Abstract
Nitride alloys of Ga, In, and Al activated by rare-earth ions  are being considered for application in nitride-based solid-state light sources. The potential applications involve using such materials as the active layer in a heterostructure design or as a fluorescent material for converting the emission from a light-emitting diode to white light. In this paper, we report luminescence from
are being considered for application in nitride-based solid-state light sources. The potential applications involve using such materials as the active layer in a heterostructure design or as a fluorescent material for converting the emission from a light-emitting diode to white light. In this paper, we report luminescence from  ,
,  ,
,  , and
, and  couple in
couple in  powder samples synthesized for this purpose. Using the photoluminescence and photoluminescence excitation responses of
powder samples synthesized for this purpose. Using the photoluminescence and photoluminescence excitation responses of  -doped
-doped  samples, the multiplet structures of these
samples, the multiplet structures of these  ions in
ions in  with tetrahedral coordination have been determined. The excitation energy transfer processes from the host and defects to
with tetrahedral coordination have been determined. The excitation energy transfer processes from the host and defects to  and from
and from  to
to  have been observed. These processes are critical for developing nitride-based luminescent materials for white-light emission.
have been observed. These processes are critical for developing nitride-based luminescent materials for white-light emission.
Export citation and abstract BibTeX RIS
During the last decade, developing efficacious white-light sources using light-emitting diodes (LEDs) of III-V nitride semiconductors has become a focus of intense research and development in lighting research.1 The conversion of electrical energy to light within a LED die occurs within the active layer sandwiched between the n- and p-type nitride layers. These n- and p-type claddings are chosen with an energy gap larger than that of the active layer for confining the e-h pairs within the active layer under forward-biasing condition. This spatial confinement of electrons and holes within the active layer enhances the probability of radiative recombination of the e-h pairs. The recombination usually results in excitonic or band-edge emission, which is ultimately converted to white light through a layer of fluorescent materials on the LED die.2
In designing such solid-state light sources, luminescent nitrides activated by  could play an important role either as a phosphor or as an active layer material. As a phosphor, they can be used for converting blue or near-ultraviolet (UV) emission from a
could play an important role either as a phosphor or as an active layer material. As a phosphor, they can be used for converting blue or near-ultraviolet (UV) emission from a  -based LED to white light in a hybrid design. As an active layer in a heterostructure design, the rare-earth (RE)-ion impurities perform as recombination centers for the e-h pairs, thus converting the band-edge emission from e-h combination to white light within the die. We are interested in the latter application of nitride-based luminescent materials. Some promising luminescence properties of RE-activated nitrides have already been discussed in the literature.3–7 The main motivation for this work was to explore the choice and concentration of
-based LED to white light in a hybrid design. As an active layer in a heterostructure design, the rare-earth (RE)-ion impurities perform as recombination centers for the e-h pairs, thus converting the band-edge emission from e-h combination to white light within the die. We are interested in the latter application of nitride-based luminescent materials. Some promising luminescence properties of RE-activated nitrides have already been discussed in the literature.3–7 The main motivation for this work was to explore the choice and concentration of  to optimize efficacy of white-light emission from such materials upon e-h recombination.
to optimize efficacy of white-light emission from such materials upon e-h recombination.
RE ions are ideal candidates as radiative recombination centers. The forbidden  transitions are relatively insensitive to the host lattice and lead to narrow band emission.8 Once the energy levels associated with the
transitions are relatively insensitive to the host lattice and lead to narrow band emission.8 Once the energy levels associated with the  configuration are identified, emission from a
configuration are identified, emission from a  ion could be accurately predicted in hosts with similar chemical composition and metal-ligand coordinations. This predictability of the emission spectrum for
ion could be accurately predicted in hosts with similar chemical composition and metal-ligand coordinations. This predictability of the emission spectrum for  transitions of
transitions of  ions has been exploited for making novel blends of luminescent materials with specific color-rendering index and color temperature.9 In this manner, phosphors with RE-ion activators revolutionized the fluorescent lighting industries in the 1960s and 1970s.
ions has been exploited for making novel blends of luminescent materials with specific color-rendering index and color temperature.9 In this manner, phosphors with RE-ion activators revolutionized the fluorescent lighting industries in the 1960s and 1970s.
Luminescent materials like  could have a similar impact on solid-state lighting. Unfortunately, spectroscopy of rare-earth ions in nitrides is still in its infancy. In order to use
could have a similar impact on solid-state lighting. Unfortunately, spectroscopy of rare-earth ions in nitrides is still in its infancy. In order to use  as fluorescent materials, a good understanding of the luminescence processes of the
as fluorescent materials, a good understanding of the luminescence processes of the  ions in nitride hosts in the visible to near-UV region is essential. In order to use these materials as active layers, the need for understanding the processes of e-h recombination in a nitride and the excitation of RE ions via energy transfer from the e-h pairs cannot be overemphasized. In this work, we focus on identifying observed transitions for the RE ions in nitride hosts and developing an understanding of how such ions are excited through photoexcitation of the host material at energies near or larger than their bandgaps.
ions in nitride hosts in the visible to near-UV region is essential. In order to use these materials as active layers, the need for understanding the processes of e-h recombination in a nitride and the excitation of RE ions via energy transfer from the e-h pairs cannot be overemphasized. In this work, we focus on identifying observed transitions for the RE ions in nitride hosts and developing an understanding of how such ions are excited through photoexcitation of the host material at energies near or larger than their bandgaps.
The spectroscopic measurements of photoluminescence (PL) and photoluminescence excitation (PLE) spectra were performed using powder samples of  .
.  was chosen as the host because of its bandgap of
was chosen as the host because of its bandgap of  . It has a wurtzite crystalline structure similar to nitrides of Ga, In, and their alloys in which the cation sites are tetrahedrally coordinated. Thus, the multiplet structures associated with the
. It has a wurtzite crystalline structure similar to nitrides of Ga, In, and their alloys in which the cation sites are tetrahedrally coordinated. Thus, the multiplet structures associated with the  configuration of the RE ions substituting for the cations are expected to be similar for all three nitrides, but the wide bandgap of
configuration of the RE ions substituting for the cations are expected to be similar for all three nitrides, but the wide bandgap of  permits probing the excited states at higher energies before the onset of strong host absorption. The chances of locating the charge transfer and
permits probing the excited states at higher energies before the onset of strong host absorption. The chances of locating the charge transfer and  absorption bands for some
absorption bands for some  ions in nitride hosts are more probable for this wide-gap material.
ions in nitride hosts are more probable for this wide-gap material.
