Abstract
This paper reports on the electrochemical lithium intercalation/deintercalation of single-crystalline  nanowires that were synthesized by a two-step approach including a hydrothermal process and a post-calcinating treatment. Techniques of X-ray diffraction, field-effect scanning electron microscopy, transmission electron microscopy, and high-resolution transmission electron microscopy were used to characterize the structure, morphology, and composition of the as-prepared nanowires. It was found that
nanowires that were synthesized by a two-step approach including a hydrothermal process and a post-calcinating treatment. Techniques of X-ray diffraction, field-effect scanning electron microscopy, transmission electron microscopy, and high-resolution transmission electron microscopy were used to characterize the structure, morphology, and composition of the as-prepared nanowires. It was found that  nanowires were of high purity with the diameter of
nanowires were of high purity with the diameter of  and the length up to
and the length up to  . The electrochemical lithium intercalation/deintercalation of the
. The electrochemical lithium intercalation/deintercalation of the  nanowires was investigated by cyclic voltammograms and galvanostatic charge-discharge methods. The results showed that the single-crystalline
nanowires was investigated by cyclic voltammograms and galvanostatic charge-discharge methods. The results showed that the single-crystalline  nanowires exhibited high initial discharge capacity of
nanowires exhibited high initial discharge capacity of  , indicating their potential application as the cathode materials of rechargeable lithium-ion batteries.
, indicating their potential application as the cathode materials of rechargeable lithium-ion batteries.
Export citation and abstract BibTeX RIS
Lithium-ion rechargeable batteries have been widely used in personal portable electronics such as in the areas of cell phones, laptops, and digital cameras.1 Among the various candidates for Li-ion intercalations, vanadium pentaoxide  has received extensive attention due to its layered structure and distinctive redox behavior.2–8 It has been found that the electrodes made from one-dimensional (1D) nanostructured
has received extensive attention due to its layered structure and distinctive redox behavior.2–8 It has been found that the electrodes made from one-dimensional (1D) nanostructured  possess high surface areas and short
possess high surface areas and short  diffusion lengths, which greatly improve the discharge capacities and enhance the reversible Li intercalation/deintercalation kinetics.9–12 For example, the initial discharge capacities of both amorphous
diffusion lengths, which greatly improve the discharge capacities and enhance the reversible Li intercalation/deintercalation kinetics.9–12 For example, the initial discharge capacities of both amorphous  nanotube and layered-lamellar
nanotube and layered-lamellar  nanorolls were about
nanorolls were about  ,3, 10 which are much higher than that of the corresponding bulk. In addition, it was found that the crystallinity of nanostructured
,3, 10 which are much higher than that of the corresponding bulk. In addition, it was found that the crystallinity of nanostructured  has a great effect on the electrode properties. The noncrystalline or low-crystalline
has a great effect on the electrode properties. The noncrystalline or low-crystalline  showed higher initial intercalation capacity than that of crystalline
showed higher initial intercalation capacity than that of crystalline  but with poor structural stability.13, 14 Recently, the hydrothermal process has been used to prepare 1D nanostructrured vanadium oxides such as wires,15, 16 ribbons,17 rods,18 belts,19 and tubes.5, 20 Herein we report on high-purity synthesis of single-crystalline
but with poor structural stability.13, 14 Recently, the hydrothermal process has been used to prepare 1D nanostructrured vanadium oxides such as wires,15, 16 ribbons,17 rods,18 belts,19 and tubes.5, 20 Herein we report on high-purity synthesis of single-crystalline  nanowires by a simple hydrothermal method and their lithium intercalation/deintercalation properties.
nanowires by a simple hydrothermal method and their lithium intercalation/deintercalation properties.
Experimental
Synthesis
The single-crystalline  nanowires were prepared via a two-step approach, which involves the precursor preparation of
