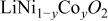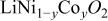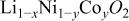Abstract
The mechanism of thermal stabilization of delithiated  by cobalt substitution for nickel was closely studied from the structural point of view by thermogravimetry, X-ray diffraction, and X-ray absorption analysis. Delithiated
by cobalt substitution for nickel was closely studied from the structural point of view by thermogravimetry, X-ray diffraction, and X-ray absorption analysis. Delithiated  with hexagonal
with hexagonal  or monoclinic
or monoclinic  structure was decomposed to a spinel phase (cubic,
structure was decomposed to a spinel phase (cubic,  at temperatures around 220°C and then converted to a rock-salt phase (cubic,
at temperatures around 220°C and then converted to a rock-salt phase (cubic,  at higher temperatures. Cobalt substitution of nickel in
at higher temperatures. Cobalt substitution of nickel in  stabilized the spinel phase, formed from the thermal decomposition of
stabilized the spinel phase, formed from the thermal decomposition of  and suppressed the decomposition of this spinel phase to a rock-salt phase. While the highly delithiated
and suppressed the decomposition of this spinel phase to a rock-salt phase. While the highly delithiated  was eventually converted to a rock salt phase with NiO structure during heating,
was eventually converted to a rock salt phase with NiO structure during heating,  spinel structure was locally formed around the cobalt ions in
spinel structure was locally formed around the cobalt ions in  The improvement of the thermal stability of highly delithiated
The improvement of the thermal stability of highly delithiated  by cobalt addition could be explained by local formation of
by cobalt addition could be explained by local formation of  spinel structure around the cobalt ions in
spinel structure around the cobalt ions in  This spinel structure around the cobalt ions was relatively stable at high temperature and therefore, depressed the decomposition of
This spinel structure around the cobalt ions was relatively stable at high temperature and therefore, depressed the decomposition of  to a rock-salt phase. © 2001 The Electrochemical Society. All rights reserved.
to a rock-salt phase. © 2001 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Due to the increased production and use of portable devices, lithium secondary batteries with extended lives and higher power output are in demand. Consequently,  has been extensively studied as a positive electrode material for these batteries because of its high specific capacity above 200 mAh/g.1
2
3
4
5 However, a few disadvantages, such as difficulty in preparation of electrochemically active form,4 capacity fading during the cycling,3 and poor thermal stability,6 are still unsolved and prevent
has been extensively studied as a positive electrode material for these batteries because of its high specific capacity above 200 mAh/g.1
2
3
4
5 However, a few disadvantages, such as difficulty in preparation of electrochemically active form,4 capacity fading during the cycling,3 and poor thermal stability,6 are still unsolved and prevent  from being used in the realistic lithium secondary batteries.
from being used in the realistic lithium secondary batteries.
In particular, the thermal instability of delithiated  has been considered as a one of the most crucial drawbacks that must be improved, because the thermal instability of delithiated
has been considered as a one of the most crucial drawbacks that must be improved, because the thermal instability of delithiated  causes safety hazards of the cell.6
7
8
9 It has been reported that delithiated
causes safety hazards of the cell.6
7
8
9 It has been reported that delithiated  is unstable at high temperatures and exothermically decomposed with oxygen liberation.6
9 The released oxygen may lead to an increase in an inner-pressure of the cell and a violent reaction with an electrolyte heated above its flash point. In addition, the heat evolved during the exothermic decomposition reaction of
is unstable at high temperatures and exothermically decomposed with oxygen liberation.6
9 The released oxygen may lead to an increase in an inner-pressure of the cell and a violent reaction with an electrolyte heated above its flash point. In addition, the heat evolved during the exothermic decomposition reaction of  may play a role as a trigger for the thermal runaway of the cell. Furthermore, the amount of lithium available in
may play a role as a trigger for the thermal runaway of the cell. Furthermore, the amount of lithium available in  i.e., specific capacity of
i.e., specific capacity of  is limited by thermal stability of
is limited by thermal stability of  because the thermal stability of delithiated
because the thermal stability of delithiated  is significantly degraded by lithium removal.
is significantly degraded by lithium removal.
In order to improve the thermal stability of cathode materials for lithium secondary batteries, substitution of other elements such as Al, Co, Mn, Mg, and/or Ti for Ni and surface coating with  or MgO have been reported.9
10
11
12
13
14 However, not only the mechanism of the thermal stabilization by the substitution and surface coating but also the detailed thermal decomposition mechanism has not been clearly understood. In previous work, we studied the thermal behavior of
or MgO have been reported.9
10
11
12
13
14 However, not only the mechanism of the thermal stabilization by the substitution and surface coating but also the detailed thermal decomposition mechanism has not been clearly understood. In previous work, we studied the thermal behavior of  and reported that delithiated
and reported that delithiated  (
( or
or  ) was thermally decomposed to a spinel
) was thermally decomposed to a spinel  phase at around 220°C and then converted to a rock-salt phase
phase at around 220°C and then converted to a rock-salt phase  at higher temperatures.15 In addition, it was also expected that the thermal stability of
at higher temperatures.15 In addition, it was also expected that the thermal stability of  could be improved by stabilization of the spinel structure and suppression of its decomposition to a rock-salt phase.
could be improved by stabilization of the spinel structure and suppression of its decomposition to a rock-salt phase.
In this study, we investigated the effect of cobalt addition on the thermal behavior of  and described the mechanism of the thermal stabilization of
and described the mechanism of the thermal stabilization of  by cobalt substitution for nickel. For this purpose, a series of
by cobalt substitution for nickel. For this purpose, a series of  and
and  were prepared by electrochemical delithiation of
were prepared by electrochemical delithiation of  and
and  and their thermal and structural characteristics were examined by thermogravimetry (TG), X-ray diffraction (XRD), and X-ray absorption (XAS) measurements.
and their thermal and structural characteristics were examined by thermogravimetry (TG), X-ray diffraction (XRD), and X-ray absorption (XAS) measurements.
Experimental

 was synthesized by reacting stoichiometric amounts of
was synthesized by reacting stoichiometric amounts of  and
and 

 was obtained by solution method using nickel and cobalt nitrate and NaOH solutions. The pellets comprised of a mixture of
was obtained by solution method using nickel and cobalt nitrate and NaOH solutions. The pellets comprised of a mixture of  and
and  were precalcined at 600°C for 12 h in a stream of oxygen. The precalcined products were powdered, pressed into pellets, and reacted at 750°C for
were precalcined at 600°C for 12 h in a stream of oxygen. The precalcined products were powdered, pressed into pellets, and reacted at 750°C for  and at 800°C for
and at 800°C for  for 24 h under oxygen flow. The reaction products were ground and stored in a desiccator. The compositions of the as-prepared
for 24 h under oxygen flow. The reaction products were ground and stored in a desiccator. The compositions of the as-prepared  and
and  were determined from the values of Ni/Co ratio and average oxidation state of
were determined from the values of Ni/Co ratio and average oxidation state of  in
in  obtained by inductively coupled plasma-Auger electron spectroscopy (ICP-AES) and iodometric titration, respectively, and are listed in Table I.
obtained by inductively coupled plasma-Auger electron spectroscopy (ICP-AES) and iodometric titration, respectively, and are listed in Table I.
Table I.
Ni/Co ratio, average oxidation states of 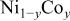 in in 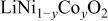 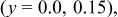 and compositions of and compositions of 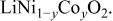 | |||
|---|---|---|---|
| Samplea | Ni/Co ratiob | Average oxidation state of 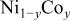 c
c | Composition |
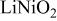 | - | 2.907 |
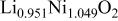 |
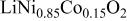 | 0.865/0.135 | 2.936 |
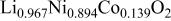 |
| a Nominal compositions of the samples are calculated from the relative content of nickel nitrate, cobalt nitrate, and lithium nitrate. | |||
| b Obtained by ICP-AES. | |||
| c Obtained by iodometric titration. | |||
The crystal structures of the as-prepared  and
and  were characterized by neutron diffraction. The neutron powder diffraction data were analyzed by Rietveld refinement analysis using the General Structure Analysis System (GSAS) program.16 The classic reliability factors, defined in the caption of Table II, were used.
were characterized by neutron diffraction. The neutron powder diffraction data were analyzed by Rietveld refinement analysis using the General Structure Analysis System (GSAS) program.16 The classic reliability factors, defined in the caption of Table II, were used.
Table II.
Rietveld refinement results for as-prepared 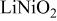 and and 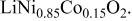 | |||||
|---|---|---|---|---|---|
| Sample |
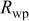 a
a | a (Å) | c (Å) | z (O) | Cationic distribution |
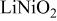 | 8.34 | 2.8813 | 14.201 | 0.2415 |
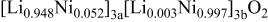 |
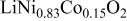 | 7.98 | 2.8718 | 14.184 | 0.2407 |
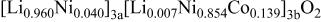 |
a 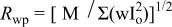 where the quality minimized is where the quality minimized is 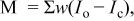 with with 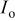 and and 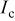 being the observed and calculated intensities, respectively, and w a weight related to the error. being the observed and calculated intensities, respectively, and w a weight related to the error. | |||||
A composite electrode was prepared by mixing 88 wt %  6 wt % acetylene black as a conductor, and 6 wt % polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) copolymers (Kynar FLEX 2801, Elf-Atochem America) as a binder dissolved in n-methyl-2-pyrrolidinone. The mixed slurry was coated onto Al foil and dried for >24 h. A three-electrode cell system was employed for the electrochemical measurements. Reference electrode and counter electrode were constructed from lithium foil, and 1 M
6 wt % acetylene black as a conductor, and 6 wt % polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) copolymers (Kynar FLEX 2801, Elf-Atochem America) as a binder dissolved in n-methyl-2-pyrrolidinone. The mixed slurry was coated onto Al foil and dried for >24 h. A three-electrode cell system was employed for the electrochemical measurements. Reference electrode and counter electrode were constructed from lithium foil, and 1 M  in propylene carbonate (PC) was used as the electrolyte. Electrochemical delithiation of
in propylene carbonate (PC) was used as the electrolyte. Electrochemical delithiation of  and
and  was carried out at room temperature in a glove box filled with purified argon.
was carried out at room temperature in a glove box filled with purified argon.