This study is focused on evaluating the performance of  under PL conditions. Irrespective of the method of generation of e-h pairs in a bulk material, these electrons and holes are expected to be at the bottom of the conduction and at the top of the valence band, respectively, in the k-space before their recombination. This state prior to e-h pair recombination can be reproduced by photoexciting the bulk material near the bandedge. At higher energies, there could be many other loss processes as the electrons and holes relax toward the bottom of the conduction band or top of the valence band, respectively, losing their energy to the host lattice through electron-phonon scattering or electron or hole capture by surface states or other nonradiative centers. Therefore, most materials show a maximum in quantum efficiency when excited near the bandedge, and then their quantum efficiency drops rapidly with increasing excitation energy. Thus, the quantum efficiency of a material near the bandedge could be a powerful indicator of the probability of radiative recombination of the e-h pairs irrespective of their method of production.
under PL conditions. Irrespective of the method of generation of e-h pairs in a bulk material, these electrons and holes are expected to be at the bottom of the conduction and at the top of the valence band, respectively, in the k-space before their recombination. This state prior to e-h pair recombination can be reproduced by photoexciting the bulk material near the bandedge. At higher energies, there could be many other loss processes as the electrons and holes relax toward the bottom of the conduction band or top of the valence band, respectively, losing their energy to the host lattice through electron-phonon scattering or electron or hole capture by surface states or other nonradiative centers. Therefore, most materials show a maximum in quantum efficiency when excited near the bandedge, and then their quantum efficiency drops rapidly with increasing excitation energy. Thus, the quantum efficiency of a material near the bandedge could be a powerful indicator of the probability of radiative recombination of the e-h pairs irrespective of their method of production.
In this work we chose to use powder samples of  for these initial spectroscopic measurements in preference to thin films by epitaxial growth techniques. First, there have been scant spectroscopic measurements of nitride powders. Second, photons passing through a thick layer of powder experience longer optical path due to multiple scattering from the interfaces between the particles and thus have a greater probability of being absorbed. Therefore, powder samples can yield satisfactory reflectance, emission, and excitation spectra at low intensity with a small amount of material compared to thin films and are used extensively for studying luminescence from conventional luminescent materials.9
for these initial spectroscopic measurements in preference to thin films by epitaxial growth techniques. First, there have been scant spectroscopic measurements of nitride powders. Second, photons passing through a thick layer of powder experience longer optical path due to multiple scattering from the interfaces between the particles and thus have a greater probability of being absorbed. Therefore, powder samples can yield satisfactory reflectance, emission, and excitation spectra at low intensity with a small amount of material compared to thin films and are used extensively for studying luminescence from conventional luminescent materials.9
The synthesis of high-purity samples of  powders, particularly free of any oxygen defects, is a challenge. The degree of oxygen contamination depends on the method of preparation and ambient conditions. The presence of these impurities could not only affect the transition probability of particular
powders, particularly free of any oxygen defects, is a challenge. The degree of oxygen contamination depends on the method of preparation and ambient conditions. The presence of these impurities could not only affect the transition probability of particular  transitions but also their transition energy. For example, various wavelengths ranging from 600 to
transitions but also their transition energy. For example, various wavelengths ranging from 600 to  have been attributed to
have been attributed to  transition of
transition of  in
in  thin films and powder samples. It is not certain whether the
thin films and powder samples. It is not certain whether the  ions are in a defective but homogeneous phase, such as
ions are in a defective but homogeneous phase, such as  , or in a minor phase of oxides in these samples. For the present study, we have adopted a solution-based approach to prepare samples of
, or in a minor phase of oxides in these samples. For the present study, we have adopted a solution-based approach to prepare samples of  of purity comparable to or better than commercially available samples.10 Although no secondary phases have been detected by X-ray diffraction measurements for the samples used in this study, measurements from energy-dispersive spectroscopy (EDS) indicate the presence of oxygen impurities. The emission spectrum from undoped
of purity comparable to or better than commercially available samples.10 Although no secondary phases have been detected by X-ray diffraction measurements for the samples used in this study, measurements from energy-dispersive spectroscopy (EDS) indicate the presence of oxygen impurities. The emission spectrum from undoped  always showed significant defect emission and the absorption edge shifts to lower energy.
always showed significant defect emission and the absorption edge shifts to lower energy.
From the PL and PLE spectra, we have observed and successfully identified various transitions between multiplet states of RE ions within the  configuration. The differences in transition energies between
configuration. The differences in transition energies between  and those observed in oxides and fluorides are very small. Results from our PLE study also indicate that
and those observed in oxides and fluorides are very small. Results from our PLE study also indicate that  ,
,  , and
, and  in
in  could be excited through energy transfer from both the host lattice and defects. Thus, when these materials are excited at energies above the bandgap, emission is observed through
could be excited through energy transfer from both the host lattice and defects. Thus, when these materials are excited at energies above the bandgap, emission is observed through  transitions of the RE-ion impurities. Because only free e-h pairs are created in the bulk and emission from the
transitions of the RE-ion impurities. Because only free e-h pairs are created in the bulk and emission from the  ions is observed on exciting the host lattice near the bandgap energy, it is reasonable to assume that upon electron and hole injection into the active layer in a heterostructure design of a LED light source, emission from the RE ions will be observed; this paper explores the underlying processes.
ions is observed on exciting the host lattice near the bandgap energy, it is reasonable to assume that upon electron and hole injection into the active layer in a heterostructure design of a LED light source, emission from the RE ions will be observed; this paper explores the underlying processes.
Experimental
The powder samples of  were prepared using a method that is an extension of the approach used first by Juza et al.11 and later improved by Garcia et al. for synthesizing an alloy of Ga and In nitrides.10 Details of the materials synthesis are discussed elsewhere.12
were prepared using a method that is an extension of the approach used first by Juza et al.11 and later improved by Garcia et al. for synthesizing an alloy of Ga and In nitrides.10 Details of the materials synthesis are discussed elsewhere.12
The room temperature PL and PLE spectra were measured with a SPEX DM 3000F spectro-fluorometer with  SPEX 1680 double monochromators. A 450W Xe-lamp was used as the light source. The photons were detected using a cooled Hamamatsu R928 photomultiplier tube. Both PL and PLE spectra were corrected for the spectral response of the setup.
SPEX 1680 double monochromators. A 450W Xe-lamp was used as the light source. The photons were detected using a cooled Hamamatsu R928 photomultiplier tube. Both PL and PLE spectra were corrected for the spectral response of the setup.
Results and Discussion
Luminescence from undoped 
The room-temperature luminescence of an undoped  sample is observed to be a broad emission around
sample is observed to be a broad emission around  (Fig. 1). In undoped
(Fig. 1). In undoped  , the broad emission band is probably composed of host emission and emissions from various point defects in the powder sample, some of the defect bands being excited directly by
, the broad emission band is probably composed of host emission and emissions from various point defects in the powder sample, some of the defect bands being excited directly by  and some indirectly via energy transfer or absorption of emitted radiation from those directly excited. A second band, whose high energy tail near