nanowires were prepared via a two-step approach, which involves the precursor preparation of  nanowires via hydrothermal treatment of polycrystalline
nanowires via hydrothermal treatment of polycrystalline  and the post-calcination of the as-synthesized
and the post-calcination of the as-synthesized  to obtain
to obtain  nanowires. In the first step, a
nanowires. In the first step, a  aqueous suspension of commercial polycrystalline
aqueous suspension of commercial polycrystalline  (
( , size of
, size of  ) and the nonionic surfactant Triton X-100 (t-octylphenyl-
) and the nonionic surfactant Triton X-100 (t-octylphenyl- ,
,  ,
,  ) was stirred for
) was stirred for  to form an orange slurry (pH 7) that was later treated in an autoclave at
to form an orange slurry (pH 7) that was later treated in an autoclave at  for
for  to prepare
to prepare  nanowires. In the second step, the as-prepared
nanowires. In the second step, the as-prepared  nanowires were post-calcinated at
nanowires were post-calcinated at  for
for  in air to engender the target product
in air to engender the target product  nanowires
nanowires
Characterization
The structure and morphology of the samples were analyzed by powder X-ray diffraction (XRD, Rigaku D/max 2500 X-ray generator with Cu  radiation), scanning electron microscopy (SEM, Philips XL-30 and JEOL JSM-6700F Field Emission), transmission electron microscopy (TEM), and high-resolution TEM (HRTEM) (Philips Tecnai F20,
radiation), scanning electron microscopy (SEM, Philips XL-30 and JEOL JSM-6700F Field Emission), transmission electron microscopy (TEM), and high-resolution TEM (HRTEM) (Philips Tecnai F20,  ).21
).21
Electrochemical measurements
Electrochemical properties of the as-prepared  nanowires were studied using two-electrode test cells with lithium metal as the counter and reference electrodes. The working electrode was fabricated by compressing a mixture of the active material (
nanowires were studied using two-electrode test cells with lithium metal as the counter and reference electrodes. The working electrode was fabricated by compressing a mixture of the active material ( nanowires,
nanowires,  ), the conductive material (acetylene black,
), the conductive material (acetylene black,  ), and binder (polytetrafluoroethylene, PTFE,
), and binder (polytetrafluoroethylene, PTFE,  ) into a nickel foam. The cell assembly was similar to our previous work22 and the electrolyte solution was
) into a nickel foam. The cell assembly was similar to our previous work22 and the electrolyte solution was 
 dissolved in a mixture of ethylene carbonate (EC), propylene carbonate (PC), and diethyl carbonate (DEC) with the volume ratio of
dissolved in a mixture of ethylene carbonate (EC), propylene carbonate (PC), and diethyl carbonate (DEC) with the volume ratio of  . The cyclic voltammograms (CV) were measured using a Solartron SI 1287 Electrochemical Interface with a scan rate of
. The cyclic voltammograms (CV) were measured using a Solartron SI 1287 Electrochemical Interface with a scan rate of  at ambient temperature. The capacity and charge-discharge cycling performance of the electrode were investigated by a galvanostatic discharge-charge method recorded at the current density of C/5
at ambient temperature. The capacity and charge-discharge cycling performance of the electrode were investigated by a galvanostatic discharge-charge method recorded at the current density of C/5  and C/20
and C/20  in the potential range of
in the potential range of  .
.
Results and discussion
Structure and morphology characterization
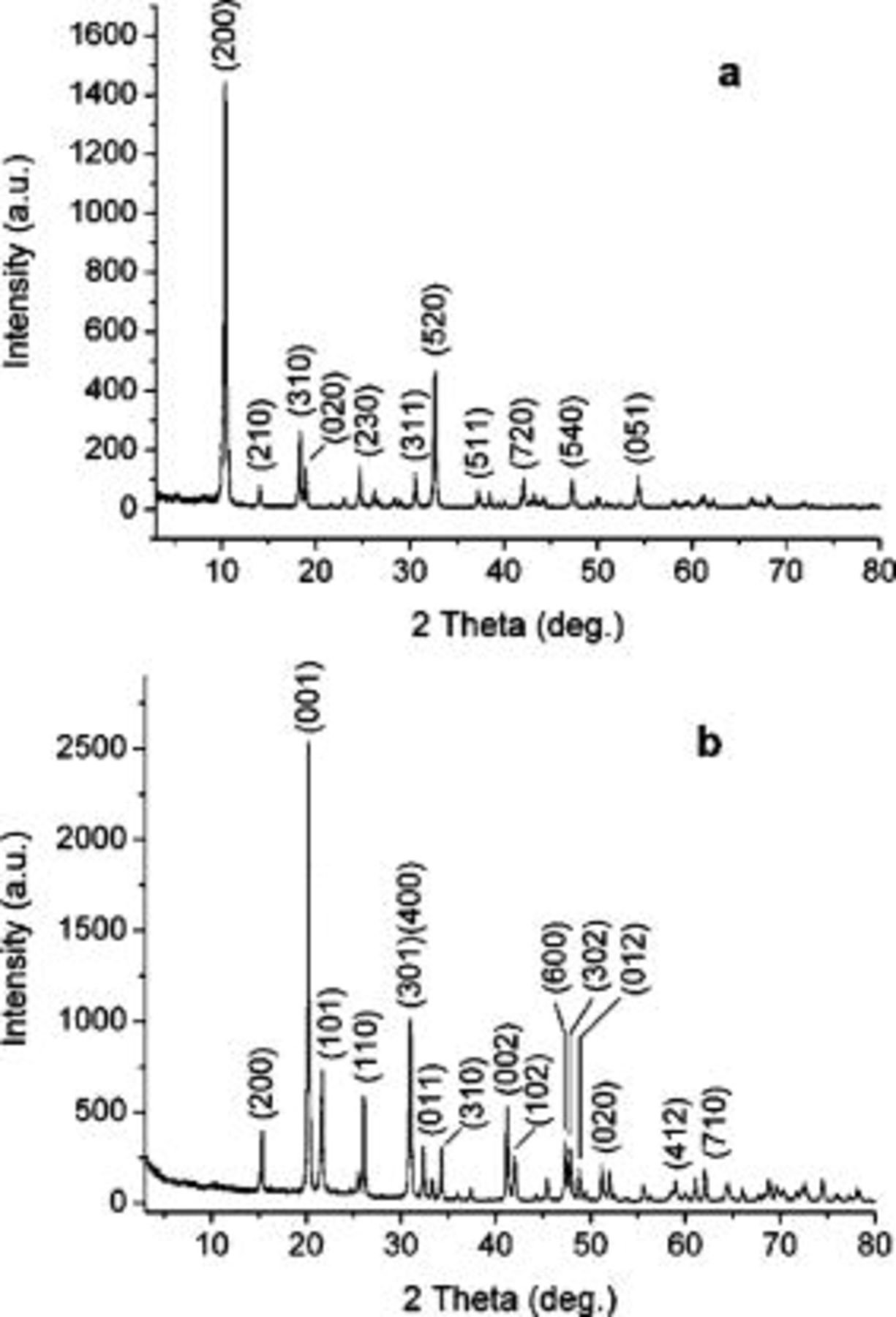
Figure 1a shows the XRD pattern of the as-prepared  nanowires. The peaks can be indexed to an orthorhombic crystalline phase
nanowires. The peaks can be indexed to an orthorhombic crystalline phase  (JCPDS no. 85-2401) with calculated lattice constants
(JCPDS no. 85-2401) with calculated lattice constants  ,
,  , and
, and  . Figure 1b shows the XRD pattern of the as-prepared
. Figure 1b shows the XRD pattern of the as-prepared  nanowires, which can be indexed to an orthorhombic phase of
nanowires, which can be indexed to an orthorhombic phase of  (JCPDS no. 41-1426) with lattice constants
(JCPDS no. 41-1426) with lattice constants  ,
,  , and
, and  . Thus, the pure phase
. Thus, the pure phase  was obtained via the thermal decomposition of
was obtained via the thermal decomposition of  .