In order to obtain a delithiated  the cell was charged to a desired composition at a constant current corresponding to a 20 h rate. After relaxation of several days, the electrode was taken out of the cell. It was then washed with tetrahydrofuran and dried thoroughly in vacuum. Thermal behavior of the charged compounds was examined up to 400°C using a TG (TGA 2050, TA Instruments) in air at a heating rate of 5°C/min. The structural change of
the cell was charged to a desired composition at a constant current corresponding to a 20 h rate. After relaxation of several days, the electrode was taken out of the cell. It was then washed with tetrahydrofuran and dried thoroughly in vacuum. Thermal behavior of the charged compounds was examined up to 400°C using a TG (TGA 2050, TA Instruments) in air at a heating rate of 5°C/min. The structural change of  as a function of temperature was investigated by means of XRD measurement with Cu Kα radiation. XAS spectra, especially X-ray absorption near edge structure (XANES), were measured before and after heating the
as a function of temperature was investigated by means of XRD measurement with Cu Kα radiation. XAS spectra, especially X-ray absorption near edge structure (XANES), were measured before and after heating the  composite electrodes at 300°C for 5 h in order to investigate the changes in the oxidation state of nickel and cobalt ions and local structure around the nickel and cobalt ions in
composite electrodes at 300°C for 5 h in order to investigate the changes in the oxidation state of nickel and cobalt ions and local structure around the nickel and cobalt ions in 
Results and Discussion
Characterization of the as-prepared  .—
.—
The neutron powder diffraction patterns of as-prepared 
 could be indexed assuming a space group of
could be indexed assuming a space group of  and the Rietveld refinement results of the patterns using the GSAS programs are listed in Table II. The structural parameters of
and the Rietveld refinement results of the patterns using the GSAS programs are listed in Table II. The structural parameters of  were refined assuming the following atomic positions: lithium-rich 3a site at 000, nickel- and cobalt-rich 3b site at 001/2, and oxygen anion site (6c) at
were refined assuming the following atomic positions: lithium-rich 3a site at 000, nickel- and cobalt-rich 3b site at 001/2, and oxygen anion site (6c) at  where
where  Compositions of as-prepared
Compositions of as-prepared  and
and  as listed in Table I, were
as listed in Table I, were  and
and  respectively, and held constant during the refinement. Detailed structural parameters, the assumption, and constraints employed during the refinements were described previously.17 The cationic distributions for
respectively, and held constant during the refinement. Detailed structural parameters, the assumption, and constraints employed during the refinements were described previously.17 The cationic distributions for  and
and  were determined as
were determined as  and
and  respectively. However, for convenience, the phases are still referred by their nominal chemical formula of
respectively. However, for convenience, the phases are still referred by their nominal chemical formula of  and
and  in the following discussion.
in the following discussion.
Electrochemical delithiation from the  .—
.—
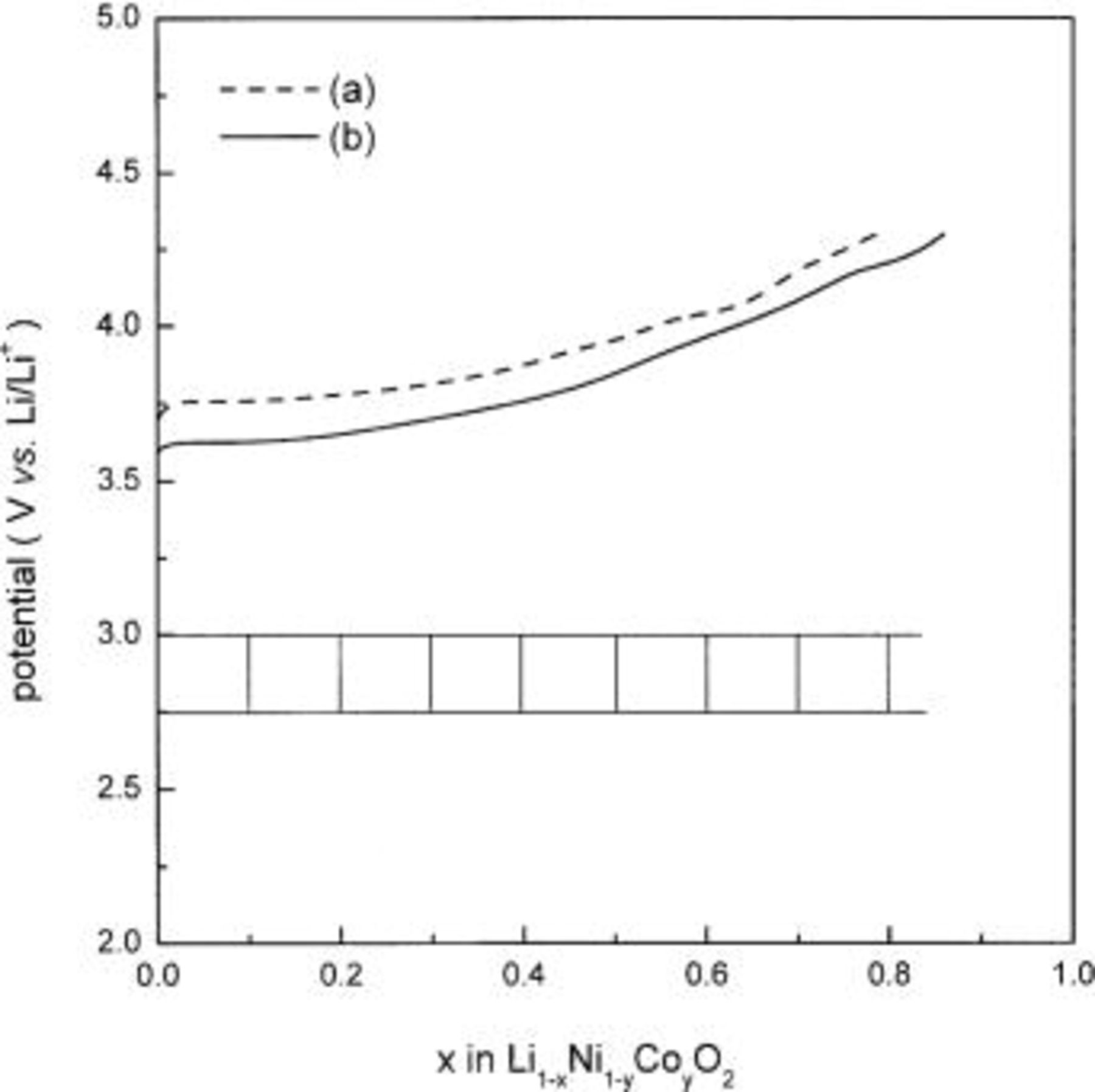
Figure 1 shows charge curves of  and
and  electrodes in 1 M
electrodes in 1 M  charged at a constant current corresponding to a 20 h charge rate. The charge curve of
charged at a constant current corresponding to a 20 h charge rate. The charge curve of  shows a few voltage plateaus at potentials of 3.66, 4.03, and 4.20 V vs.
shows a few voltage plateaus at potentials of 3.66, 4.03, and 4.20 V vs.  and these are reported to be caused by phase transitions of
and these are reported to be caused by phase transitions of  on lithium deintercalation.3 However,
on lithium deintercalation.3 However,  shows a monotonous charge curve until 0.8Li are removed, and it means a single phase is maintained during the lithium deintercalation in this composition range.
shows a monotonous charge curve until 0.8Li are removed, and it means a single phase is maintained during the lithium deintercalation in this composition range.
Figure 1. Continuous charge curves of (a)  and (b)
and (b)  obtained during the constant-current charging at a 20 h rate in 1 M
obtained during the constant-current charging at a 20 h rate in 1 M  in PC. Bar shown in the inset indicates the compositions at which the thermal and structural analysis was carried out.
in PC. Bar shown in the inset indicates the compositions at which the thermal and structural analysis was carried out.
In order to investigate the thermal behavior of delithiated  and
and  thermal analysis using TG and structural analysis using XRD and XAS were carried out. The bar shown in the inset of Fig. 1 indicates the compositions at which the thermal and structural analysis was conducted.
thermal analysis using TG and structural analysis using XRD and XAS were carried out. The bar shown in the inset of Fig. 1 indicates the compositions at which the thermal and structural analysis was conducted.