and some indirectly via energy transfer or absorption of emitted radiation from those directly excited. A second band, whose high energy tail near  is shown, appears to be developing in the near-infrared region. Although no additional crystalline phases are suggested from the X-ray diffraction measurements, EDS measurements show the presence of oxygen defects in the lattice.
is shown, appears to be developing in the near-infrared region. Although no additional crystalline phases are suggested from the X-ray diffraction measurements, EDS measurements show the presence of oxygen defects in the lattice.
Figure 1. Room-temperature PL spectrum of  and undoped
and undoped  corresponding to excitation at
corresponding to excitation at  .
.
From remission measurements, the absorption edge of the undoped  sample appears to be shifted to lower energy,
sample appears to be shifted to lower energy, 
 ,12 compared to the bandgap of
,12 compared to the bandgap of  ,
,  . It is worthwhile to understand the origin of this shift of the bandedge. Trinkler and Brezina attribute the main PL excitation band in
. It is worthwhile to understand the origin of this shift of the bandedge. Trinkler and Brezina attribute the main PL excitation band in  ceramics at
ceramics at  to oxygen-related centers.13 An oxygen atom substituting for a nitrogen atom,
to oxygen-related centers.13 An oxygen atom substituting for a nitrogen atom,  , creates a shallow donor level (the Kroger-Vink notation for point defects in solids is used where superscripts
, creates a shallow donor level (the Kroger-Vink notation for point defects in solids is used where superscripts  , ', and • represent neutral, negative, and positive charge states and subscripts the atom being substituted). From an electronic structure point of view, a neutral point defect
, ', and • represent neutral, negative, and positive charge states and subscripts the atom being substituted). From an electronic structure point of view, a neutral point defect  implies a positively charged defect
implies a positively charged defect  and an electron in a donor level. Thus, the oxygen atoms substituting for the nitrogen atoms in III-V semiconductors lead to n-type conductivity.
and an electron in a donor level. Thus, the oxygen atoms substituting for the nitrogen atoms in III-V semiconductors lead to n-type conductivity.
Considering the nature of chemical bonding Ga and O atoms, it is obvious that this donor level would have the characteristics of an antibonding  -hybridized state of Ga and O, because all the bonding states are fully occupied and belong to occupied states in the valence band. The antibonding levels of
-hybridized state of Ga and O, because all the bonding states are fully occupied and belong to occupied states in the valence band. The antibonding levels of  belong to the unoccupied conduction band states of the host material. In this sense, this impurity-induced donor level due to an oxygen atom substituting for a nitrogen atom can be viewed as a perturbed state at the bottom of the conduction band. Due to the positively charged point defect
belong to the unoccupied conduction band states of the host material. In this sense, this impurity-induced donor level due to an oxygen atom substituting for a nitrogen atom can be viewed as a perturbed state at the bottom of the conduction band. Due to the positively charged point defect  instead of
instead of  , the corresponding energy level is lowered into the bandgap. This donor level associated with
, the corresponding energy level is lowered into the bandgap. This donor level associated with  holding the electron released from the substitution process ultimately frees the electron with increasing temperature and contributes to the n-type behavior.
holding the electron released from the substitution process ultimately frees the electron with increasing temperature and contributes to the n-type behavior.
However, this picture of a localized level associated with an  center is valid at a substantially low concentration of oxygen impurity at donor concentration of
center is valid at a substantially low concentration of oxygen impurity at donor concentration of  which normally corresponds to typical dopant concentration in intentionally doped samples for device application. At the level of oxygen concentration present (2–10%) in the samples used in this study, these localized levels lead to extended states and energy bands. Because this orbital is obtained due to the lowering of a state from the conduction band, the corresponding energy level lies close in energy to the bottom of the conduction band, and the associated wave function has significant overlap with other extended states at the bottom of the conduction band. From first-order perturbation theory, it can be easily shown that the first-order correction to the wave function leads to a significant mixture of the donor wave function with the extended states near the bottom of the conduction band. Thus, with increasing oxygen concentration, these perturbed wave functions overlap through the admixture of the extended states of the host and start forming an energy band. Unlike the deep level or the localized impurity states, the states associated with
which normally corresponds to typical dopant concentration in intentionally doped samples for device application. At the level of oxygen concentration present (2–10%) in the samples used in this study, these localized levels lead to extended states and energy bands. Because this orbital is obtained due to the lowering of a state from the conduction band, the corresponding energy level lies close in energy to the bottom of the conduction band, and the associated wave function has significant overlap with other extended states at the bottom of the conduction band. From first-order perturbation theory, it can be easily shown that the first-order correction to the wave function leads to a significant mixture of the donor wave function with the extended states near the bottom of the conduction band. Thus, with increasing oxygen concentration, these perturbed wave functions overlap through the admixture of the extended states of the host and start forming an energy band. Unlike the deep level or the localized impurity states, the states associated with  rapidly lead to delocalized states with increasing oxygen concentration. This is essentially reflected in the results obtained from band-structure calculations using supercells ranging from typical unit cells to
rapidly lead to delocalized states with increasing oxygen concentration. This is essentially reflected in the results obtained from band-structure calculations using supercells ranging from typical unit cells to  supercells.14 From this perspective, the host in this study could be considered as a crystalline and homogeneous material having the composition
supercells.14 From this perspective, the host in this study could be considered as a crystalline and homogeneous material having the composition  with a bandgap near
with a bandgap near  .
.
Luminescence from  in
in 
 is considered as a candidate for blue emission.
is considered as a candidate for blue emission.  is used as a blue emitter in cathode ray tube (CRT) applications (
is used as a blue emitter in cathode ray tube (CRT) applications ( at
at  ). Figure 1 compares the room-temperature PL spectra of
). Figure 1 compares the room-temperature PL spectra of  with that of an undoped
with that of an undoped  sample excited at
sample excited at  from a Xe lamp. The narrow-band f-f intraconfigurational transitions associated with
from a Xe lamp. The narrow-band f-f intraconfigurational transitions associated with  are clearly contrasted with the broad-band emission from undoped
are clearly contrasted with the broad-band emission from undoped  due to lattice defects and impurities. The spectroscopic assignments of the emission peaks are made by comparisons with data from the literature for
due to lattice defects and impurities. The spectroscopic assignments of the emission peaks are made by comparisons with data from the literature for  thin film15 and
thin film15 and  powder samples.16
powder samples.16
The strongest emission with the peak wavelength of  is attributed to the
is attributed to the  transition of
transition of  . The blue emission near
. The blue emission near  is assigned to the hypersensitive
is assigned to the hypersensitive  transition. In addition to these two major peaks, there are two other peaks assigned to
transition. In addition to these two major peaks, there are two other peaks assigned to  and
and  transitions near 360 and
transitions near 360 and  , respectively. All the observed transitions are listed in Table I, along with their spectroscopic assignments.
, respectively. All the observed transitions are listed in Table I, along with their spectroscopic assignments.
Table I. Summary of intraconfigurational transitions of  ,
,  , and
, and  in
in  .
.
 ion ion | λ (nm) at room temperature | Transitions assignments |
|---|---|---|
| Tm | 356 |