.
Figure 1. XRD patterns of the as-synthesized (a) precursor  nanowires and (b) product
nanowires and (b) product  nanowires.
nanowires.
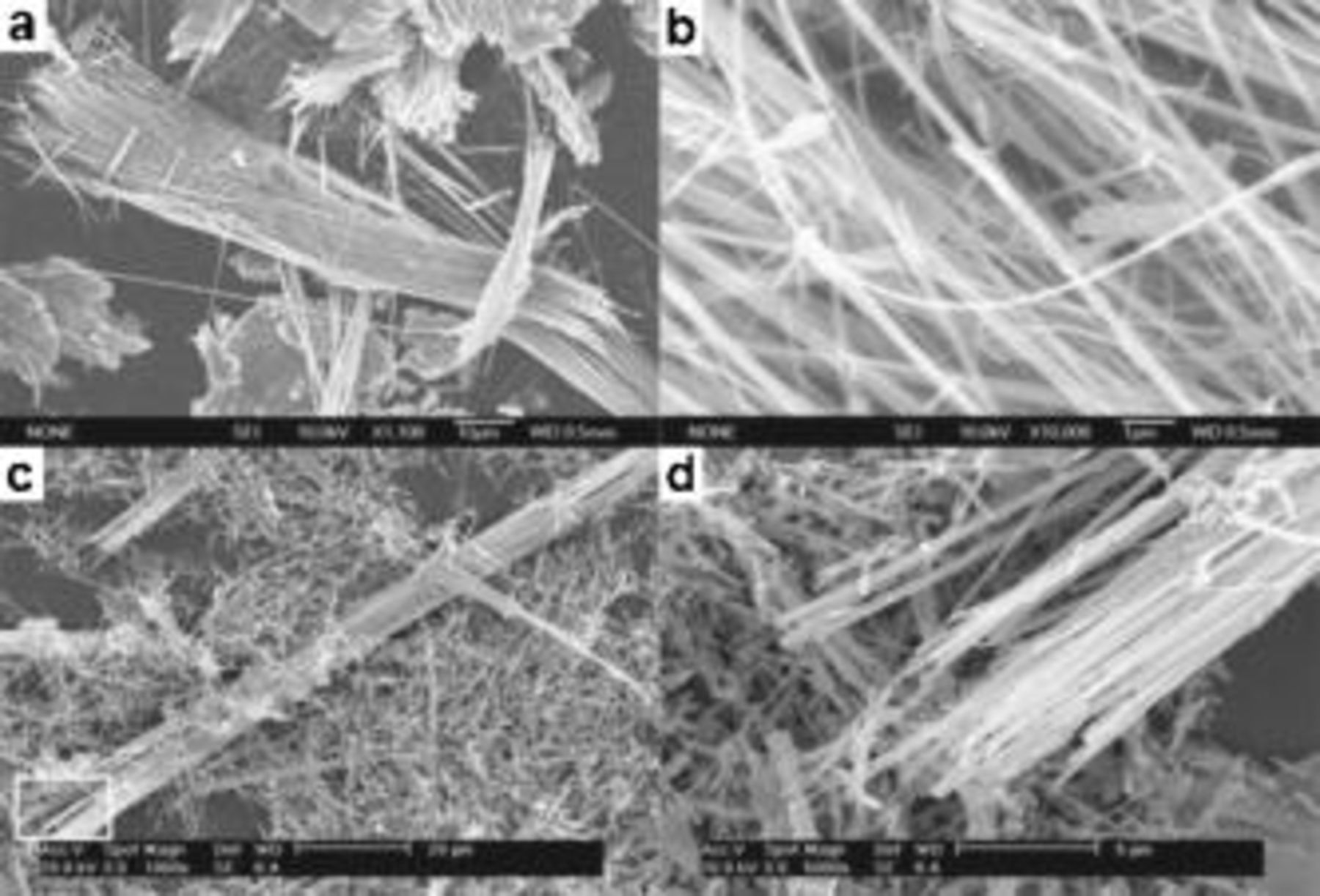
The FESEM image in Fig. 2a shows that the as-prepared  consists of uniform nanowires with the lengths up to
consists of uniform nanowires with the lengths up to  . The typical diameter of the nanowires was found to be
. The typical diameter of the nanowires was found to be  at a higher magnification (Fig. 2b). Note that one of the nanowires did not break up even when it bent to about 90°, indicating the good flexibility of the nanowires. The FESEM images of the resultant
at a higher magnification (Fig. 2b). Note that one of the nanowires did not break up even when it bent to about 90°, indicating the good flexibility of the nanowires. The FESEM images of the resultant  were shown in Fig. 2c and 2d. It can be observed that the morphology of the
were shown in Fig. 2c and 2d. It can be observed that the morphology of the  is similar to the precursor
is similar to the precursor  , which is reasonably attributed to the shape memory effect during the thermal decomposition.