Thermal decomposition of  .—
.—
Figure 2 shows the thermogravimetric analysis (TGA) results for washed composite electrodes containing a series of 
 acetylene black, and PVDF-HFP copolymer at a heating rate of 5°C/min in an air atmosphere. In general, weight losses measured with the TGA were found to be almost entirely due to oxygen liberated from the samples.6
7
8 In previous work, we studied the thermal behavior of
acetylene black, and PVDF-HFP copolymer at a heating rate of 5°C/min in an air atmosphere. In general, weight losses measured with the TGA were found to be almost entirely due to oxygen liberated from the samples.6
7
8 In previous work, we studied the thermal behavior of  and reported that delithiated
and reported that delithiated  was thermally decomposed to a spinel phase at around 220°C and then converted to a rock-salt phase at higher temperature.15 The phase transitions of
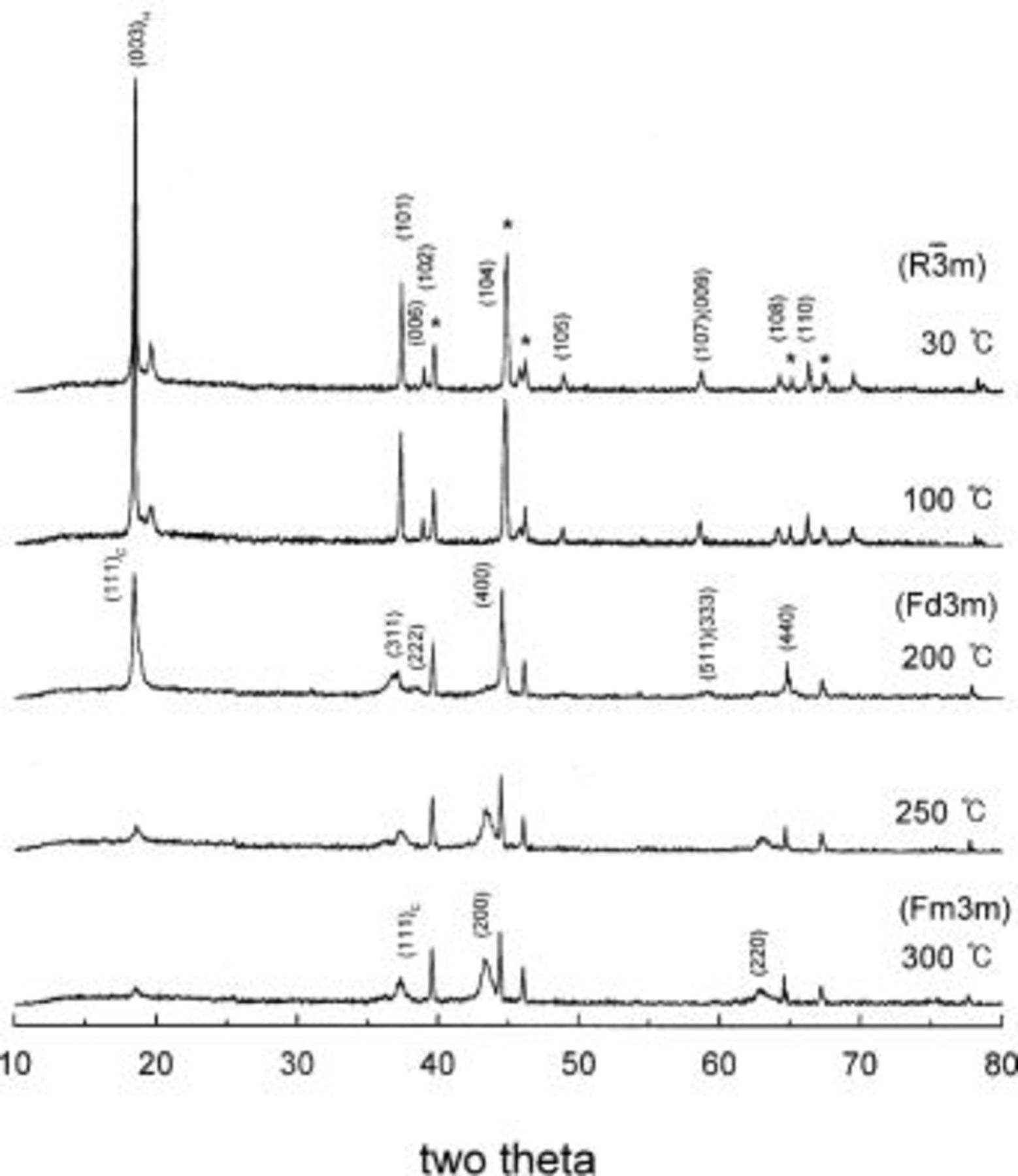
was thermally decomposed to a spinel phase at around 220°C and then converted to a rock-salt phase at higher temperature.15 The phase transitions of  with temperature were observed by high-temperature XRD analysis, and the representative XRD patterns of
with temperature were observed by high-temperature XRD analysis, and the representative XRD patterns of  at various temperatures are shown in Fig. 3. As the temperature is increased, the trigonal phases with space group of
at various temperatures are shown in Fig. 3. As the temperature is increased, the trigonal phases with space group of  transformed into a spinel phase
transformed into a spinel phase  at 200°C and then converted to a rock-salt phase
at 200°C and then converted to a rock-salt phase  at 300°C. More details about the phase transitions of
at 300°C. More details about the phase transitions of  with temperature have been described earlier.15
with temperature have been described earlier.15
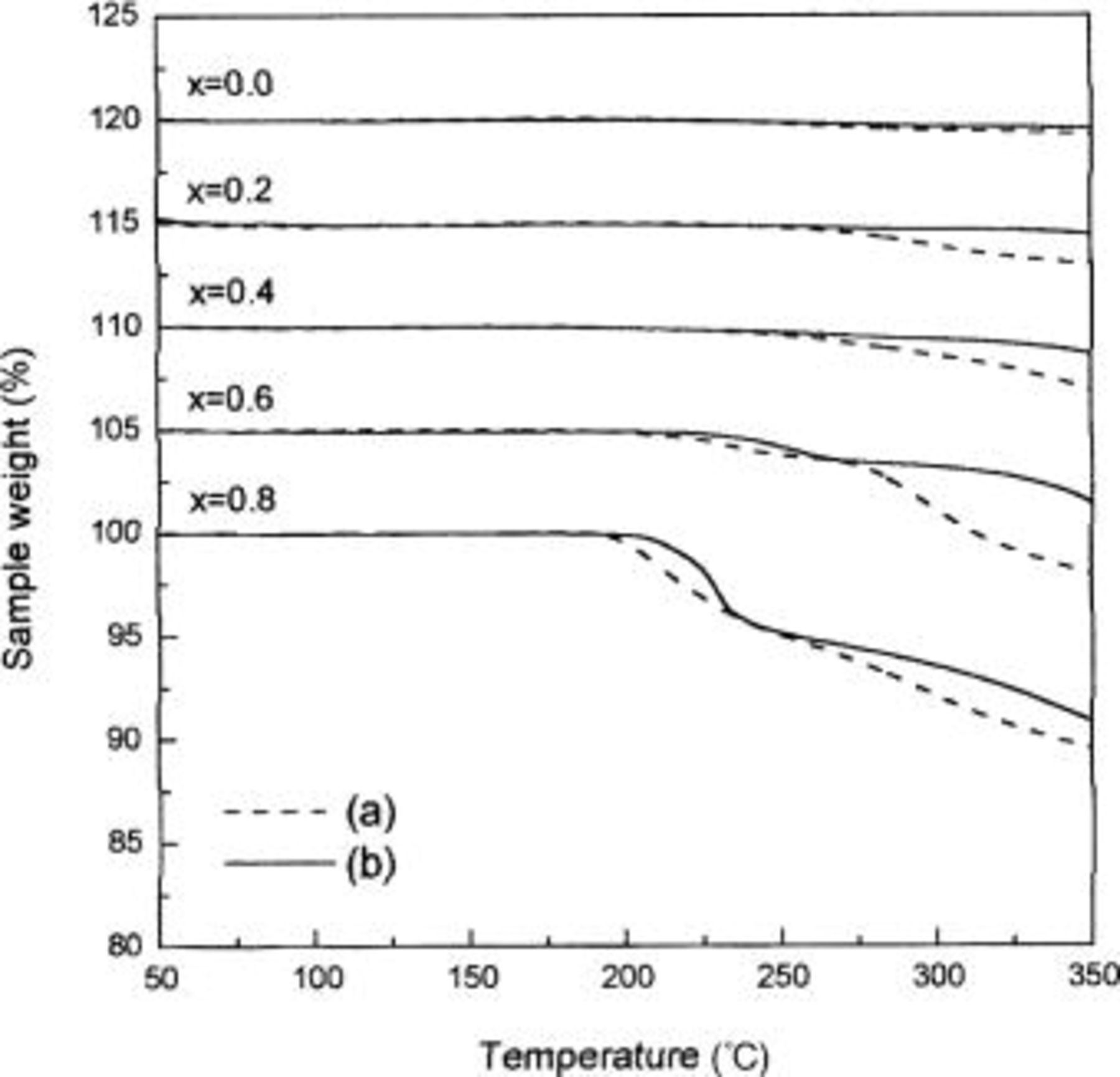
Figure 2. TGA results for (a)  and (b)
and (b)  composites in air atmosphere at a heating rate of 5°C/min. The data have been offset vertically by 5% sequentially for clarity.
composites in air atmosphere at a heating rate of 5°C/min. The data have been offset vertically by 5% sequentially for clarity.
Figure 3. XRD patterns of  during heating at a rate of 5°C/min. (*) Al foil and sample holder, Pt.
during heating at a rate of 5°C/min. (*) Al foil and sample holder, Pt.
Therefore, it could be noted that the weight loss at around 220°C in TG curves of  shown in Fig. 2a resulted from the phase transition of
shown in Fig. 2a resulted from the phase transition of  to a spinel. For the samples with
to a spinel. For the samples with  it is suggested that there is no release of oxygen to form a spinel; no weight loss is observed. However, for
it is suggested that there is no release of oxygen to form a spinel; no weight loss is observed. However, for  the conversion of
the conversion of  to a spinel is accompanied by oxygen release because there are more than four oxygen atoms for three cations in such samples. In addition, the weight losses at temperatures higher than the transformation temperature to spinel are due to the formation of a rock-salt phase. The temperatures at which the transformation to a rock salt occurred significantly decreased with x in
to a spinel is accompanied by oxygen release because there are more than four oxygen atoms for three cations in such samples. In addition, the weight losses at temperatures higher than the transformation temperature to spinel are due to the formation of a rock-salt phase. The temperatures at which the transformation to a rock salt occurred significantly decreased with x in  as reported previously.15
as reported previously.15
 was thermally decomposed at temperatures higher than those of
was thermally decomposed at temperatures higher than those of  as shown in Fig. 2b, and it indicated that the thermal stability of
as shown in Fig. 2b, and it indicated that the thermal stability of  was improved by cobalt substitution for nickel. The increase in the decomposition temperature by cobalt addition is more remarkable for the transformation to a rock-salt phase than that to a spinel phase. Therefore, it could be noted that cobalt ions stabilized the spinel phase, formed from thermal decomposition of trigonal
was improved by cobalt substitution for nickel. The increase in the decomposition temperature by cobalt addition is more remarkable for the transformation to a rock-salt phase than that to a spinel phase. Therefore, it could be noted that cobalt ions stabilized the spinel phase, formed from thermal decomposition of trigonal  and suppressed decomposition of this phase to a rock-salt phase.
and suppressed decomposition of this phase to a rock-salt phase.