|
| 481 |

| |
| 655 |

| |
| 792 |

| |
| Tb | 378 |

|
| 416 |

| |
| 435 |

| |
| 484 |

| |
| 542 |

| |
| 585 |

| |
| 621 |

| |
| Eu | 364 |

|
| 378 |

| |
| 465 |

| |
| 526 |

| |
| 536 |

| |
| 592 |

| |
| 610 |

| |
| 655 |

| |
| 707 |

|
When  is doped with
is doped with  , the emission spectrum is dominated by emission from
, the emission spectrum is dominated by emission from  and the defect emission is significantly suppressed. This is indicative of successful energy transfer from the host or the excited states of the defect to
and the defect emission is significantly suppressed. This is indicative of successful energy transfer from the host or the excited states of the defect to  .
.
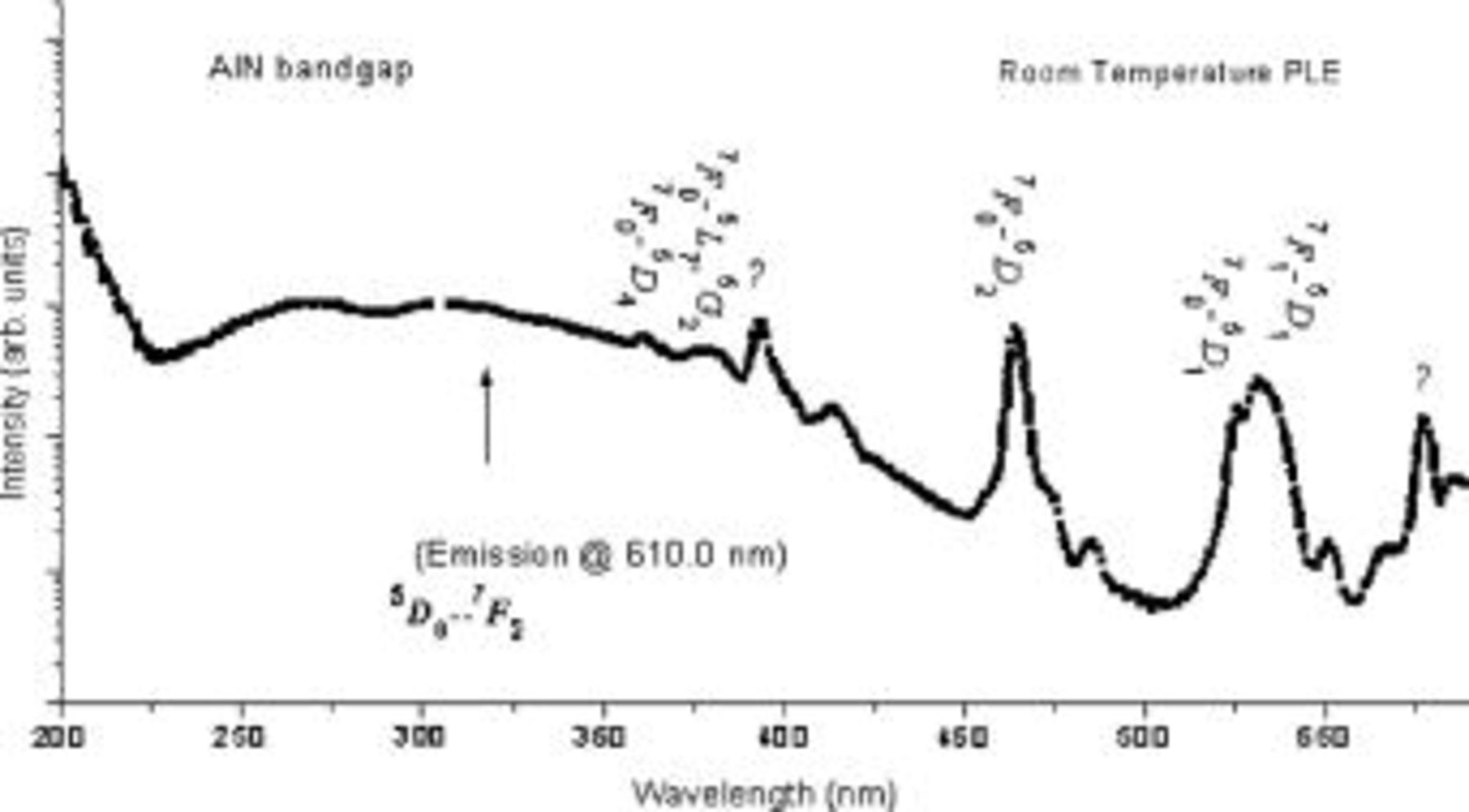
In order to have a better understanding of the excitation processes leading to the  intraconfigurational emissions, the UV-visible excitation spectra were measured with the emission wavelength fixed at
intraconfigurational emissions, the UV-visible excitation spectra were measured with the emission wavelength fixed at  (corresponding to the
(corresponding to the  transition). The PLE spectrum is shown in Fig. 2. It clearly shows that this transition can be excited in a broad range from 300 to
transition). The PLE spectrum is shown in Fig. 2. It clearly shows that this transition can be excited in a broad range from 300 to  . Two weak but well-defined peaks are superimposed upon this broad excitation profile. These two peaks are easily identified to be
. Two weak but well-defined peaks are superimposed upon this broad excitation profile. These two peaks are easily identified to be  and
and  associated with intraconfigurational transition within the
associated with intraconfigurational transition within the  manifold. These two peaks overlap with the broad-band emission from undoped
manifold. These two peaks overlap with the broad-band emission from undoped  . This is one of the conditions for energy transfer from one radiative center to another. Most likely, nonradiative energy transfer from the host or defect states excites
. This is one of the conditions for energy transfer from one radiative center to another. Most likely, nonradiative energy transfer from the host or defect states excites  from the ground state,
from the ground state, to
to  and
and  states, from which it relaxes to the emitting state,
states, from which it relaxes to the emitting state,  .
.
Figure 2. Room-temperature PLE spectrum of the  transition in
transition in  at
at  .
.
A broad excitation band with a peak around  is also observed. An excitation peak near
is also observed. An excitation peak near  was reported earlier to be associated with oxygen-associated defect centers.13 Considering the absorption edge of
was reported earlier to be associated with oxygen-associated defect centers.13 Considering the absorption edge of  , as determined from the remission spectrum of the same sample, this excitation band is tentatively attributed to the excitation of the
, as determined from the remission spectrum of the same sample, this excitation band is tentatively attributed to the excitation of the  host with oxygen impurities,
host with oxygen impurities,  . At this excitation wavelength, absorption of a photon most likely promotes an electron from the ground state to the excited state of the host lattice. These states are probably perturbed p-like states of nitrogen and s-like states of aluminum, respectively. The source of perturbation could be oxygen defects occupying the nitrogen sites or
. At this excitation wavelength, absorption of a photon most likely promotes an electron from the ground state to the excited state of the host lattice. These states are probably perturbed p-like states of nitrogen and s-like states of aluminum, respectively. The source of perturbation could be oxygen defects occupying the nitrogen sites or  occupying the aluminum sites. In the former case, the 3s-like levels of aluminum ions surrounding the oxygen impurities are lowered into the gap region. This process may explain the modified bandgap of these materials estimated from reflectance measurements. In the latter case, the 2p-like levels of nitrogen ions coordinated to the RE ions move into the band gap due to lower Madelung potential experienced by these ions because of the substantially larger
occupying the aluminum sites. In the former case, the 3s-like levels of aluminum ions surrounding the oxygen impurities are lowered into the gap region. This process may explain the modified bandgap of these materials estimated from reflectance measurements. In the latter case, the 2p-like levels of nitrogen ions coordinated to the RE ions move into the band gap due to lower Madelung potential experienced by these ions because of the substantially larger  bond length compared to that of