, which is reasonably attributed to the shape memory effect during the thermal decomposition.
Figure 2. The representative FESEM images of (a, b)  nanowires and (c, d)
nanowires and (c, d)  nanowires at different magnifications. (d) Taken from the rectangle marked in (c).
nanowires at different magnifications. (d) Taken from the rectangle marked in (c).
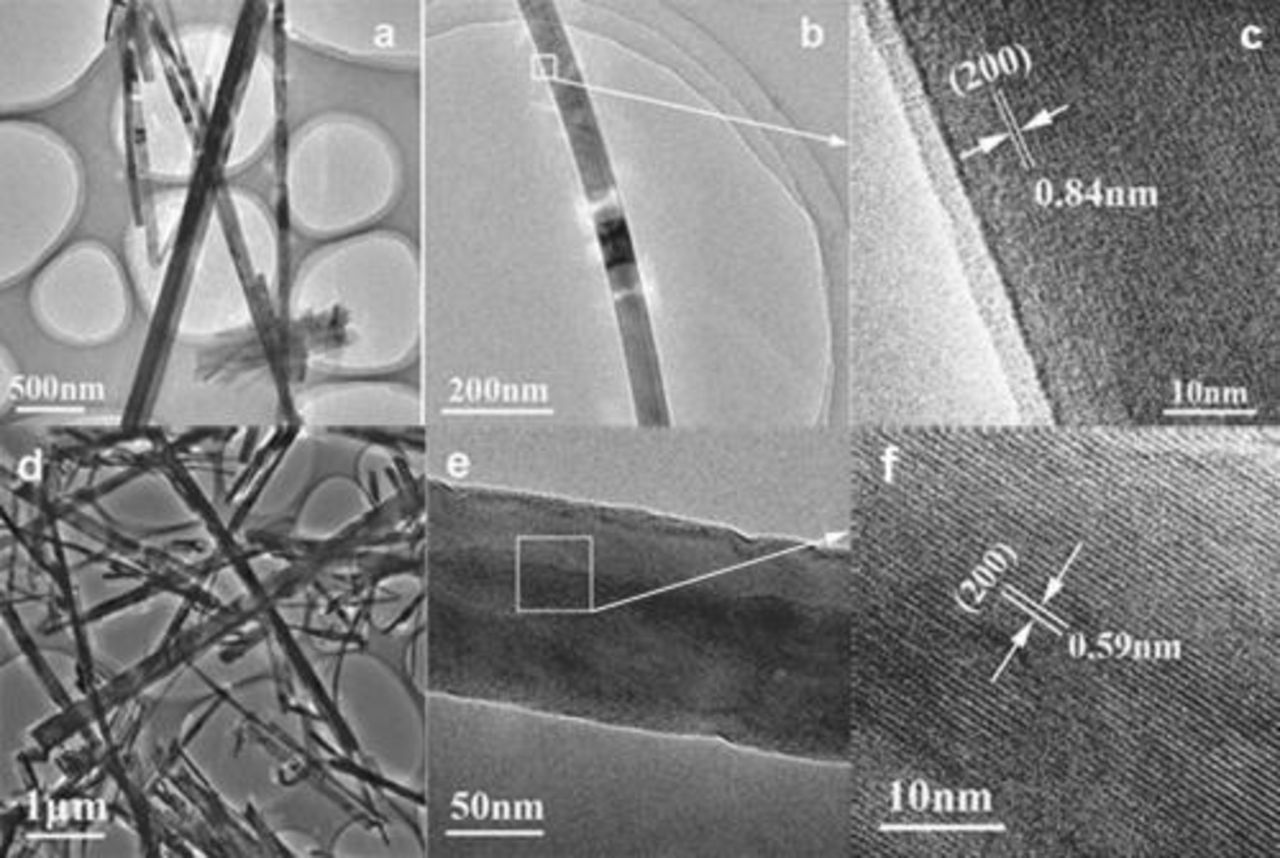
Further insight into the morphology and microstructure of the nanowires was investigated by TEM and HRTEM (Fig. 3). Figure 3a shows the typical TEM image of the as-synthesized  nanowires. Figure 3c exhibits the HRTEM image of the edge of a single nanowire shown in Fig. 3b. It is observed that the interplanar spacing is
nanowires. Figure 3c exhibits the HRTEM image of the edge of a single nanowire shown in Fig. 3b. It is observed that the interplanar spacing is  , which corresponds to the (200) planes of
, which corresponds to the (200) planes of  . The shape of
. The shape of  nanowires, as shown in Fig. 3d, 3e and 3f, is similar to that of
nanowires, as shown in Fig. 3d, 3e and 3f, is similar to that of  nanowires. A single nanowire is exhibited in Fig. 3e, and the HRTEM image of its body part marked with square is demonstrated in Fig. 3f. The observed interplanar spacing of
nanowires. A single nanowire is exhibited in Fig. 3e, and the HRTEM image of its body part marked with square is demonstrated in Fig. 3f. The observed interplanar spacing of  agrees well with the value between the (200) planes of orthorhombic
agrees well with the value between the (200) planes of orthorhombic  crystals. The clear lattice structure was observed extending to the edges (not shown), indicating the single-crystalline structure of
crystals. The clear lattice structure was observed extending to the edges (not shown), indicating the single-crystalline structure of  nanowires.
nanowires.