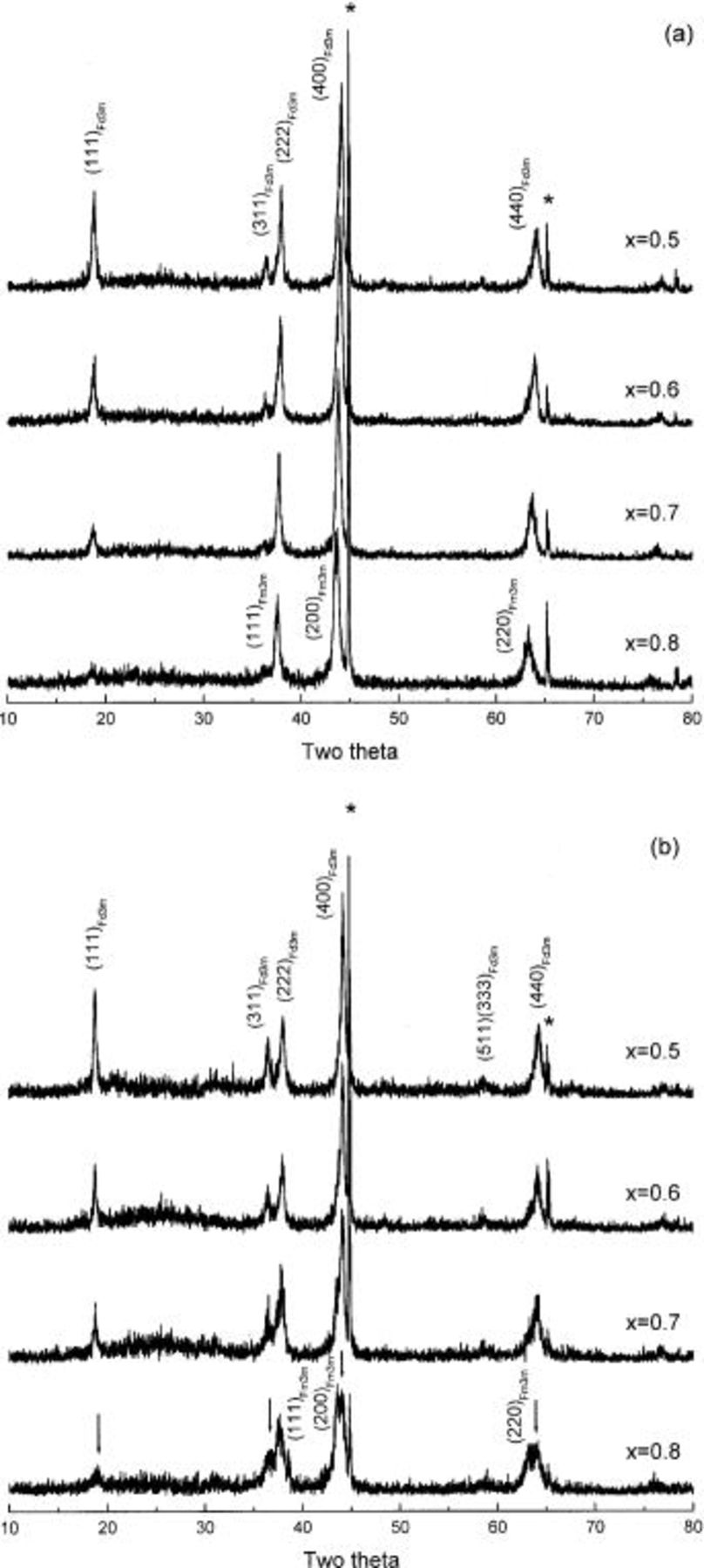
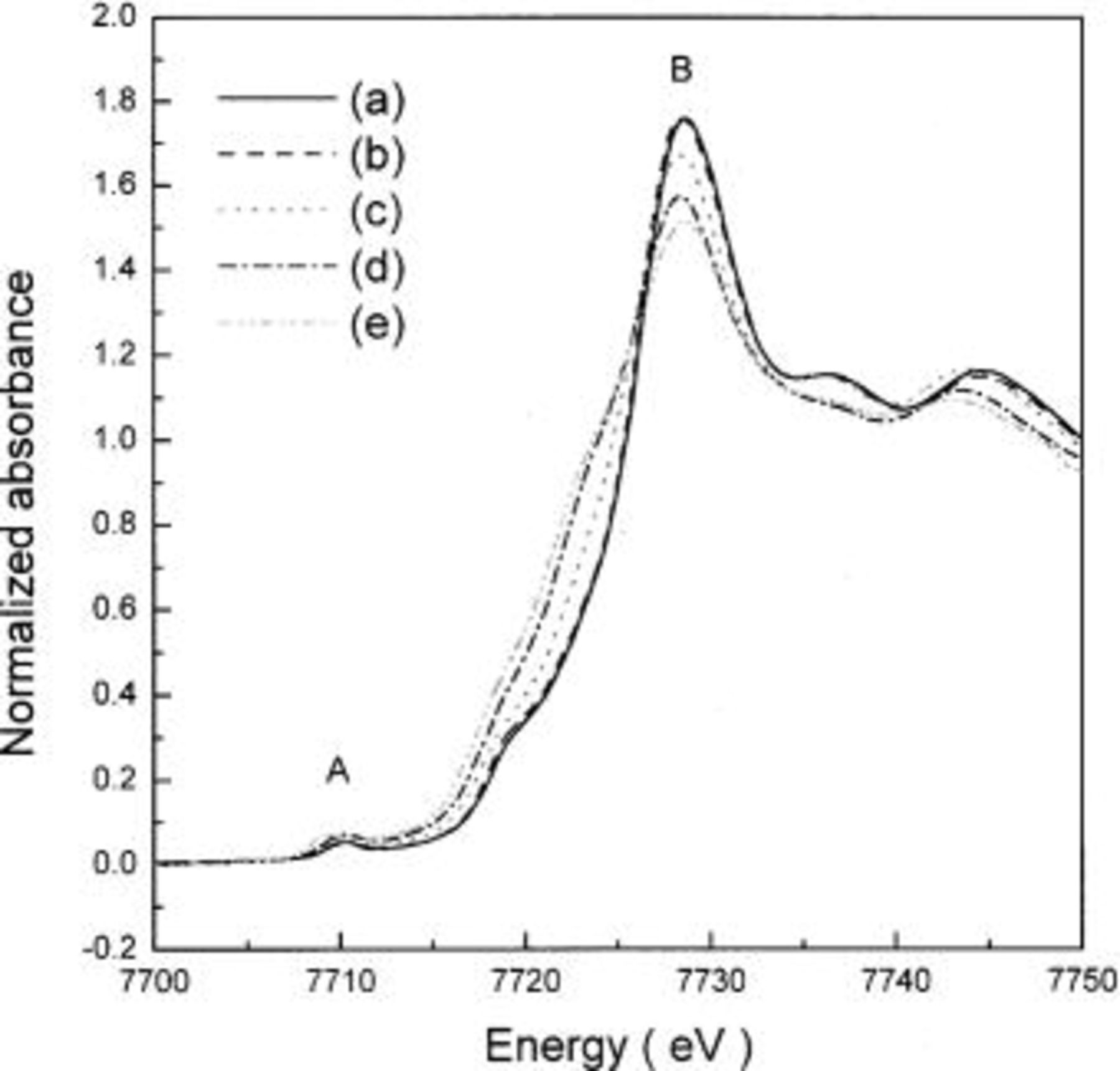
In order to investigate the effect of cobalt substitution for nickel on the phase transition from spinel to rock-salt phase, XRD patterns of  and
and  were measured after heating at 300°C for 5 h.
were measured after heating at 300°C for 5 h.  heated at 300°C for 5 h showed an XRD pattern of spinel with space group of
heated at 300°C for 5 h showed an XRD pattern of spinel with space group of  as shown in Fig. 4a, because little decomposition to a rock-salt phase occurred at this temperature. The temperature of decomposition of
as shown in Fig. 4a, because little decomposition to a rock-salt phase occurred at this temperature. The temperature of decomposition of  to a rock-salt phase tends to decrease with x; therefore, further decomposition of
to a rock-salt phase tends to decrease with x; therefore, further decomposition of  to a rock-salt phase was expected to proceed during heating at 300°C for 5 h with increase in x. For
to a rock-salt phase was expected to proceed during heating at 300°C for 5 h with increase in x. For  the decomposition to rock-salt phase was almost complete during heating at 300°C for 5 h, therefore, the XRD pattern of heat-treated
the decomposition to rock-salt phase was almost complete during heating at 300°C for 5 h, therefore, the XRD pattern of heat-treated  could be indexed to the rock-salt phase with a space group of
could be indexed to the rock-salt phase with a space group of  as shown in Fig. 4a.
as shown in Fig. 4a.
Figure 4. XRD patterns of (a)  and (b)
and (b)  after heating at 300°C for 5 h. (*) Al foil.
after heating at 300°C for 5 h. (*) Al foil.
As shown in Fig. 4b, XRD patterns of  after heating at 300°C for 5 h are similar to those of
after heating at 300°C for 5 h are similar to those of  The XRD pattern of heat-treated
The XRD pattern of heat-treated  could be indexed assuming a spinel structure, and further decomposition to a rock-salt phase was observed for the samples with higher x. However, contrary to
could be indexed assuming a spinel structure, and further decomposition to a rock-salt phase was observed for the samples with higher x. However, contrary to  in addition to a rock-salt phase, there is also a small amount of spinel phase still observed in the heat-treated
in addition to a rock-salt phase, there is also a small amount of spinel phase still observed in the heat-treated  This can be seen by the remnants of
This can be seen by the remnants of 

 and
and  peaks, marked by downward pointing arrows, in XRD pattern of
peaks, marked by downward pointing arrows, in XRD pattern of  after heating at 300°C for 5 h.
after heating at 300°C for 5 h.
From the XRD patterns of  and
and  heated at 300°C for 5 h, it could be noted that cobalt ions stabilized the spinel phase, formed from the thermal decomposition of
heated at 300°C for 5 h, it could be noted that cobalt ions stabilized the spinel phase, formed from the thermal decomposition of  and shifted the onset of the decomposition of this spinel phase to a rock-salt phase to higher temperatures.
and shifted the onset of the decomposition of this spinel phase to a rock-salt phase to higher temperatures.