bond length compared to that of  . We also observed this broad excitation band peaked around 245 nm in the PLE spectra of samples of
. We also observed this broad excitation band peaked around 245 nm in the PLE spectra of samples of  doped with other RE ions such as
doped with other RE ions such as  12 and
12 and  , which is discussed in detail in the following sections. Thus, this excitation peak is probably associated with transitions in the perturbed environment of the
, which is discussed in detail in the following sections. Thus, this excitation peak is probably associated with transitions in the perturbed environment of the  and the transfer of energy from the corresponding excited states to the
and the transfer of energy from the corresponding excited states to the  is quite efficient. Because defect emission is still observed with 250 nm excitation in other samples of
is quite efficient. Because defect emission is still observed with 250 nm excitation in other samples of  , it is also possible that excitation of
, it is also possible that excitation of  ions proceeds at this wavelength with sequential energy transfers from the host to defects to
ions proceeds at this wavelength with sequential energy transfers from the host to defects to  ions. The two excitation mechanisms were discussed in detail in Ref. 12. Alternatively, upon excitation at
ions. The two excitation mechanisms were discussed in detail in Ref. 12. Alternatively, upon excitation at  , free electron and hole pairs are generated which radiatively recombine preferably at the RE ions sites; it is very difficult to distinguish experimentally this nonradiative process from the other nonradiative energy-transfer processes. Irrespective of the exact mechanism, it appears that the
, free electron and hole pairs are generated which radiatively recombine preferably at the RE ions sites; it is very difficult to distinguish experimentally this nonradiative process from the other nonradiative energy-transfer processes. Irrespective of the exact mechanism, it appears that the  ion successfully harvests excitation from the host. This observation is particularly encouraging for its application as a LED light source.
ion successfully harvests excitation from the host. This observation is particularly encouraging for its application as a LED light source.
Figure 2 indicates that  could also be excited in a broad range of spectrum from 300 to
could also be excited in a broad range of spectrum from 300 to  . Excitation spectrum associated with the defect emission at
. Excitation spectrum associated with the defect emission at  could also be contributing partly to this broadband. But this contribution is expected to be very weak considering the strong RE emission at this wavelength. In this broad wavelength range from 300 to
could also be contributing partly to this broadband. But this contribution is expected to be very weak considering the strong RE emission at this wavelength. In this broad wavelength range from 300 to  , the absorption of photons most likely promotes defects from the ground states to the excited states other than those excited at
, the absorption of photons most likely promotes defects from the ground states to the excited states other than those excited at  . The
. The  ions are subsequently excited from the ground state via nonradiative energy transfer from these excited point defects.
ions are subsequently excited from the ground state via nonradiative energy transfer from these excited point defects.
Luminescence from  in
in 
The  transition in the yellow-green region from
transition in the yellow-green region from  has often been utilized as the green component in white-light-emitting blends of luminescent materials. Figure 3 shows the room-temperature PL spectra of an
has often been utilized as the green component in white-light-emitting blends of luminescent materials. Figure 3 shows the room-temperature PL spectra of an  sample excited at
sample excited at  . The narrow band
. The narrow band  intra configurational transitions associated with the
intra configurational transitions associated with the  appear to be superimposed on a background emission from the host lattice. Although emission from the defects in the host lattice is stronger than that for
appear to be superimposed on a background emission from the host lattice. Although emission from the defects in the host lattice is stronger than that for  , the emission from
