Abstract
The stringent environmental requirements regarding the mobility energy usage are forcing most automakers to develop hybrid electric vehicles, which allows for a more efficient and thus less polluting use of fossil combustibles. A vast deployment of such vehicles involves producing and recycling of batteries on the thousand tons per year scale. Present Li-ion technologies involve the use of fluorinated binders, which are costly, and the use of environmentally unfriendly volatile organic compounds for the processing, which are difficult to recycle. In this paper, it is shown that the fluorinated binders can be replaced with greener and cost-effective polymers derived from cellulose.
Export citation and abstract BibTeX RIS
The development of greener, cheaper, and safer batteries is considered today as a goal of strategic importance for battery technology. The realization of such kinds of batteries could open new possibilities for the application of these devices, thus making their massive utilization in the automotive industry possible, with tremendous consequences for the battery market.
Since a few years ago, numerous groups have been studying and developing new electrode and electrolyte materials with suitable characteristics for the realization of greener batteries, and promising results have already been achieved.1, 2 However, to realize batteries with such kinds of properties, not only active and electrolyte materials have to be taken into account but, in general, all the battery components and even the process to realize the batteries that need to be considered and improved. Several studies are now also focused on the improvement of the inactive materials as well as of the electrode production.1, 2 In this context, a key role is certainly played by the binder. As a matter of fact, the binder is not only responsible for the binding of the active materials and the conductive agent to the metal current collectors, but it also strongly affects the electrode processing. Consequently, the improvement of the binder must necessarily be considered as a key point for the development of new greener batteries.
An interesting example of the influence of the binder for the development of green batteries is observed when the preparation of electrodes based on lithium iron phosphate  is considered.
is considered.  displays high stability of the capacity during prolonged cycling; it is environment-friendly, cheap, and safe.3–5 Because of these characteristics, today it is considered as a very attractive cathodic material and a promising candidate for the development of greener and cheaper batteries. So far, however, most of the research and the development in composite cathodes, and also for those based on
displays high stability of the capacity during prolonged cycling; it is environment-friendly, cheap, and safe.3–5 Because of these characteristics, today it is considered as a very attractive cathodic material and a promising candidate for the development of greener and cheaper batteries. So far, however, most of the research and the development in composite cathodes, and also for those based on  , have been focused on the use of fluorinated binders and practically all commercial lithium-ion batteries are made using poly(vinylidene fluoride) (PVDF) as the binder. However, this polymer is costly (industrial cost in the multiton scale is around 15–18 EUR/kg); it requires the use of volatile organic compounds that are often toxic (like
, have been focused on the use of fluorinated binders and practically all commercial lithium-ion batteries are made using poly(vinylidene fluoride) (PVDF) as the binder. However, this polymer is costly (industrial cost in the multiton scale is around 15–18 EUR/kg); it requires the use of volatile organic compounds that are often toxic (like  -methyl pyrrolidone) in the processing, and it is not easily disposable at the end of the battery life. Therefore, when the overall electrode preparation process is considered, clearly only the use of a safe and cheap material such as
-methyl pyrrolidone) in the processing, and it is not easily disposable at the end of the battery life. Therefore, when the overall electrode preparation process is considered, clearly only the use of a safe and cheap material such as  is insufficient for the development of greener batteries. Also, the introduction of alternative binders, as well as an improved preparation procedure, is necessary.
is insufficient for the development of greener batteries. Also, the introduction of alternative binders, as well as an improved preparation procedure, is necessary.
Recently, alternative binders have been introduced for the manufacture of anodes for lithium-ion batteries.6–18 Among them, one of the most interesting is certainly the sodium salt of carboxymethyl cellulose (CMC). CMC is produced by the insertion of carboxy methyl groups in natural cellulose. The presence of these groups makes the CMC water-soluble. This is certainly the greatest advantage of CMC because it allows processing in aqueous slurries rather than in polluting, health and environment unfriendly, volatile organic-compound-based slurries. The second great advantage of CMC resides in its easy disposability at the end of the life of the battery. Once the electrode is extracted, the active electrode material can be easily recovered by pyrolysis of the binder. Last but not least is the material cost. The CMC industrial price is about 1–2 EUR/kg, i.e., about 1 order of magnitude lower than PVDF.