Figure 3. Typical TEM and HRTEM images of (a-c)  nanowires and (d-f)
nanowires and (d-f)  nanowires. (c) Taken from the square marked in (b). (f) Taken from the square marked in (e).
nanowires. (c) Taken from the square marked in (b). (f) Taken from the square marked in (e).
Electrochemical performance
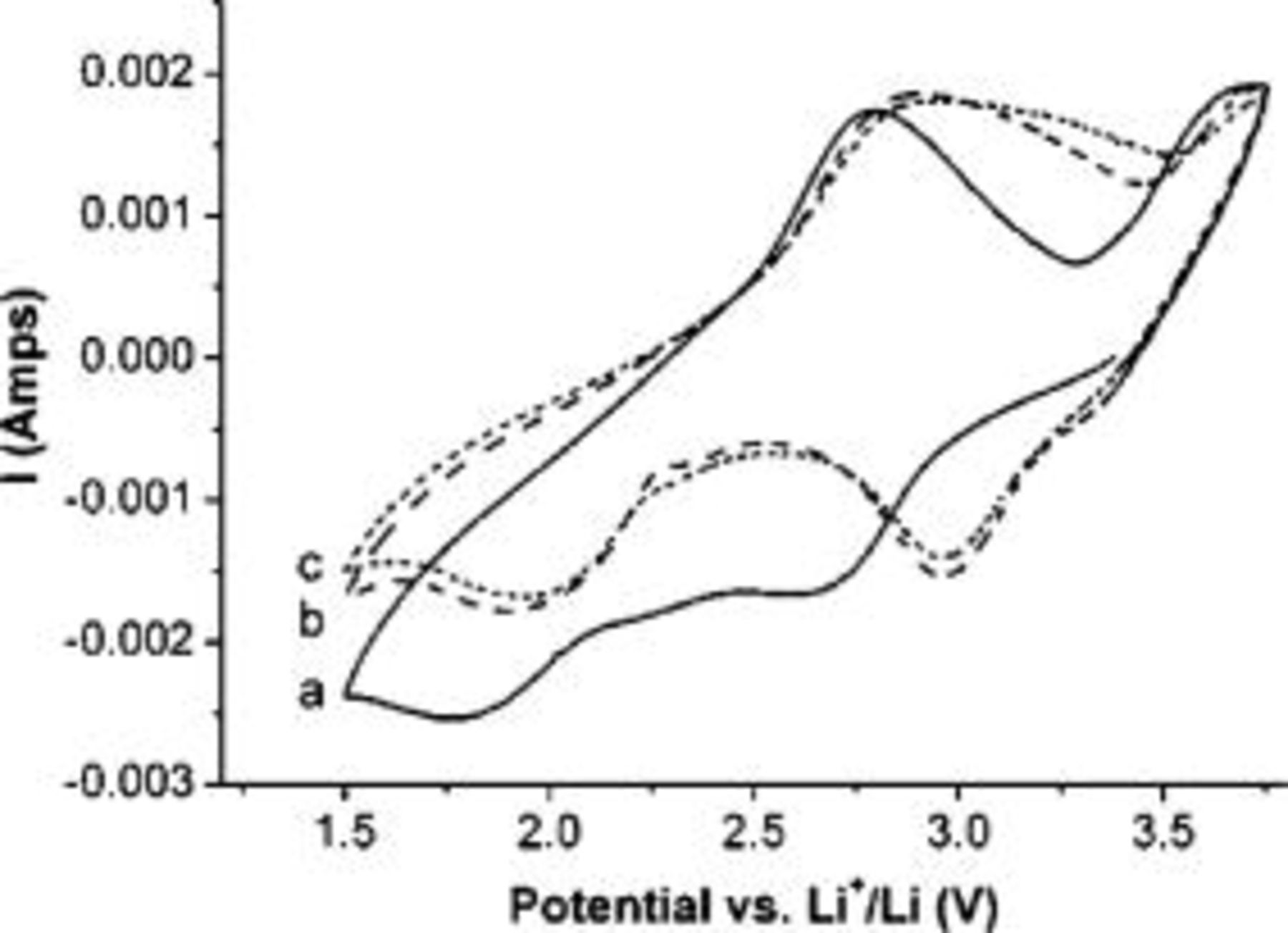
Figure 4 shows the CVs of the electrode made from  nanowires at a scan rate of
nanowires at a scan rate of  . It can be seen that during the cathodic scanning in the first cycle, two distinctive peaks are observed at 2.65 and
. It can be seen that during the cathodic scanning in the first cycle, two distinctive peaks are observed at 2.65 and  vs
vs  , being assigned to a complicated multistep lithium intercalation process.10 In the following anodic scanning, two peaks were observed at around 2.76 and
, being assigned to a complicated multistep lithium intercalation process.10 In the following anodic scanning, two peaks were observed at around 2.76 and  (vs
(vs  ), corresponding to the lithium extraction processes. The
), corresponding to the lithium extraction processes. The  intercalation and deintercalation process can be expressed by
intercalation and deintercalation process can be expressed by
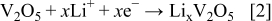
Figure 4. CVs of the electrodes made by  nanowires. (a) The first cycle (solid line), (b) the second cycle (dash line), and (c) the third cycle (dot line), with the scan rate of
nanowires. (a) The first cycle (solid line), (b) the second cycle (dash line), and (c) the third cycle (dot line), with the scan rate of  .