, the emission from  appears to be quite efficient. Various emission peaks have been given spectroscopic assignments (Table I) by comparisons with data from the literature for
appears to be quite efficient. Various emission peaks have been given spectroscopic assignments (Table I) by comparisons with data from the literature for  .8, 15 The strongest emission near
.8, 15 The strongest emission near  is attributed to the
is attributed to the  transition of
transition of  . This transition will be useful for application of this material as a yellowish-green emitter in solid-state lighting applications.
. This transition will be useful for application of this material as a yellowish-green emitter in solid-state lighting applications.
Figure 3. Room-temperature PL spectrum of  corresponding to the excitation wavelength of
corresponding to the excitation wavelength of  .
.
The PLE spectrum corresponding to the  emission is shown in Fig. 4. Similar to
emission is shown in Fig. 4. Similar to  , the PLE spectrum consists of a broad-band peaking at
, the PLE spectrum consists of a broad-band peaking at  , another broad-band profile extending to almost
, another broad-band profile extending to almost  , and sharp peaks which could be assigned to
, and sharp peaks which could be assigned to  transitions associated with
transitions associated with  . These peaks overlap with the broad-band emission from the host lattice and are most likely involved in energy transfer from the host or defect states to
. These peaks overlap with the broad-band emission from the host lattice and are most likely involved in energy transfer from the host or defect states to  . As mentioned earlier, the broad excitation peak is most likely associated with the perturbed states of p-like states of nitrogen and s-like states of aluminum, respectively.
. As mentioned earlier, the broad excitation peak is most likely associated with the perturbed states of p-like states of nitrogen and s-like states of aluminum, respectively.
Figure 4. Room-temperature PLE spectrum for the  transition in
transition in  at
at  .
.
Luminescence from  in
in 
Figure 5 shows the room-temperature PL spectra of an  sample. The excitation wavelengths were fixed at 250 (solid line) and
sample. The excitation wavelengths were fixed at 250 (solid line) and  (dashed line). In contrast to other samples discussed, the emission from the defect states appears to be quite strong with
(dashed line). In contrast to other samples discussed, the emission from the defect states appears to be quite strong with  excitation. Nevertheless, in the spectral range from 550 to
excitation. Nevertheless, in the spectral range from 550 to  , one observes sharp multiplet transitions within the f manifold. Upon exciting
, one observes sharp multiplet transitions within the f manifold. Upon exciting  directly at
directly at  corresponding to a transition from the ground level,
corresponding to a transition from the ground level,  , to an excited level,
, to an excited level,  , a well-resolved, clean spectrum for
, a well-resolved, clean spectrum for  in
in  is obtained with almost no defect emission. In Table I, a list of observed
is obtained with almost no defect emission. In Table I, a list of observed  transitions for
transitions for  in this sample is provided.
in this sample is provided.
Figure 5. Room-temperature PL spectrum of  for excitations at 250 and
for excitations at 250 and  .
.
In Fig. 5 the strongest emission with the peak wavelength near 610 nm is attributed to the hypersensitive  transition of
transition of  . This transition is particularly interesting for application of this material as a red emitter in solid-state lighting applications. The peak energy for this
. This transition is particularly interesting for application of this material as a red emitter in solid-state lighting applications. The peak energy for this  hypersensitive transition in
hypersensitive transition in  has been reported at energies varying within
has been reported at energies varying within  depending on the method of preparation. It is usually observed at
depending on the method of preparation. It is usually observed at  in
in  and
and  thin films. This emission is reported to be at
thin films. This emission is reported to be at  in
in  prepared by radio frequency (rf)-sputtering17 or at
prepared by radio frequency (rf)-sputtering17 or at  along with a shoulder at
along with a shoulder at  by Hirata et al. in
by Hirata et al. in  powder prepared by a combustion method.18 Hao19 reports this transition occurs at
