 | - | |
| 150 | 0.09 | 0.0032 | 0.0026 | 0.0016 | 0.0015 | - |
| 0.22 | 0.018 | 0.015 | 0.012 | 0.0077 | - | |
| 0.39 | 0.060 | 0.050 | 0.040 | 0.025 | - | |
| 170 | 0.06 | 0.0018 | 0.0005 | - | - | - |
| 0.12 | 0.0023 | 0.0013 | - | - | - | |
Figure 8. Proton conductivity of Nafion/silica hybrid membranes at 25°C as a function of water vapor activity.
It is well known that proton conduction in Nafion membrane is governed by two mechanisms. One is a proton hopping (Grotthus) mechanism, and the other is a pure migration of hydrated protons  species]. It has been suggested that transport of
species]. It has been suggested that transport of  by a hopping mechanism contributes more to conduction at high water content, but little protonic hopping is expected at low water content.4
by a hopping mechanism contributes more to conduction at high water content, but little protonic hopping is expected at low water content.4
In 5 wt % Nafion/silica hybrid membranes, excess water molecules are likely to be involved in hydrating incorporated silica located in clusters. Thus, even at the same overall water content, the water available for either the hopping mechanism or to solvate protons for migration may be lower. It is reasonable to assume that this could lead to the interruption of both conduction mechanisms and thus decrease the conductivity, especially at low water content. However, a new conduction mechanism may be present, due to the presence of the incorporated silica. If the intercluster proton hopping along the large hydrative silica particles is significant, then conductivity should increase. Our results suggest that this intercluster hopping in Nafion/silica hybrid membranes is not significant at 25°C.
Turning our attention now to the membranes with higher silica content, the composition analysis reported earlier shows a rather constant  content throughout the film except on the surfaces of the membrane. With a two-phase model, the parallel resistances would be dominated by the higher conducting phase (assumed to be the Nafion/silica phase), and thus we would expect to see the same conductivity as would be seen for the membranes with no excess surface silica layer, e.g., with the 5 wt % content. The fact that lower conductivities are observed in samples with higher silica content suggests that water may be preferentially distributed into the silica-rich phase.
content throughout the film except on the surfaces of the membrane. With a two-phase model, the parallel resistances would be dominated by the higher conducting phase (assumed to be the Nafion/silica phase), and thus we would expect to see the same conductivity as would be seen for the membranes with no excess surface silica layer, e.g., with the 5 wt % content. The fact that lower conductivities are observed in samples with higher silica content suggests that water may be preferentially distributed into the silica-rich phase.
Proton conductivity of Nafion/silica hybrid membranes above 100°C
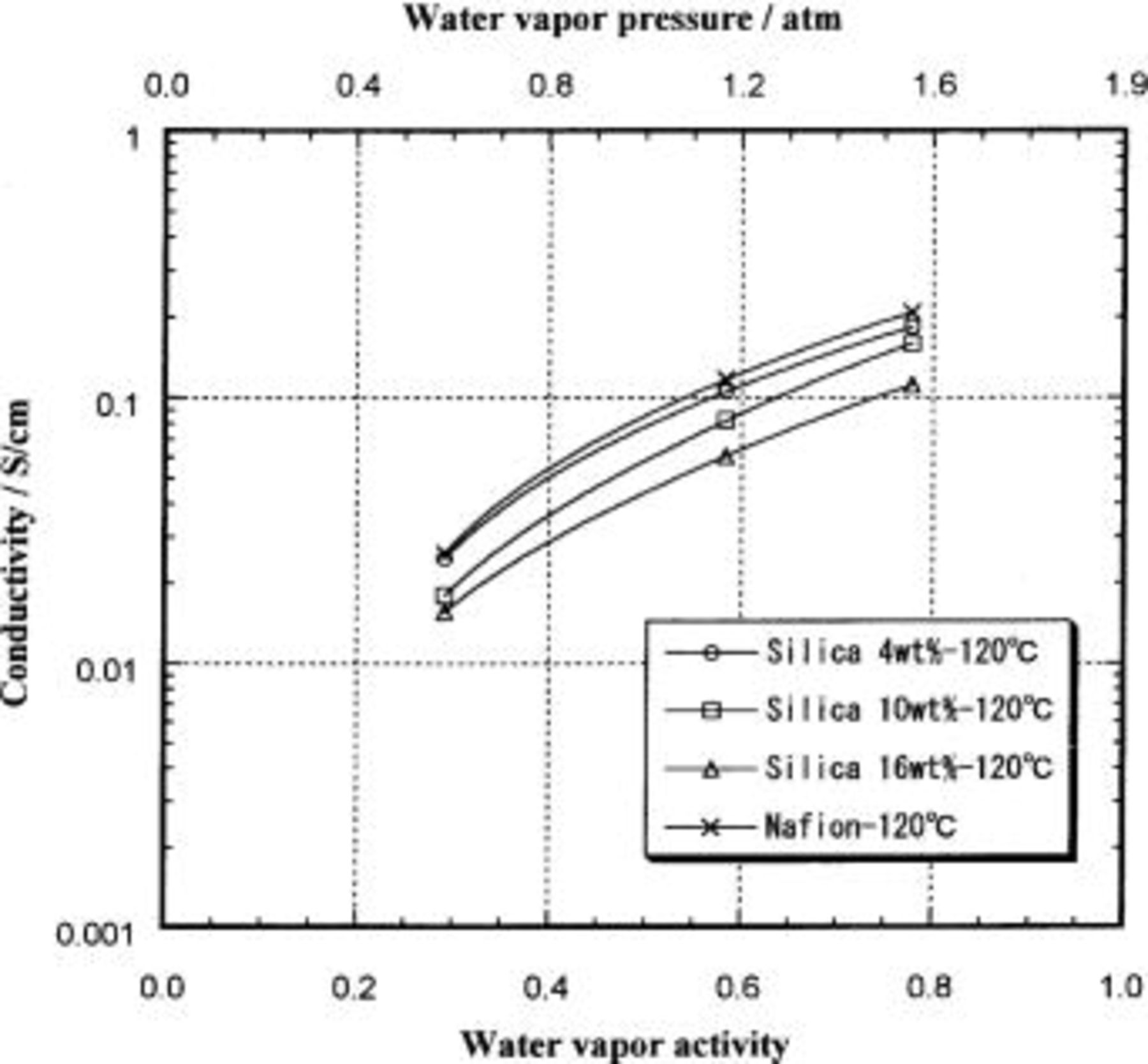
As mentioned above, the impedance spectrum of the Nafion/silica hybrid membranes was measured using a four-probe technique. The data for all temperatures and water vapor activities show that the impedance of the hybrid membranes, as with unmodified Nafion membrane,21 is purely resistive from 1 to  The conductivity of the hybrid membranes at 120°C is shown in Fig. 9 as a function of water vapor activity. The conductivity at all temperatures is equal to or lower than that of the unmodified Nafion membrane at all water vapor activities considered, in spite of the higher water content in the hybrid membranes at water activities above 0.1 (at 120°C). The intercluster proton hopping is apparently insufficiently active to contribute to the conductivity even at the higher temperatures. The observed decrease in conductivity with high silica content may follow the same explanation given above for the data taken at 25°C.