.
As lithium ions were inserted into the layers of  , the phase transformation consecutively occurred from
, the phase transformation consecutively occurred from 
 to
to 
 ,
, 
 ,
, 
 , and
, and 
 .9, 12 Among various phases of
.9, 12 Among various phases of  ,
,  can be recovered to the pristine
can be recovered to the pristine  through lithium deintercalation, while
through lithium deintercalation, while  and
and  (rock-salt type structure) were formed irreversibly. However, for battery application,
(rock-salt type structure) were formed irreversibly. However, for battery application,  and
and  can be reversibly cycled.23, 24
can be reversibly cycled.23, 24
Note that the cathodic pattern in the second cycle is remarkably distinct from that in the first cycle. The main difference is the two-peaks shifting from  and
and  , suggesting that irreversible phase transformation occurred during the lithium insertion process.11 In the following CV cycles, there are no substantial changes in the peak potential and curve shape, which remain similar to those in the second cycle, indicating the reversibility of electrochemical lithium insertion/extraction.
, suggesting that irreversible phase transformation occurred during the lithium insertion process.11 In the following CV cycles, there are no substantial changes in the peak potential and curve shape, which remain similar to those in the second cycle, indicating the reversibility of electrochemical lithium insertion/extraction.
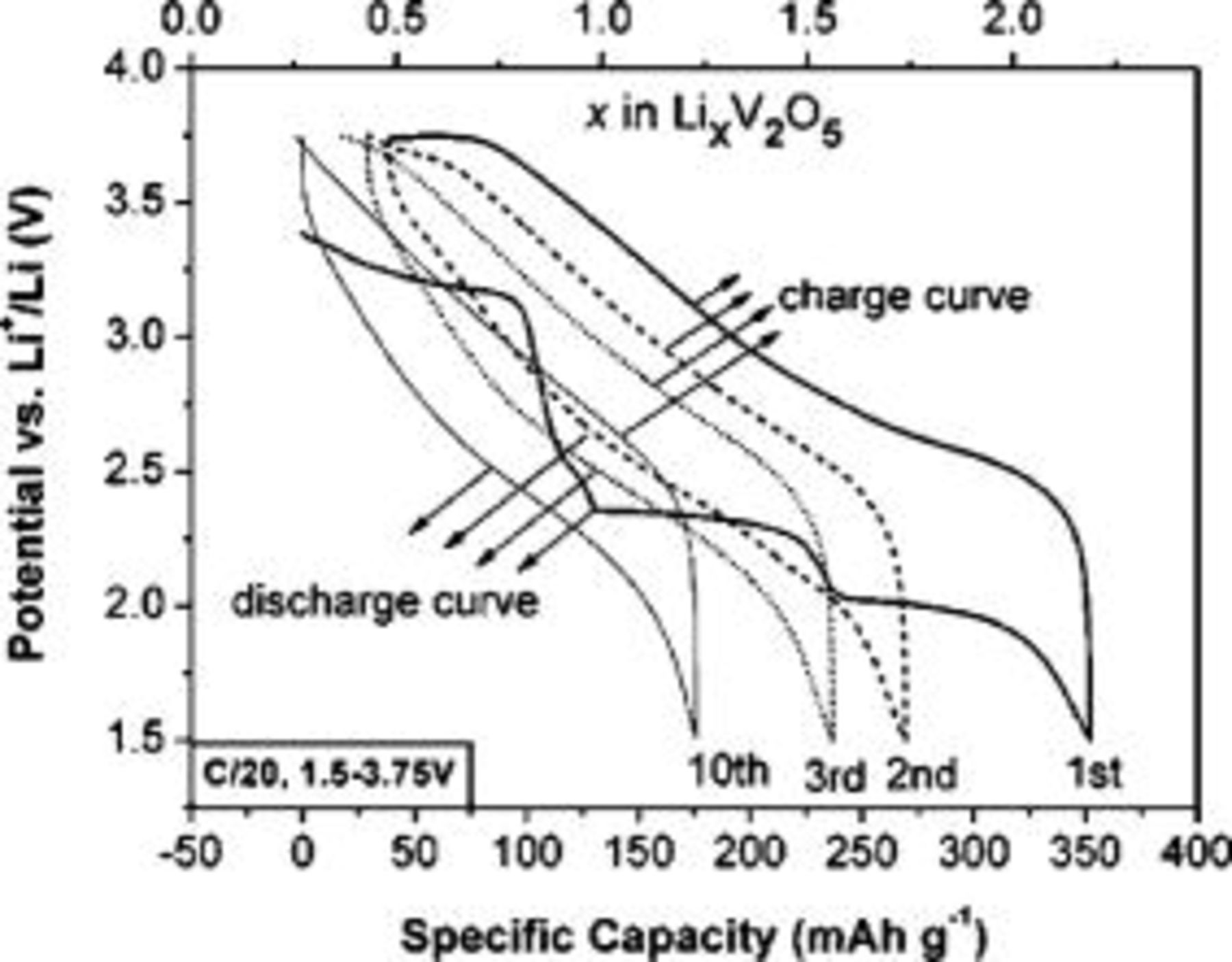
Figure 5 illustrates the first three and the tenth discharge-charge cycles of the electrodes made from  nanowires at a constant current density of C/20
nanowires at a constant current density of C/20  at room temperature. There are three obvious voltage plateaus in the first discharge curve, which is the typical characteristic of crystalline orthorhombic
at room temperature. There are three obvious voltage plateaus in the first discharge curve, which is the typical characteristic of crystalline orthorhombic  discharge pattern.11, 25 The initial discharge capacity was found to be
discharge pattern.11, 25 The initial discharge capacity was found to be  , which corresponds to an intercalation of about
, which corresponds to an intercalation of about 
 into
into 
 , revealing the formation of
, revealing the formation of 
 . Note that the second discharge curve exhibits an obvious indistinct discharge plateau which is different from that in the first cycle, indicating a irreversible phase transformation of
. Note that the second discharge curve exhibits an obvious indistinct discharge plateau which is different from that in the first cycle, indicating a irreversible phase transformation of  . As a result, the discharge capacity dropped to
. As a result, the discharge capacity dropped to  in the second cycle. The third and the following discharge-charge cycles (such as the tenth cycle) are reversible and similar to the second one with less loss of capacities, which confirms the reversibility of lithium intercalation/deintercalation in