Considering these advantages, clearly the introduction of CMC as a binder in combination with  could be an important step for the improvement of the preparation of cathodic material and the development of greener and cost-effective lithium-ion batteries. So far, only a few groups have reported on the manufacture of
could be an important step for the improvement of the preparation of cathodic material and the development of greener and cost-effective lithium-ion batteries. So far, only a few groups have reported on the manufacture of  cathodes using CMC as a slurry thickener and as a binder.19–22 The results reported so far showed that
cathodes using CMC as a slurry thickener and as a binder.19–22 The results reported so far showed that  can be processed in aqueous slurries and that CMC could be used as an alternative binder for this cathodic material. However, even if these results were certainly very encouraging, the long-term performance of
can be processed in aqueous slurries and that CMC could be used as an alternative binder for this cathodic material. However, even if these results were certainly very encouraging, the long-term performance of  electrodes containing CMC as the binder was not fully investigated and well defined.
electrodes containing CMC as the binder was not fully investigated and well defined.
This paper is focused on the study of  -based electrodes using CMC as a binder. The morphology and electrochemical performance of electrodes prepared with two different drying procedures have been investigated. For comparison,
-based electrodes using CMC as a binder. The morphology and electrochemical performance of electrodes prepared with two different drying procedures have been investigated. For comparison,  -based electrodes containing PVDF were also considered. The results are reported in this paper.
-based electrodes containing PVDF were also considered. The results are reported in this paper.
Experimental
The carbon-coated  was provided by a commercial supplier (Südchemie, Germany) and was used as delivered. The average particle size diameter was
was provided by a commercial supplier (Südchemie, Germany) and was used as delivered. The average particle size diameter was  and the carbon content was 2.3%. Sodium CMC was provided by Dow Wolff Cellulosics (Walocel CRT 2000 PPA 12) with a degree of substitution of 1.2. As a conducting agent, carbon black (Super-P, TIMCAL), with an average particle size of 30 nm, was used.
and the carbon content was 2.3%. Sodium CMC was provided by Dow Wolff Cellulosics (Walocel CRT 2000 PPA 12) with a degree of substitution of 1.2. As a conducting agent, carbon black (Super-P, TIMCAL), with an average particle size of 30 nm, was used.
For the preparation of the electrodes, CMC was dissolved in high purity deionized water to obtain a 2.5 wt % solution and equilibrated for 1 h at room temperature with magnetic stirring. The required amount of Super-P was then added and the mixture further equilibrated for one more hour with magnetic stirring. By adding more water, the viscosity was decreased before the addition of  . The selected amount of active material was added and then immediately dispersed with a high energy stirrer (Ultra-Turrax, IKA) for 30 min. The so-obtained slurry was cast immediately on an etched aluminum foil (
. The selected amount of active material was added and then immediately dispersed with a high energy stirrer (Ultra-Turrax, IKA) for 30 min. The so-obtained slurry was cast immediately on an etched aluminum foil ( ,
,  , etched with 5 wt % KOH,
, etched with 5 wt % KOH,  , 60 s) by using a laboratory scale doctor blade. The wet film thickness was
, 60 s) by using a laboratory scale doctor blade. The wet film thickness was  , and the wet foil was immediately predried in an atmospheric oven with stagnant air at
, and the wet foil was immediately predried in an atmospheric oven with stagnant air at  for 12 h. From the so-obtained electrode tapes, disk electrodes with a diameter of 12 mm were cut out. The punched electrodes were dried at two different temperatures (120 and
for 12 h. From the so-obtained electrode tapes, disk electrodes with a diameter of 12 mm were cut out. The punched electrodes were dried at two different temperatures (120 and  ) under vacuum for 24 h. The composition of the dried electrode was 88 wt %
) under vacuum for 24 h. The composition of the dried electrode was 88 wt %  , 7 wt % Super-P, and 5 wt % of CMC. Benchmark electrodes whose compositions were 85 wt %
, 7 wt % Super-P, and 5 wt % of CMC. Benchmark electrodes whose compositions were 85 wt %  , 10 wt % Super-P, and 5 wt % of PVDF (Kynar Flex 761) were prepared as described in Ref. 23. The average mass loading of the electrodes containing CMC was
, 10 wt % Super-P, and 5 wt % of PVDF (Kynar Flex 761) were prepared as described in Ref. 23. The average mass loading of the electrodes containing CMC was  and
and  for the electrodes with PVDF. The corresponding average density of the electrodes was
for the electrodes with PVDF. The corresponding average density of the electrodes was  (CMC) and
(CMC) and  (PVDF), displaying wet film thickness/dry coating thickness ratios of approximately 1:5 for CMC and 1:4 for PVDF. All the electrochemical tests were carried out in a three-electrode Swagelok cell. The cells were assembled in an M Braun glove box with oxygen and water contents lower than 1 ppm. Metallic lithium (Chemetall) was used for both the counter and reference electrodes. As a separator, a stack of Freudenberg fleeces (FS2226) drenched in
(PVDF), displaying wet film thickness/dry coating thickness ratios of approximately 1:5 for CMC and 1:4 for PVDF. All the electrochemical tests were carried out in a three-electrode Swagelok cell. The cells were assembled in an M Braun glove box with oxygen and water contents lower than 1 ppm. Metallic lithium (Chemetall) was used for both the counter and reference electrodes. As a separator, a stack of Freudenberg fleeces (FS2226) drenched in  of the electrolyte was used. The electrolyte was 1 M
of the electrolyte was used. The electrolyte was 1 M  in a 30:70 volume mixture of ethylene carbonate and diethyl carbonate with a water content of less than 10 ppm.