X-ray absorption analysis of  .—
.—
In order to investigate the thermal behavior of  and the mechanism of thermal stabilization of this phase by cobalt addition, changes in oxidation states of nickel and cobalt ions and local structures around these ions in
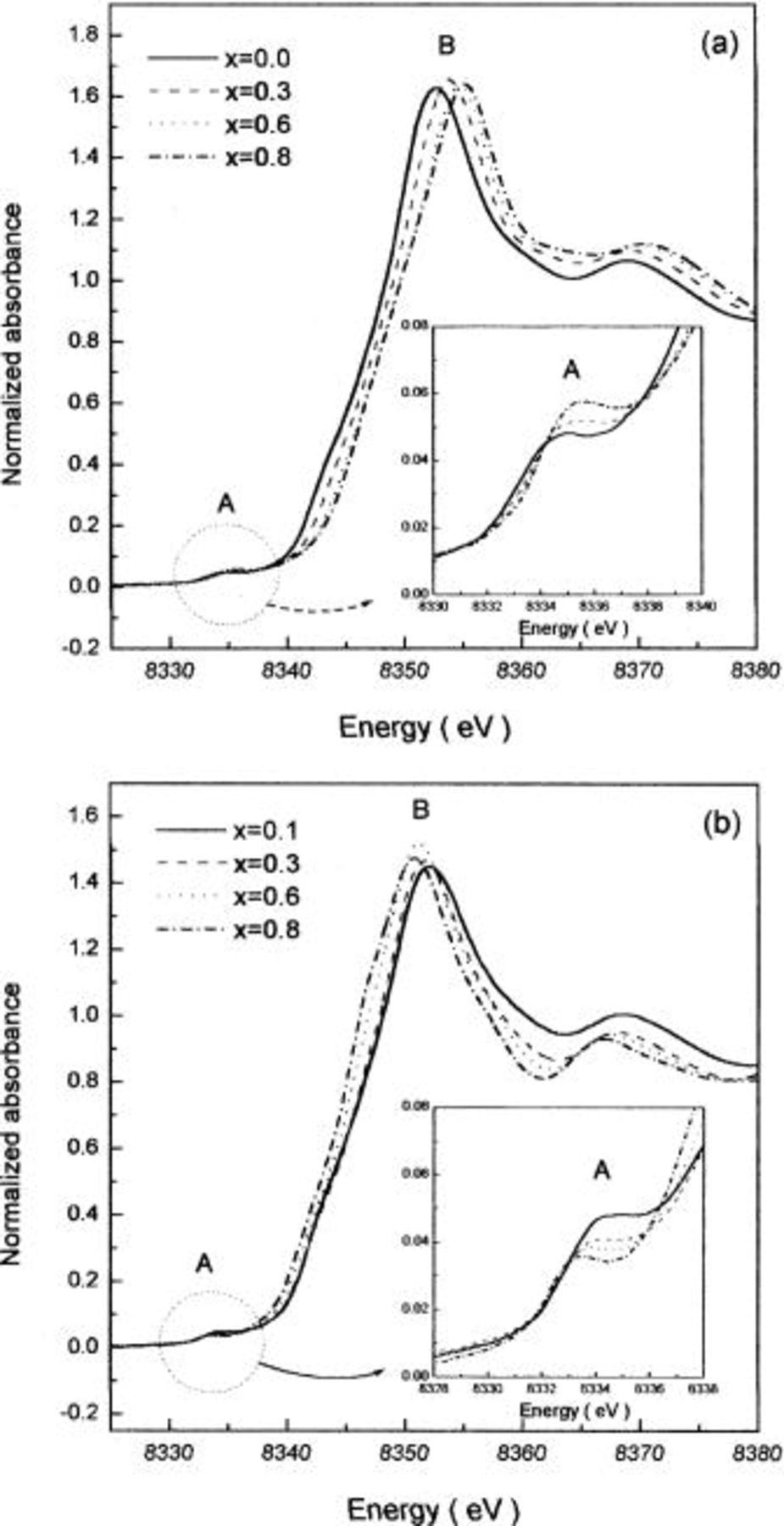
and the mechanism of thermal stabilization of this phase by cobalt addition, changes in oxidation states of nickel and cobalt ions and local structures around these ions in  with temperature were studied by XAS. Figure 5 shows the Ni K-edge XANES spectra of
with temperature were studied by XAS. Figure 5 shows the Ni K-edge XANES spectra of  both before and after heating at 300°C at 5 h. Two peaks were observed in the Ni K-edge XANES spectra of
both before and after heating at 300°C at 5 h. Two peaks were observed in the Ni K-edge XANES spectra of  in Fig. 5a. A weak pre-edge (peak A) represents the transition of the 1s electron to unoccupied 3d orbitals of
in Fig. 5a. A weak pre-edge (peak A) represents the transition of the 1s electron to unoccupied 3d orbitals of  ions. The
ions. The  transition, which is formally forbidden, becomes dipole-allowed because of a combination of strong 3d-4p mixing and an overlap of the metal 3d orbitals with the oxygen 2p orbitals due to the noncentrosymmetric environment of slightly distorted
transition, which is formally forbidden, becomes dipole-allowed because of a combination of strong 3d-4p mixing and an overlap of the metal 3d orbitals with the oxygen 2p orbitals due to the noncentrosymmetric environment of slightly distorted  octahedral site.17
18
19
20 A strong main-edge (peak B) appears due to the electric dipole-allowed transition of a 1s core electron to a unoccupied 4p orbitals.17
18
19
20 As x is increased, both the pre-edge and the main edge shifts to a higher energy region, suggesting that
octahedral site.17
18
19
20 A strong main-edge (peak B) appears due to the electric dipole-allowed transition of a 1s core electron to a unoccupied 4p orbitals.17
18
19
20 As x is increased, both the pre-edge and the main edge shifts to a higher energy region, suggesting that  ions were oxidized to
ions were oxidized to  ions upon lithium deintercalation.
ions upon lithium deintercalation.
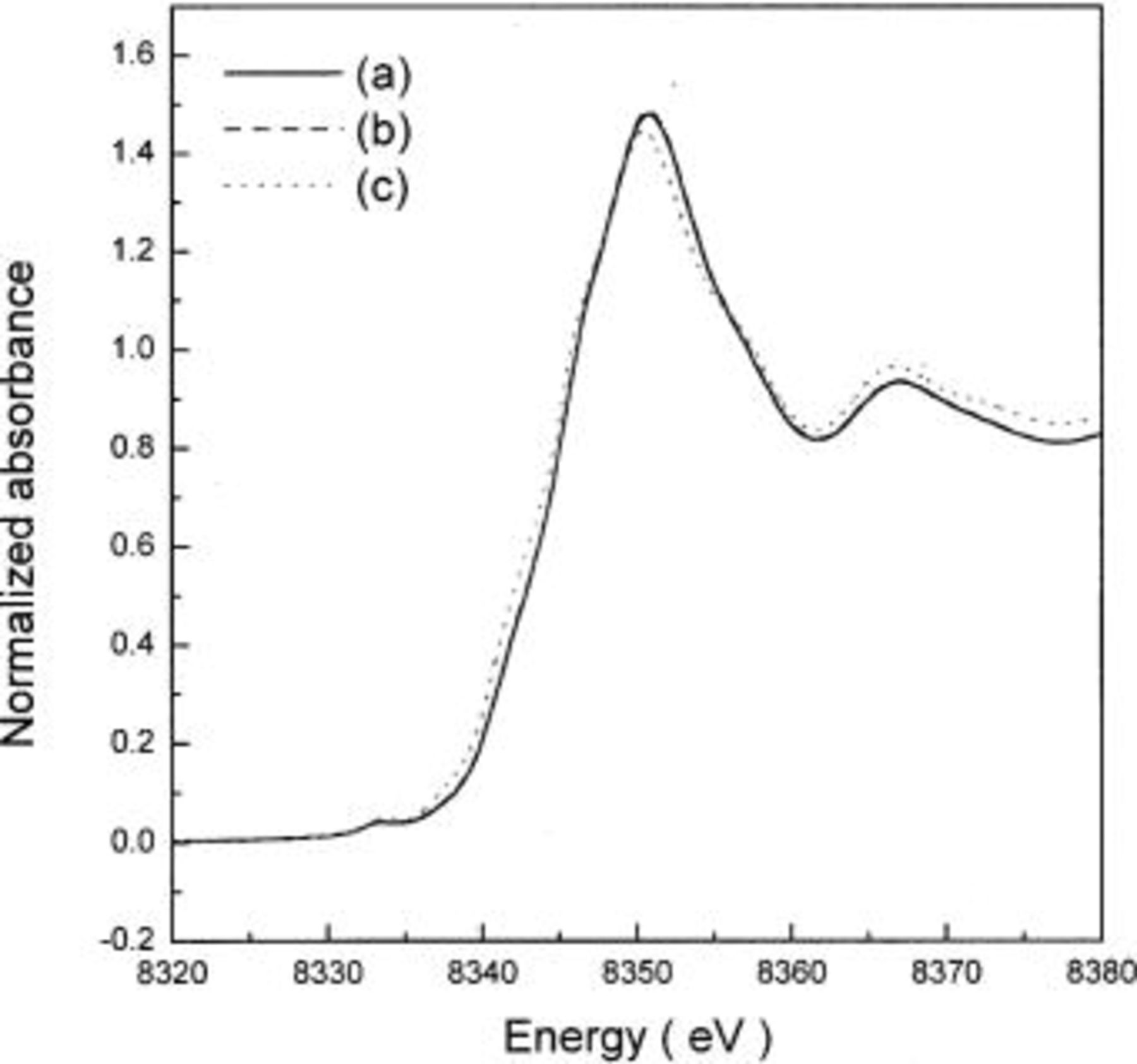
Figure 5. Ni K-edge XANES spectra of  (a) before and (b) after heating at 300°C for 5 h. The insets show expanded views of the region containing the
(a) before and (b) after heating at 300°C for 5 h. The insets show expanded views of the region containing the  transitions.
transitions.
Delithiated  was thermally decomposed to a rock-salt phase at high temperature, because highly oxidized nickel ions are unstable and reduced to divalent state. The decomposition reaction was reported to proceed according to the following reaction8
15
was thermally decomposed to a rock-salt phase at high temperature, because highly oxidized nickel ions are unstable and reduced to divalent state. The decomposition reaction was reported to proceed according to the following reaction8
15
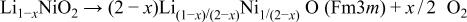
Therefore, the oxidation state of nickel ions in  must be reduced to values lower than +3 during the decomposition to a rock-salt phase, and the oxidation state of nickel ions in heated
must be reduced to values lower than +3 during the decomposition to a rock-salt phase, and the oxidation state of nickel ions in heated  decreases with x after the decomposition.
decreases with x after the decomposition.