The conductivity of the hybrid membranes at 120°C is shown in Fig. 9 as a function of water vapor activity. The conductivity at all temperatures is equal to or lower than that of the unmodified Nafion membrane at all water vapor activities considered, in spite of the higher water content in the hybrid membranes at water activities above 0.1 (at 120°C). The intercluster proton hopping is apparently insufficiently active to contribute to the conductivity even at the higher temperatures. The observed decrease in conductivity with high silica content may follow the same explanation given above for the data taken at 25°C.
Figure 9. Proton conductivity of Nafion/silica hybrid membranes at 120°C as a function of water vapor activity.
Our study shows that the proton conductivity in Nafion/silica hybrid membranes is equal to or lower than unmodified Nafion under all conditions investigated. On the other hand, fuel cell results reported in the literature17 18 show improved performance at elevated temperatures. Although these results appear to be in conflict with the data reported here, the enhanced fuel cell performance may be due to other effects than simply the membrane conductivity, such as lower electrode polarization, or mitigation of water diffusion and electro-osmotic transport effects. The more complex conditions of the fuel cell make it difficult to isolate the singular effect of the membrane conductivity.
It should also be pointed out that all of the conductivity measurements reported here are in the plane of the sample, not through the plane as in the case of a fuel cell geometry. For the membranes with higher silica content that have silica-rich surface layers, it is likely that the through-plane conductivity would be lower than reported here.
Mauritz et al.10 have also showed that the ultimate tensile strength of a Nafion/silica hybrid membrane increases with increasing silica content at 50% relative humidity and 25°C. The hybrid membranes have an ultimate tensile strength of  at low silica content (around 5 wt %) and of
at low silica content (around 5 wt %) and of  at silica contents around 16 wt %, as compared to approximately
at silica contents around 16 wt %, as compared to approximately  for unmodified Nafion 1200 equivalent weight (EW) membrane. Young's modulus also clearly increased with increasing silica content. Therefore, the hybrid membrane, especially with a silica content around 5 wt %, could also be useful because of its increased mechanical strength, without sacrificing proton conductivity. Consequently, the hybrid membrane should have greater durability in the long-term fuel cell operation. An advantage of enhanced mechanical properties may also be realized by fabricating thinner membranes, less than 50 μm, which should lead to improved fuel cell performance due to reduction of internal resistance and minimization of water management difficulties.
for unmodified Nafion 1200 equivalent weight (EW) membrane. Young's modulus also clearly increased with increasing silica content. Therefore, the hybrid membrane, especially with a silica content around 5 wt %, could also be useful because of its increased mechanical strength, without sacrificing proton conductivity. Consequently, the hybrid membrane should have greater durability in the long-term fuel cell operation. An advantage of enhanced mechanical properties may also be realized by fabricating thinner membranes, less than 50 μm, which should lead to improved fuel cell performance due to reduction of internal resistance and minimization of water management difficulties.
These sol-gel derived hybrid membranes can be made in an endless variety of ways according to the considerable number of variables for the in situ reactions: pH, water:TEOS ratio, solvent type, temperature, use of other metal alkoxides, thermal history, etc. It may be possible to obtain a more optimal structure by selective control of these variables, and further improvements in this area are likely.
Conclusions
We report experimental findings for proton conductivity and water content in sol-gel derived Nafion/silica hybrid membranes at 25, 120, 150, and 170°C as a function of temperature and water vapor activity. The hybrid membranes have higher water content at 25 and 120°C than the unmodified Nafion membrane, but not at 150 and 170°C. Despite the higher water content, the proton conductivity in the hybrid membranes is lower than, or equal to, that in unmodified Nafion under all conditions investigated. The proton conductivity of a hybrid membrane decreases with increasing silica content under all experimental conditions of this study.
Acknowledgments
N.M. is a visiting scholar at Case Western Reserve University supported by Asahi Kasei Co., Ltd. This work is supported in part by a DARPA Cooperative Research Agreement F 30602-97-2-0311. The characterization of membrane samples by the Analytical Research Laboratory at Asahi Kasei Co., Ltd., is also appreciated.
Asahi Kasei Company, Limited, assisted in meeting the publication costs of this article.