in the second cycle. The third and the following discharge-charge cycles (such as the tenth cycle) are reversible and similar to the second one with less loss of capacities, which confirms the reversibility of lithium intercalation/deintercalation in  .23 In a word, the results of CV and galvanostatic measurement are consistent not only in the occurrence of irreversible phase transformation in the first cycle, but also in the reversibility of lithium intercalation/deintercalation in the second and the following cycles.
.23 In a word, the results of CV and galvanostatic measurement are consistent not only in the occurrence of irreversible phase transformation in the first cycle, but also in the reversibility of lithium intercalation/deintercalation in the second and the following cycles.
Figure 5. Electrode discharge-charge curves of  nanowires at the first cycle (black solid line), the second cycle (dash line), the third cycle (dotted line), and the tenth cycle (gray solid line) recorded at C/20
nanowires at the first cycle (black solid line), the second cycle (dash line), the third cycle (dotted line), and the tenth cycle (gray solid line) recorded at C/20  .
.
As the current density increased from C/20  to C/5
to C/5  , the initial capacity decreased from
, the initial capacity decreased from  . It is reported that the theoretical discharge capacities of
. It is reported that the theoretical discharge capacities of  for
for  and
and  for
for  are rarely reached for the crystalline
are rarely reached for the crystalline  .10 In our experiments, however, high initial discharge capacities were obtained with
.10 In our experiments, however, high initial discharge capacities were obtained with  nanowire electrodes, indicating the formation of
nanowire electrodes, indicating the formation of 
 at both two current densities.
at both two current densities.
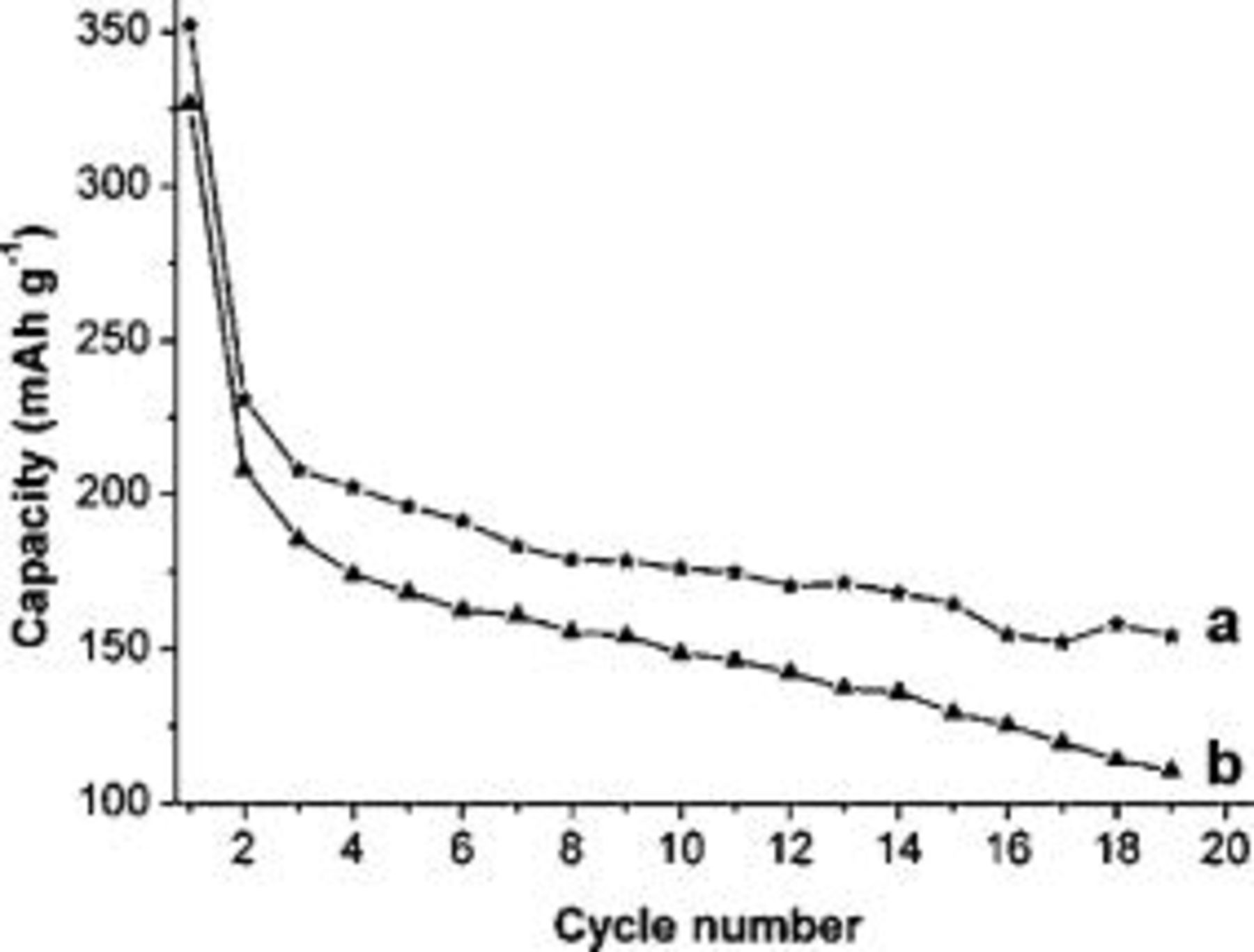
Figure 6 shows the curves of discharge capacity vs cycle number for the electrodes made from  nanowires at two different charge-discharge current densities (C/20 and C/5). The capacities decreased with the increasing cycle number, and a rapid capacity loss is observed in the second cycle at both current densities. After the second cycle, the capacity decay was measured. Thus, the lithium ions in
nanowires at two different charge-discharge current densities (C/20 and C/5). The capacities decreased with the increasing cycle number, and a rapid capacity loss is observed in the second cycle at both current densities. After the second cycle, the capacity decay was measured. Thus, the lithium ions in  can be reversibly inserted/extracted. In the case of the current density of C/5, the capacity decays faster than that of C/20, indicating that the electrode cycle life of
can be reversibly inserted/extracted. In the case of the current density of C/5, the capacity decays faster than that of C/20, indicating that the electrode cycle life of  nanowires still needs improvement. It was reported that the doped
nanowires still needs improvement. It was reported that the doped  with Mn and Mo were found of better electrochemical performance than the undoped ones.26, 27 Thus, the doped
with Mn and Mo were found of better electrochemical performance than the undoped ones.26, 27 Thus, the doped  nanowires might be a solution to further enhance the electrochemical properties of vanadium oxides nanowires.
nanowires might be a solution to further enhance the electrochemical properties of vanadium oxides nanowires.