Figure 5b shows the Ni K-edge XANES spectra of  after heating at 300°C for 5 h. For all compositions, both the pre-edge and the main edge were located in a lower energy region than those of
after heating at 300°C for 5 h. For all compositions, both the pre-edge and the main edge were located in a lower energy region than those of  and it shows that the reduction of nickel ions in
and it shows that the reduction of nickel ions in  to
to  occurred during heating. Reduction of nickel ions led to oxygen evolution from the
occurred during heating. Reduction of nickel ions led to oxygen evolution from the  in order to satisfy the charge neutrality in the oxides. Decreases in both the pre-edge and the main-edge energies with x were also observed in the XANES spectra of
in order to satisfy the charge neutrality in the oxides. Decreases in both the pre-edge and the main-edge energies with x were also observed in the XANES spectra of  after heating, indicating that the oxidation state of nickel ions in the heated
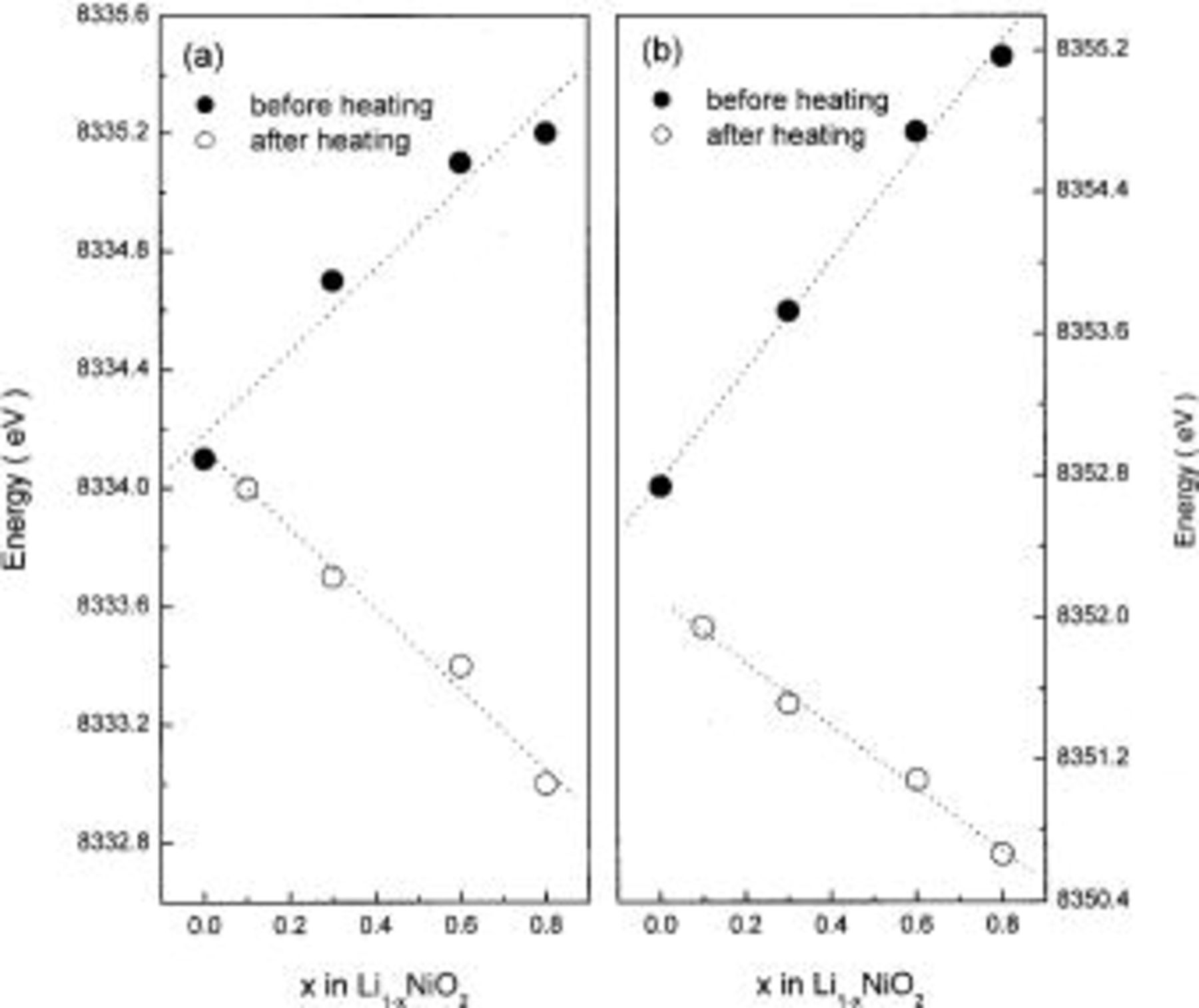
after heating, indicating that the oxidation state of nickel ions in the heated  decreased with x. Figure 6 clearly shows the changes in the pre-edge and the main-edge energies in the Ni K-edge XANES spectra of
decreased with x. Figure 6 clearly shows the changes in the pre-edge and the main-edge energies in the Ni K-edge XANES spectra of  which correspond to the changes in the oxidation state of nickel ions, before and after heating 300°C for 5 h, as a function of x.
which correspond to the changes in the oxidation state of nickel ions, before and after heating 300°C for 5 h, as a function of x.
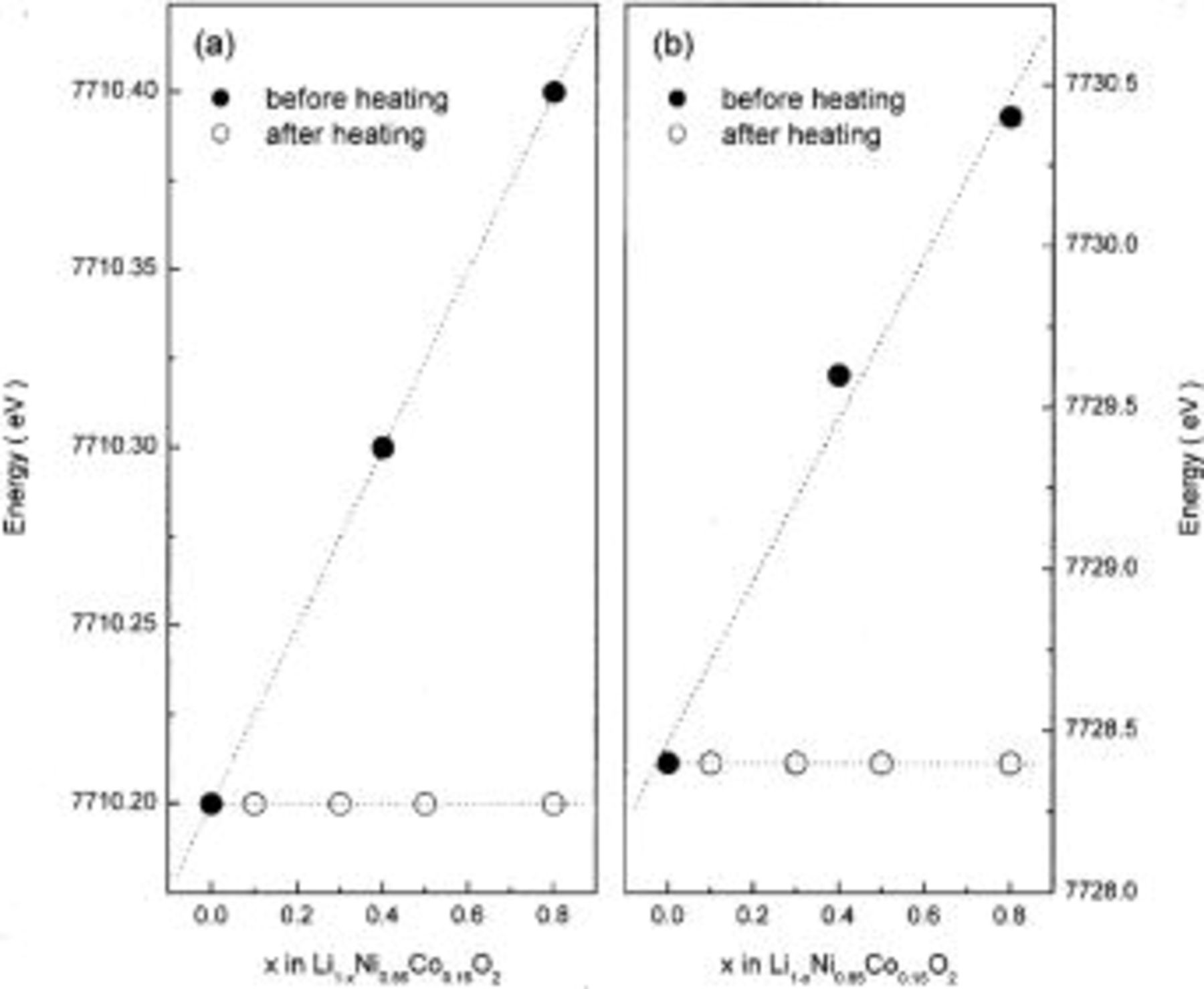
Figure 6. (a) Pre-edge and (b) main-edge energies in the Ni K-edge XANES spectra of  before and after heating at 300°C for 5 h.
before and after heating at 300°C for 5 h.