in a 30:70 volume mixture of ethylene carbonate and diethyl carbonate with a water content of less than 10 ppm.
The C-rate test was done at room temperature between 4.2 and 2.8 V with a constant charge rate of C/10 (ca.  ) and the discharge rate was varied from C/10 (ca.
) and the discharge rate was varied from C/10 (ca.  ) to 5C
) to 5C  . After the C-rate test, constant current–constant voltage (CCCV) tests were carried out with a current density corresponding to a 1C rate at room temperature. Both measurement types were performed at
. After the C-rate test, constant current–constant voltage (CCCV) tests were carried out with a current density corresponding to a 1C rate at room temperature. Both measurement types were performed at  with a BaSyTec MDS battery test system or with a Maccor Battery tester 4300.
with a BaSyTec MDS battery test system or with a Maccor Battery tester 4300.
A  source was used for the
source was used for the  Mössbauer spectroscopy investigations. The samples were placed in thin-walled poly(vinyl chloride) sealed containers with a weight of about
Mössbauer spectroscopy investigations. The samples were placed in thin-walled poly(vinyl chloride) sealed containers with a weight of about  . The measurements were run in the usual transmission geometry. Both the source and the absorber were kept at room temperature. The total counting time per spectrum was about 8 h. The values reported were referred to as α-iron.
. The measurements were run in the usual transmission geometry. Both the source and the absorber were kept at room temperature. The total counting time per spectrum was about 8 h. The values reported were referred to as α-iron.
Results and Discussion
The introduction of aqueous processing for the electrode preparation is limited by the stability in water of the used active material. If the active materials do not show satisfactory stability in water, during the preparation process its structure could be modified with a negative effect on the performance of the electrode.
Carbon-coated  performance is known to suffer from humidity absorption that leads to the formation of LiOH and the oxidation of Fe from the bivalent to the trivalent state. Recently, some papers19–21 have shown that when properly postdried,
performance is known to suffer from humidity absorption that leads to the formation of LiOH and the oxidation of Fe from the bivalent to the trivalent state. Recently, some papers19–21 have shown that when properly postdried,  can be processed in water. However, considering the fact that the presence of ppm level of water can deteriorate the overall cell performance, processing this material in aqueous environments obviously presents the risk of losing some capacity. For this reason, great care was taken to identify the optimum drying procedure. Two different drying temperatures (120 and
can be processed in water. However, considering the fact that the presence of ppm level of water can deteriorate the overall cell performance, processing this material in aqueous environments obviously presents the risk of losing some capacity. For this reason, great care was taken to identify the optimum drying procedure. Two different drying temperatures (120 and  ) were selected and
) were selected and  electrodes using CMC binder were prepared. For comparison,
electrodes using CMC binder were prepared. For comparison,  electrodes using PVDF as a binder were also prepared and tested.
electrodes using PVDF as a binder were also prepared and tested.