powder prepared by a combustion method.18 Hao19 reports this transition occurs at  in
in  . Kitai20 describes this emission as occurring at
. Kitai20 describes this emission as occurring at  in
in  thin-film devices. In oxide phosphors
thin-film devices. In oxide phosphors  ,
,  , and
, and  , these transitions are known to occur at 611, 619, and
, these transitions are known to occur at 611, 619, and  , respectively. These variations appear to suggest that the intensity of this hypersensitive transition is not only dependent on the host lattice but also depends on structural modifications in the local environment. Considering that our sample has higher oxygen impurity concentration compared to thin films grown epitaxially with oxygen-free precursors, the peak energy at
, respectively. These variations appear to suggest that the intensity of this hypersensitive transition is not only dependent on the host lattice but also depends on structural modifications in the local environment. Considering that our sample has higher oxygen impurity concentration compared to thin films grown epitaxially with oxygen-free precursors, the peak energy at  indicates that the
indicates that the  ion segregate in an oxygen-rich environment.
ion segregate in an oxygen-rich environment.
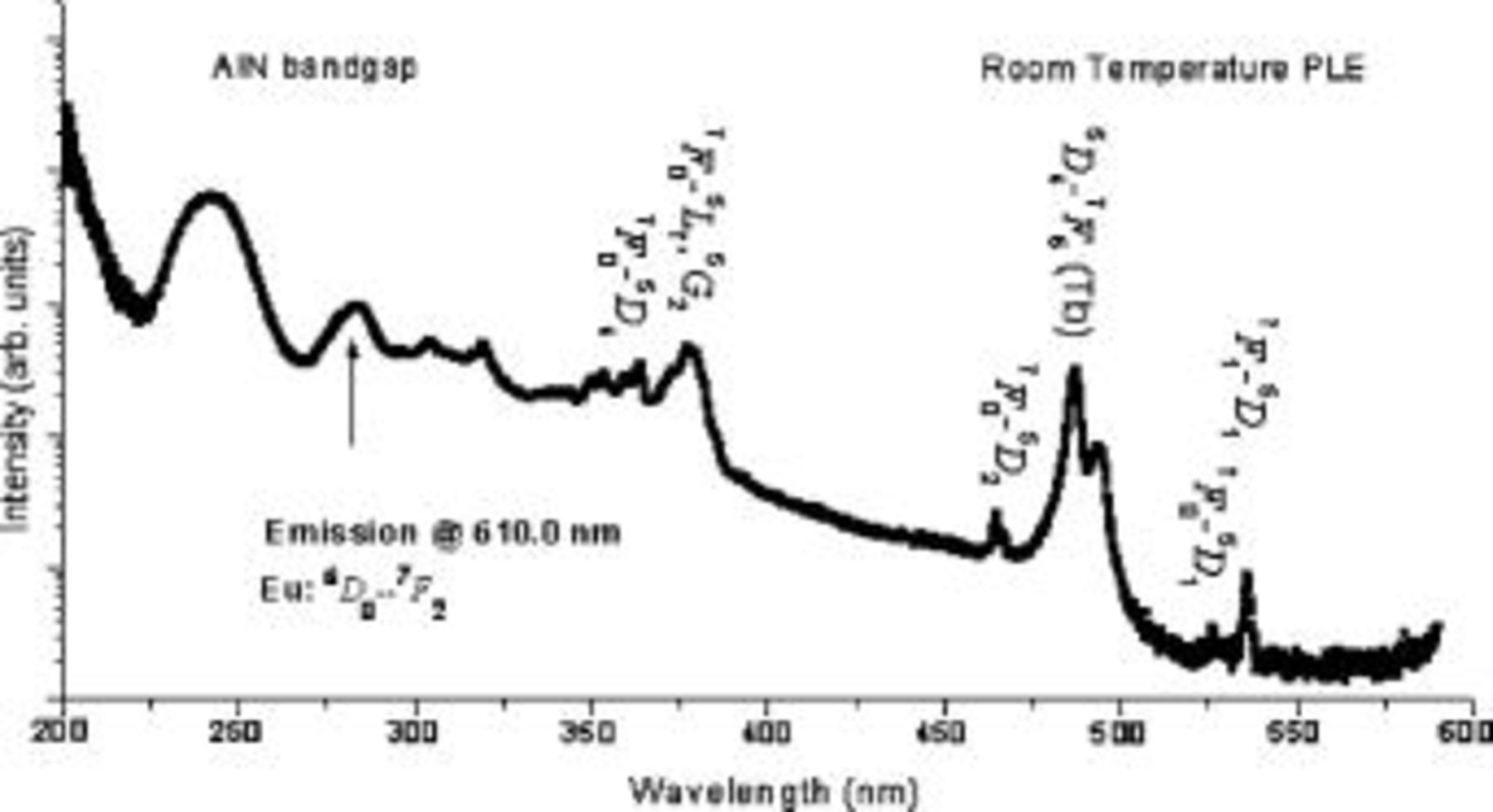
To determine the excitation processes leading to the  intraconfigurational transitions, the UV-visible excitation spectrum was measured with the emission wavelength fixed at
intraconfigurational transitions, the UV-visible excitation spectrum was measured with the emission wavelength fixed at  (corresponding to the
(corresponding to the  transition). The PLE spectra (Fig. 6) suggests similar excitation processes, namely, excitation via defects between 300 and to
transition). The PLE spectra (Fig. 6) suggests similar excitation processes, namely, excitation via defects between 300 and to  , excitation via energy transfer from the host around
, excitation via energy transfer from the host around  , and direct excitation via its own excited state levels within the
, and direct excitation via its own excited state levels within the  manifold. However, the broad peak near
manifold. However, the broad peak near  is not as pronounced as in the case of
is not as pronounced as in the case of  and
and  .
.
Figure 6. Room-temperature PLE spectrum of the  transition in
transition in  .
.
Luminescence from  codoped with
codoped with  and
and  —Evidence of energy transfer from
—Evidence of energy transfer from  to
to 
Figures 7a and 7b show the room-temperature PL spectra of an  sample codoped with
sample codoped with  and
and  . The narrow-band f-f intraconfigurational transitions associated with both
. The narrow-band f-f intraconfigurational transitions associated with both  and
and  are clearly observed. The spectrum between 300 and
are clearly observed. The spectrum between 300 and  (Fig. 7a) is dominated by the intraconfigurational f-f transitions of
(Fig. 7a) is dominated by the intraconfigurational f-f transitions of  , while between 580 and
, while between 580 and  (Fig. 7b) the f-f transitions involving
(Fig. 7b) the f-f transitions involving  are clearly observed. The assignments of different transitions are indicated in the figures. These results indicate that in the codoped sample both Tb and Eu are optically active in the trivalent state.
are clearly observed. The assignments of different transitions are indicated in the figures. These results indicate that in the codoped sample both Tb and Eu are optically active in the trivalent state.
Figure 7. Room-temperature PL spectrum of an  sample codoped with (a) Tb and Eu in the wavelength range between 300 and
sample codoped with (a) Tb and Eu in the wavelength range between 300 and  and (b)
and (b)  and
and  in the wavelength range between 580 and
in the wavelength range between 580 and  .
.
Our main interest in the  couple is to explore if energy transfer from one RE ion to another occurs in a nitride host. This information is critical for designing a single emitting system that could generate white light with one sensitizer and multiple activators. The energy transfer from
couple is to explore if energy transfer from one RE ion to another occurs in a nitride host. This information is critical for designing a single emitting system that could generate white light with one sensitizer and multiple activators. The energy transfer from  to
to  has been previously observed in
has been previously observed in  and
and  codoped
codoped  .21 This process of
.21 This process of  excitation is attributed to
excitation is attributed to