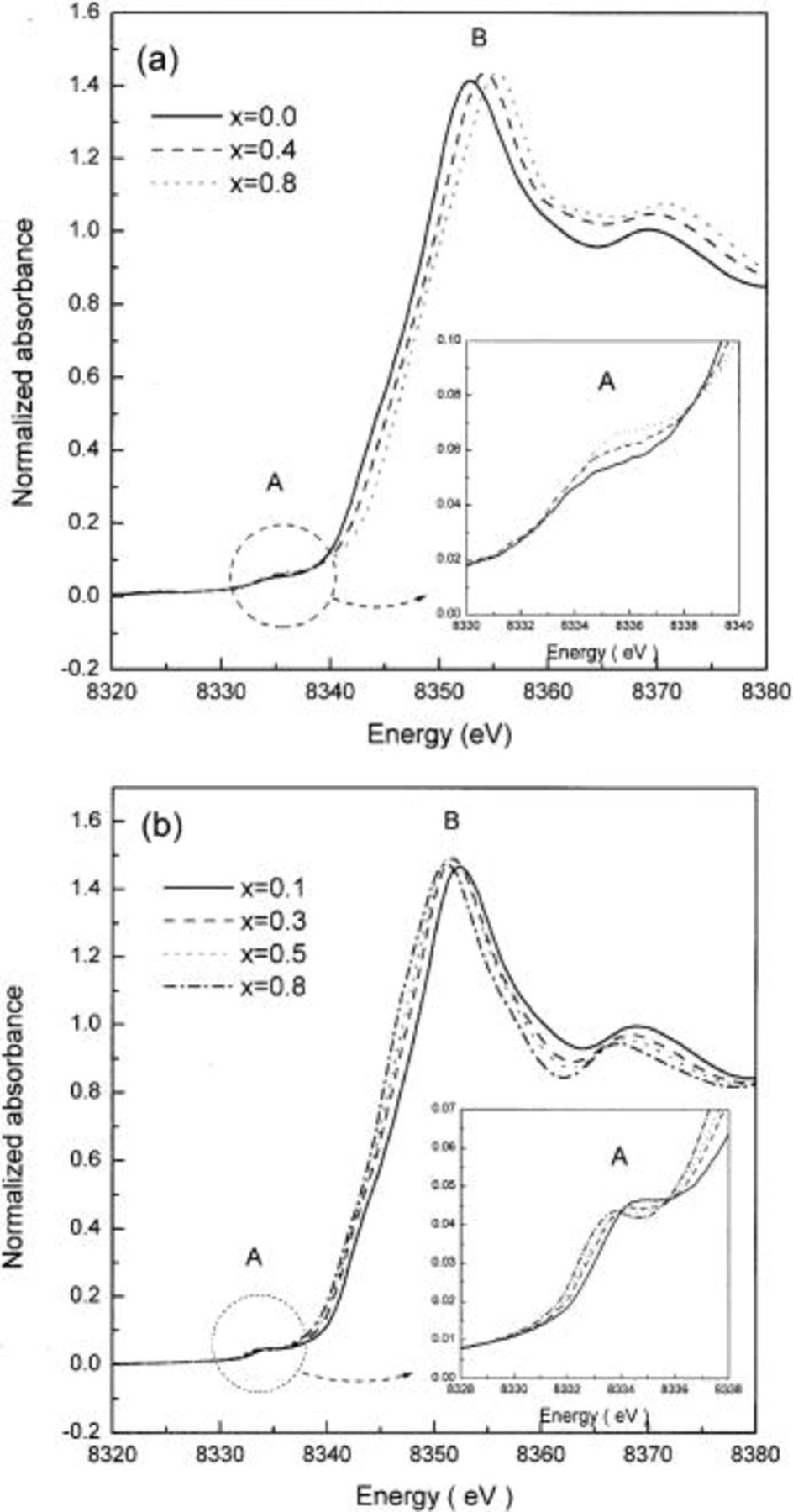
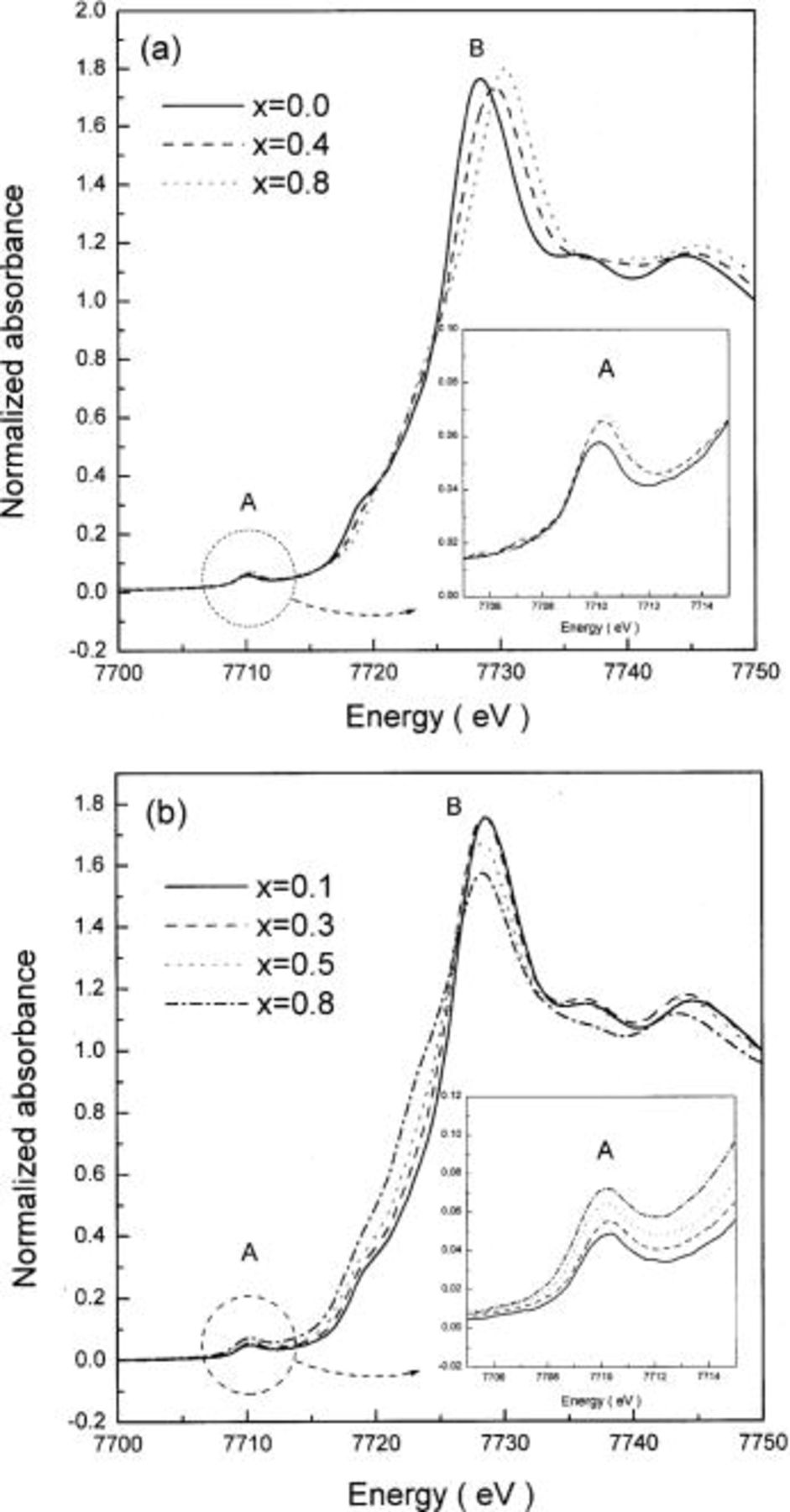
Figures 7 and 8 show the Ni K-edge XANES spectra of  and the energies of the pre-edge and the main edge in the spectra before and after heating at 300°C for 5 h as a function of x, respectively. The Ni K-edge XANES spectra of
and the energies of the pre-edge and the main edge in the spectra before and after heating at 300°C for 5 h as a function of x, respectively. The Ni K-edge XANES spectra of  as a function of lithium content both before and after heating were identical to that of
as a function of lithium content both before and after heating were identical to that of  presented in Fig. 5. The spectra showed that the nickel ions in
presented in Fig. 5. The spectra showed that the nickel ions in  have the same local surroundings and show the same behavior on lithium deintercalation and temperature as in
have the same local surroundings and show the same behavior on lithium deintercalation and temperature as in  In addition, both the Ni K-edge XANES spectra of highly delithiated
In addition, both the Ni K-edge XANES spectra of highly delithiated  and
and  after heating at 300°C for 5 h were in good agreement with that of NiO, as shown in Fig. 9. Therefore, it should be noted that the oxidation states of the nickel ions in the delithiated
after heating at 300°C for 5 h were in good agreement with that of NiO, as shown in Fig. 9. Therefore, it should be noted that the oxidation states of the nickel ions in the delithiated  and
and  were reduced to the values lower than +3, and the local structures around the nickel ions in these oxides were converted to that in NiO with rock-salt structure during heating.
were reduced to the values lower than +3, and the local structures around the nickel ions in these oxides were converted to that in NiO with rock-salt structure during heating.
Figure 7. Ni K-edge XANES spectra of  (a) before and (b) after heating at 300°C for 5 h. The inset shows expanded views of the region containing the
(a) before and (b) after heating at 300°C for 5 h. The inset shows expanded views of the region containing the  transitions.
transitions.
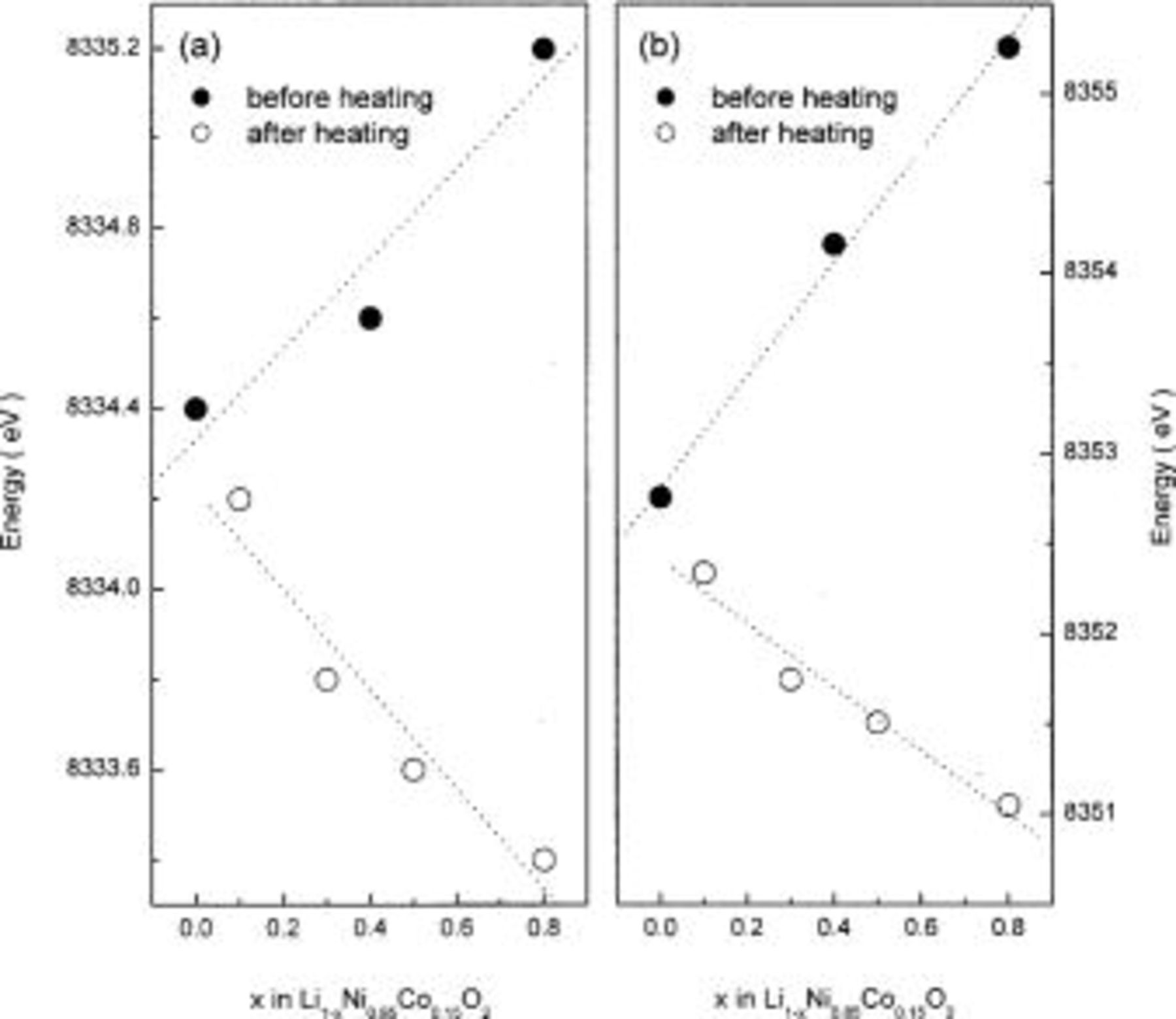
Figure 8. (a) Pre-edge and (b) main-edge energies in the Ni K-edge XANES spectra of  before and after heating at 300°C for 5 h.
before and after heating at 300°C for 5 h.