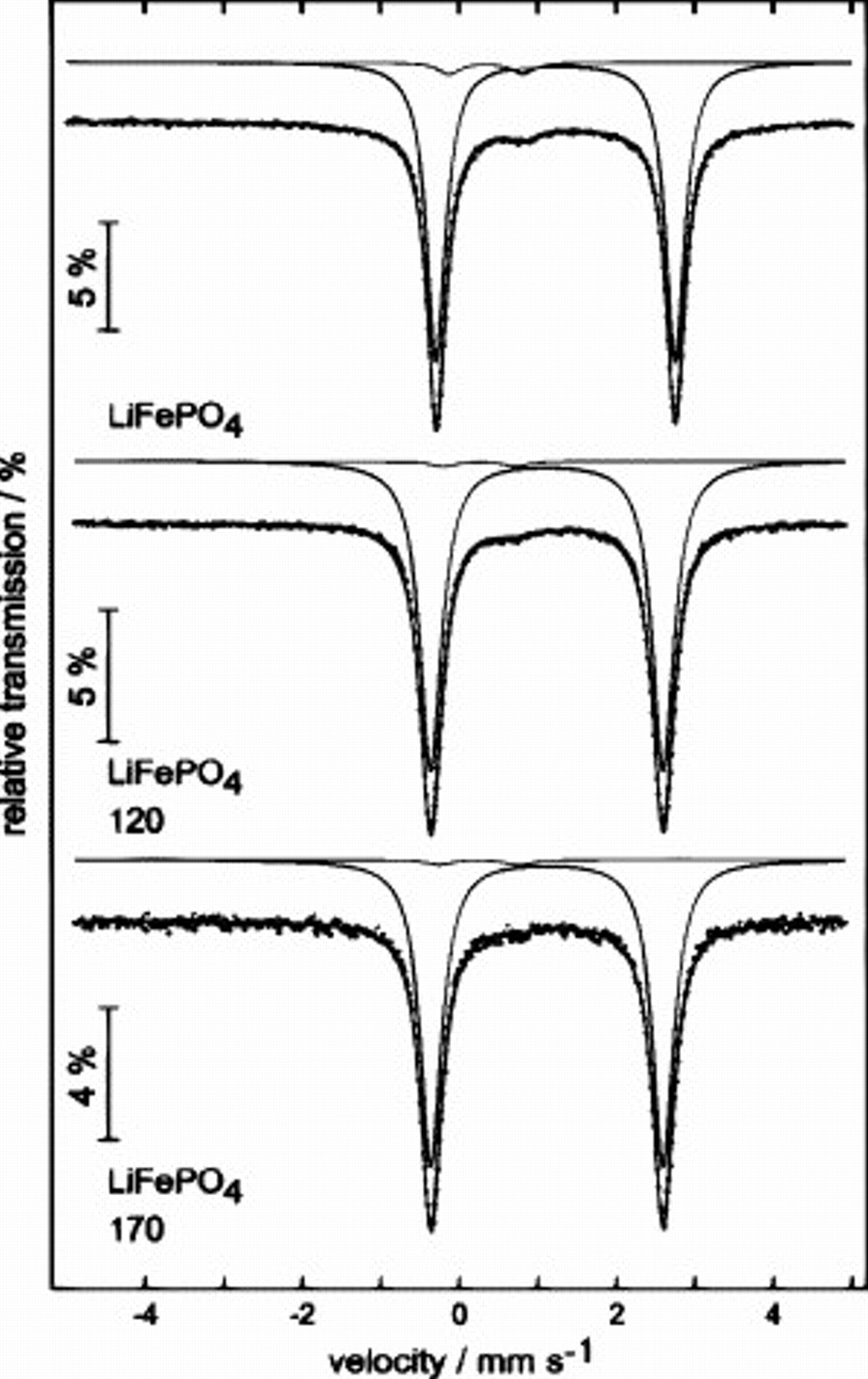
To verify if the use of the aqueous slurries during the preparation of the electrodes containing CMC would affect the structure of the pristine  and to optimize the drying procedure, we took advantage of Mössbauer spectroscopy. As shown in Fig. 1, the recorded spectra showed that the use of aqueous slurries during the electrode preparation practically does not modify the oxidation state of iron in
and to optimize the drying procedure, we took advantage of Mössbauer spectroscopy. As shown in Fig. 1, the recorded spectra showed that the use of aqueous slurries during the electrode preparation practically does not modify the oxidation state of iron in  . As a matter of fact, the spectra of the electrodes containing CMC are comparable with those of pristine
. As a matter of fact, the spectra of the electrodes containing CMC are comparable with those of pristine  . As reported in Table I, the isomer shift
. As reported in Table I, the isomer shift  and the quadrupolar splitting
and the quadrupolar splitting  of the three considered samples agree with the values reported in the literature24, 25 and indicate that all the iron present in the samples is in the
of the three considered samples agree with the values reported in the literature24, 25 and indicate that all the iron present in the samples is in the  state of charge. These results clearly indicate that a fast processing in water and a correct drying procedure are able to prevent the structural modification of
state of charge. These results clearly indicate that a fast processing in water and a correct drying procedure are able to prevent the structural modification of  . Moreover, no significant differences have been observed between the electrodes dried at 120 and
. Moreover, no significant differences have been observed between the electrodes dried at 120 and  , indicating that both temperatures were suitable for the electrode preparation process. On the basis of these results, the use of aqueous binders, in particular CMC, instead of the most expensive and volatile organic compounds-based PVDF therefore appears possible.