In order to determine if the red emitting  ion could be sensitized by
ion could be sensitized by  the UV-visible excitation spectrum was measured with the emission wavelength fixed at
the UV-visible excitation spectrum was measured with the emission wavelength fixed at  (corresponding to the
(corresponding to the  transition) while the excitation wavelength was scanned between 200 and
transition) while the excitation wavelength was scanned between 200 and  (Fig. 8). In addition to narrow excitation peaks originating from
(Fig. 8). In addition to narrow excitation peaks originating from  , there is a peak which clearly belongs to the
, there is a peak which clearly belongs to the  transition associated with
transition associated with  . This transition could be used for transferring energy from
. This transition could be used for transferring energy from  to
to  while using excitation features of
while using excitation features of  to optimize the host lattice for maximum efficacy.
to optimize the host lattice for maximum efficacy.
Figure 8. Room-temperature PLE spectrum of the  transition in
transition in  .
.
Conclusions
In this work, effective intraconfigurational f-f emissions from  powders doped with different RE ions including
powders doped with different RE ions including  ,
,  ,
,  , and
, and  couple were demonstrated. Both PL and PLE responses of the different samples were systematically investigated to determine the electronic structures of RE ions in the nitride host. The PLE studies of
couple were demonstrated. Both PL and PLE responses of the different samples were systematically investigated to determine the electronic structures of RE ions in the nitride host. The PLE studies of  -,
-,  -, and
-, and  -doped
-doped  indicate that the RE transitions could be excited through both host lattice and defects in the
indicate that the RE transitions could be excited through both host lattice and defects in the  host. Energy transfer between different RE ions is also observed in
host. Energy transfer between different RE ions is also observed in  codoped
codoped  . Our results support the idea of using
. Our results support the idea of using  -doped
-doped  as a suitable active layer in a heterostructure design.
as a suitable active layer in a heterostructure design.
Acknowledgments
This manuscript was prepared with the support of the U.S. Department of Energy, under award no. DE-FC26-04NT42274 . However, any opinions, findings, conclusions, or recommendations expressed herein are those of the authors and do not necessarily reflect the views of the DOE.
OSRAM SYLVANIA assisted in meeting the publication costs of this article.