Figure 9. Ni K-edge XANES spectra of (a)  and (b)
and (b)  after heating at 300°C for 5 h, and (c) NiO.
after heating at 300°C for 5 h, and (c) NiO.
Changes in oxidation state of cobalt ions and local structure around the cobalt ions in  both before and after heating at 300°C for 5 h were also investigated by Co K-edge XANES analysis. The Co K-edge XANES spectra of
both before and after heating at 300°C for 5 h were also investigated by Co K-edge XANES analysis. The Co K-edge XANES spectra of  as a function of lithium content are shown in Fig. 10a and are identical to the previously reported Co K-edge XANES spectra of
as a function of lithium content are shown in Fig. 10a and are identical to the previously reported Co K-edge XANES spectra of  17
18 This indicates that the cobalt ions in
17
18 This indicates that the cobalt ions in  have the same local surroundings and show the same behavior on lithium deintercalation as in
have the same local surroundings and show the same behavior on lithium deintercalation as in  As x is increased, both the pre-edge and the main edge in the Co K-edge XANES spectra of
As x is increased, both the pre-edge and the main edge in the Co K-edge XANES spectra of  shift to a higher energy region.
shift to a higher energy region.  ions in
ions in  were oxidized to
were oxidized to  ions on lithium deintercalation.
ions on lithium deintercalation.
Figure 10. Co K-edge XANES spectra of  (a) before and (b) after heating at 300°C for 5 h. The inset shows expanded views of the region containing
(a) before and (b) after heating at 300°C for 5 h. The inset shows expanded views of the region containing  transitions.
transitions.
Figure 10b shows the Co K-edge XANES spectra of  after heating at 300°C for 5 h as a function of x. While both the pre-edge and the main edge in the Ni K-edge XANES spectra of
after heating at 300°C for 5 h as a function of x. While both the pre-edge and the main edge in the Ni K-edge XANES spectra of  and
and  shifted to the energy regions lower than those of
shifted to the energy regions lower than those of  after heating, the Co K-edge XANES spectra of heated
after heating, the Co K-edge XANES spectra of heated  had the same pre-edge and main-edge energies as those of pristine
had the same pre-edge and main-edge energies as those of pristine  as shown in Fig. 10b. This indicates that while the oxidation state of nickel ions in
as shown in Fig. 10b. This indicates that while the oxidation state of nickel ions in  and
and  was reduced to the values lower than +3 after heating requiring the reduction of
was reduced to the values lower than +3 after heating requiring the reduction of  to
to  the reduction of cobalt ions took place only to the oxidation state of +3 at this temperature. The pre-edge and the main-edge energies in the Co K-edge XANES spectra of
the reduction of cobalt ions took place only to the oxidation state of +3 at this temperature. The pre-edge and the main-edge energies in the Co K-edge XANES spectra of  both before and after heating were plotted in Fig. 11 for clarity.
both before and after heating were plotted in Fig. 11 for clarity.
Figure 11. (a) Pre-edge and (b) main-edge energies in the Co K-edge XANES spectra of  before and after heating at 300°C for 5 h.
before and after heating at 300°C for 5 h.
 ion is much more readily reducible than
ion is much more readily reducible than  ion, and this is consistent with the fact that
ion, and this is consistent with the fact that  is easily synthesized while stoichiometric
is easily synthesized while stoichiometric  is difficult to synthesize. Therefore, contrary to nickel ions in delithiated
is difficult to synthesize. Therefore, contrary to nickel ions in delithiated  and
and  which are easily reduced to
which are easily reduced to  ions, the reduction of cobalt ions to the oxidation state below +3 is very difficult even at high temperature. It indicates that cobalt ions in
ions, the reduction of cobalt ions to the oxidation state below +3 is very difficult even at high temperature. It indicates that cobalt ions in  can hold more oxygen in oxides at high temperature compared with nickel ions and therefore, suppress the thermal decomposition of
can hold more oxygen in oxides at high temperature compared with nickel ions and therefore, suppress the thermal decomposition of  to a rock-salt phase accompanied by oxygen release.
to a rock-salt phase accompanied by oxygen release.
Figure 12 shows the Co K-edge XANES spectra of 
 after heating at 300°C for 5 h. The Co K-edge XANES spectra of the heated
after heating at 300°C for 5 h. The Co K-edge XANES spectra of the heated  and heated
and heated  were very similar to those of
were very similar to those of  and
and  spinel, respectively, and the Co K-edge XANES spectrum of heated
spinel, respectively, and the Co K-edge XANES spectrum of heated  showed the intermediate feature between the
showed the intermediate feature between the  and the
and the  spinel. The electronic structure of cobalt ions and the local structure around the cobalt ions in the heated
spinel. The electronic structure of cobalt ions and the local structure around the cobalt ions in the heated  and
and  are very similar to those in
are very similar to those in  and
and  respectively. For the heated
respectively. For the heated  some cobalt ions are in the same surroundings as in the
some cobalt ions are in the same surroundings as in the  and the others are in the
and the others are in the  From these results, it can be said that the local structure around the cobalt ions in
From these results, it can be said that the local structure around the cobalt ions in  changed to that in the mixed phase of
changed to that in the mixed phase of  and
and  during heating at 300°C for 5 h, and the fraction of the
during heating at 300°C for 5 h, and the fraction of the  spinel increased with x.
spinel increased with x.
Figure 12. Co K-edge XANES spectra of (a) heated  (b)
(b)  (c) heated
(c) heated  (d) heated
(d) heated  and (e)
and (e) 
It has been reported that delithiated  is decomposed to
is decomposed to  and
and  spinel on heating according to the following reaction6
spinel on heating according to the following reaction6
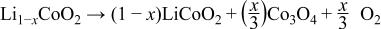
Because the cobalt ions in  have the same local surroundings as in the
have the same local surroundings as in the  as mentioned previously, the local structural changes around the cobalt ions in
as mentioned previously, the local structural changes around the cobalt ions in  with temperature may be similar to those in
with temperature may be similar to those in  Therefore, it can be noted that
Therefore, it can be noted that  and
and  structures are locally formed around the cobalt ions in
structures are locally formed around the cobalt ions in  during heating, and the relative amount of
during heating, and the relative amount of  structure increases as x increases.
structure increases as x increases.
In highly oxidized  NiO and
NiO and  structure may be dominantly formed around the nickel and cobalt ions at high temperatures, respectively. Therefore, the heated
structure may be dominantly formed around the nickel and cobalt ions at high temperatures, respectively. Therefore, the heated  shows the Ni K-edge XANES spectrum similar to that of NiO, as shown in Fig. 9, and the Co K-edge XANES spectrum similar to that of
shows the Ni K-edge XANES spectrum similar to that of NiO, as shown in Fig. 9, and the Co K-edge XANES spectrum similar to that of  as shown in Fig. 12. The XRD patterns of heated
as shown in Fig. 12. The XRD patterns of heated  as shown in Fig. 4b, in which the diffraction peaks of the spinel still remained, could be explained by coexistence of rock-salt phase of NiO structure and spinel phase of
as shown in Fig. 4b, in which the diffraction peaks of the spinel still remained, could be explained by coexistence of rock-salt phase of NiO structure and spinel phase of  structure locally formed around the cobalt ions.
structure locally formed around the cobalt ions.
From the Ni and Co K-edge XANES analysis of  and
and  it can be noted that the decomposition of
it can be noted that the decomposition of  to a rock-salt phase was suppressed by cobalt substitution for nickel.
to a rock-salt phase was suppressed by cobalt substitution for nickel.  structure was locally formed around the cobalt ions in
structure was locally formed around the cobalt ions in  and this spinel structure is relatively stable at high temperatures.
and this spinel structure is relatively stable at high temperatures.
While  spinel structure was locally formed around the cobalt ions in the heated
spinel structure was locally formed around the cobalt ions in the heated  the cobalt ions in the heated
the cobalt ions in the heated  showed an oxidation state of +3, which is slightly higher than the oxidation state (+2.67) of cobalt ions in stoichiometric
showed an oxidation state of +3, which is slightly higher than the oxidation state (+2.67) of cobalt ions in stoichiometric  spinel. It might be due to the incorporation of a small amount of
spinel. It might be due to the incorporation of a small amount of  and/or
and/or  within the spinel structure locally formed around the cobalt ions in the heated
within the spinel structure locally formed around the cobalt ions in the heated  However, further studies should be pursued to obtain accurate structural information about this spinel structure formed around the cobalt ions in the heated
However, further studies should be pursued to obtain accurate structural information about this spinel structure formed around the cobalt ions in the heated 
Conclusions
The thermal stability of highly delithiated  was improved by cobalt substitution for nickel. The thermal stabilization mechanism was explained by local structural changes of
was improved by cobalt substitution for nickel. The thermal stabilization mechanism was explained by local structural changes of  by cobalt addition.
by cobalt addition.
Delithiated  was thermally decomposed to a spinel around 220°C and then turned into a rock-salt phase at higher temperature. Because
was thermally decomposed to a spinel around 220°C and then turned into a rock-salt phase at higher temperature. Because  spinel structure was locally formed around the cobalt ions added in the highly delithiated
spinel structure was locally formed around the cobalt ions added in the highly delithiated  and this spinel structure was relatively stable at high temperature, the decomposition of spinel phase, formed from a thermal decomposition of
and this spinel structure was relatively stable at high temperature, the decomposition of spinel phase, formed from a thermal decomposition of  was suppressed by cobalt addition.
was suppressed by cobalt addition.
Acknowledgments
The authors thank Korea Basic Science Institute for TG measurements and LG Chemical for financial support. This study has been supported by the Brain Korea 21 Projects.
Yonsei University assisted in meeting the publication costs of this article.