, indicating that both temperatures were suitable for the electrode preparation process. On the basis of these results, the use of aqueous binders, in particular CMC, instead of the most expensive and volatile organic compounds-based PVDF therefore appears possible.
Figure 1. Mössbauer spectra of  as it is (pristine material) and in CMC-based electrodes dried at 120 and
as it is (pristine material) and in CMC-based electrodes dried at 120 and  . The spectra were recorded at room temperature.
. The spectra were recorded at room temperature.
Table I. Parameters used to fit the Mössbauer spectra at room temperature of  as it is (pristine material) and in CMC-based electrodes dried at 120 and
as it is (pristine material) and in CMC-based electrodes dried at 120 and  .
.  : Isomer shift;
: Isomer shift;  : Quadrupolar splitting;
: Quadrupolar splitting;  : Linewidth.
: Linewidth.
| Compound | δ 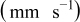 |
 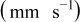 | Γ 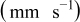 |
|---|---|---|---|
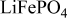 | 1.23 (1) | 2.97 (1) | 0.31 (1) |
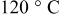 | 1.22 (1) | 2.96 (1) | 0.33 (1) |
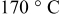 | 1.22 (1) | 2.96 (1) | 0.32 (1) |
When the morphology of the prepared electrodes is considered, another important advantage of the use of aqueous slurries during the electrode preparation can also be observed. As shown in the scanning electron microscope (SEM) images reported in Fig. 2, the electrode containing CMC (Fig. 2a) displays a different morphology with respect to that based on PVDF (Fig. 2b). The electrode containing CMC displays a more homogeneous surface and a more uniform distribution over the current collector, whereas the electrodes containing PVDF appear less homogeneous and with a higher number of aggregate particles. This difference seems to indicate that after the manufacturing process, CMC-based electrodes are more compact than those based on PVDF. The higher compactness of the electrodes containing CMC certainly represents an advantage in view of their use in batteries because they might not need the further additional processing (roll-pressing) required for  electrodes based on PVDF. This postpreparation treatment is, in fact, needed to increase the compactness, i.e., to reduce the porosity, of the electrodes to improve their performance inside the batteries. Electrodes based on CMC already display a high compactness that would allow for a reduction or even the elimination of this postpreparation treatment during the manufacturing process, with a consequent reduction in cost and process time for the batteries.
electrodes based on PVDF. This postpreparation treatment is, in fact, needed to increase the compactness, i.e., to reduce the porosity, of the electrodes to improve their performance inside the batteries. Electrodes based on CMC already display a high compactness that would allow for a reduction or even the elimination of this postpreparation treatment during the manufacturing process, with a consequent reduction in cost and process time for the batteries.
Figure 2. SEM images of  electrodes based on (a) CMC and (b) PVDF.
electrodes based on (a) CMC and (b) PVDF.