Figure 6. Variation of discharge capacities vs cycle numbers for the electrodes of  nanowires at the discharge/charge current densities of (a) C/20
nanowires at the discharge/charge current densities of (a) C/20  and (b) C/5
and (b) C/5  .
.
Figure 7 displays the SEM of the cathode mixture which was removed from the nanowire electrode after 30 discharge-charge cycles at the current density of C/5. It can be observed that the nanowire morphology of the active materials can still be preserved during the numerous charge-discharge cycles. The diameter of the nanowires is a little wider and the surface is rougher than that of the as-synthesized  nanowires, which might be due to the coating of the binder (polytetrafluoroethylene, PTFE). In addition, a lot of chunky material is visible in Fig. 7. An explanation follows. The cathode mixture after cycling experiments was prepared by ultrasonic vibrating the electrode in ethanol. As a result, the active material of
nanowires, which might be due to the coating of the binder (polytetrafluoroethylene, PTFE). In addition, a lot of chunky material is visible in Fig. 7. An explanation follows. The cathode mixture after cycling experiments was prepared by ultrasonic vibrating the electrode in ethanol. As a result, the active material of  nanowires was taken out accompanied by the chunky conductive material (acetylene black, ATB) and the binder. The SEM observation indicates the rigid structural feature of
nanowires was taken out accompanied by the chunky conductive material (acetylene black, ATB) and the binder. The SEM observation indicates the rigid structural feature of  nanowires, which can be tolerant of the repeated lithium intercalation/deintercalation processes, and thus is beneficial to holding the capacity of the battery.
nanowires, which can be tolerant of the repeated lithium intercalation/deintercalation processes, and thus is beneficial to holding the capacity of the battery.
Figure 7. SEM of  nanowires after 30 discharge/charge cycles at C/5
nanowires after 30 discharge/charge cycles at C/5  .
.
Conclusions
In summary, single-crystalline  nanowires were fabricated via a two-step route including a hydrothermal process and a post-calcination treatment, which can be extended to the large-scale synthesis of
nanowires were fabricated via a two-step route including a hydrothermal process and a post-calcination treatment, which can be extended to the large-scale synthesis of  nanowires. The electrochemical measurements showed that the electrodes made by the as-synthesized orthorhombic
nanowires. The electrochemical measurements showed that the electrodes made by the as-synthesized orthorhombic  nanowires exhibited a high initial discharge capacity of
nanowires exhibited a high initial discharge capacity of  . The improved electrochemical properties are attributed to the intrinsic structure feature of single-crystalline
. The improved electrochemical properties are attributed to the intrinsic structure feature of single-crystalline  nanowires.
nanowires.
Acknowledgment
This work was supported by National Key Basic Research Program (2005CB623607), the Ministry of Education (2004164), and Tianjin City Project (05YFJMJC00300).
Nankai University assisted in meeting the publication costs of this article.