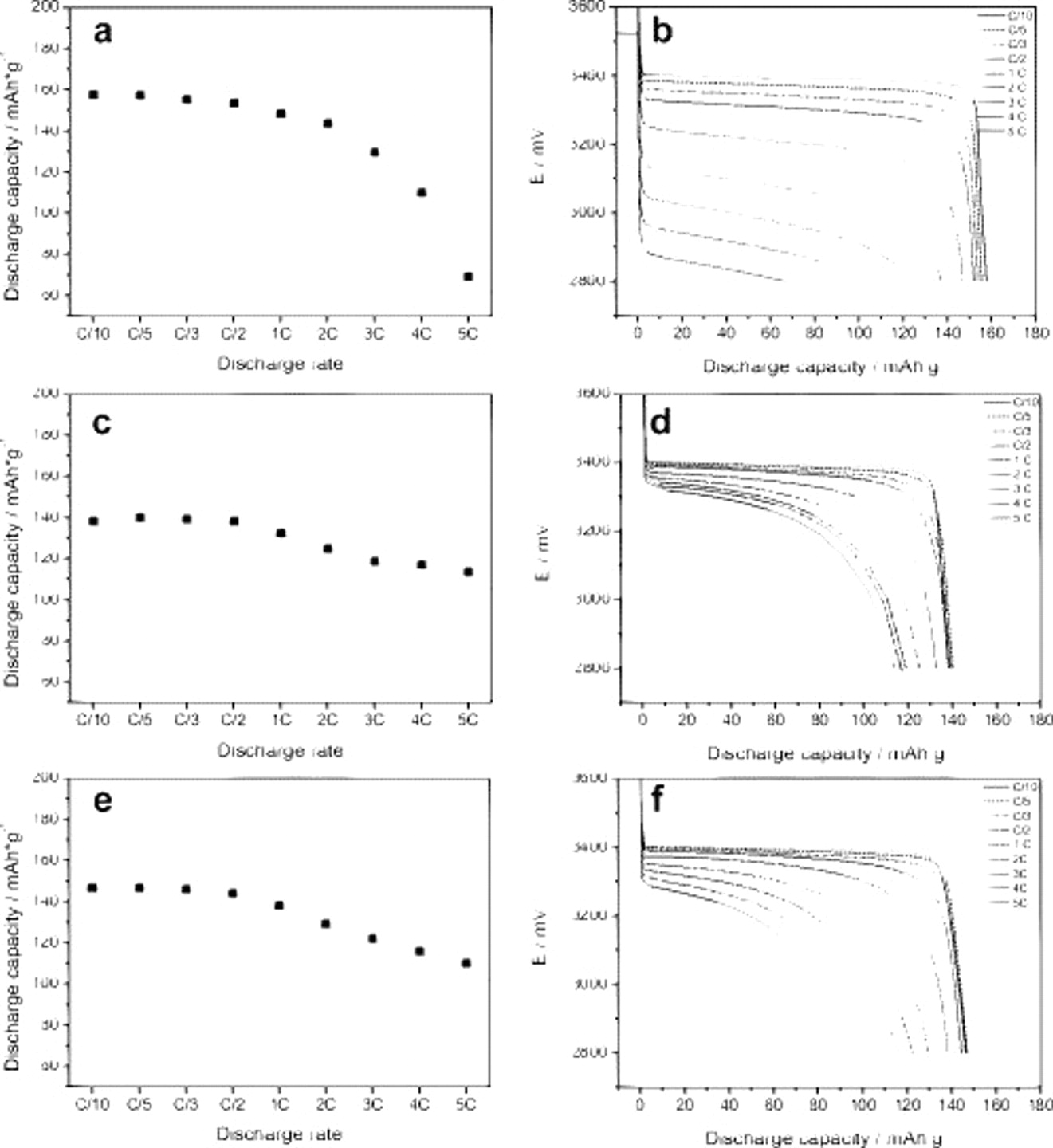
Figure 3 shows the rate performance and the discharge voltage profiles recorded during the tests performed at various currents ranging from C/10 (ca.  ) to 5C
) to 5C  . The tests were performed on PVDF-based (Fig. 3a and 3b) electrodes and CMC-based electrodes dried at
. The tests were performed on PVDF-based (Fig. 3a and 3b) electrodes and CMC-based electrodes dried at  (Fig. 3c and 3d) and
(Fig. 3c and 3d) and  (Fig. 3e and 3f). Any postpreparation treatment was applied for all tested electrodes. As shown in the figure (Fig. 3a, 3c and 3e), the discharge capacity is dependent upon the discharge rate for all the considered electrodes but especially for the PVDF-based electrode. In fact, although at low to medium rates (from C/10 to 2C) the electrode containing PVDF displays the higher values of specific capacity (between 140 and
(Fig. 3e and 3f). Any postpreparation treatment was applied for all tested electrodes. As shown in the figure (Fig. 3a, 3c and 3e), the discharge capacity is dependent upon the discharge rate for all the considered electrodes but especially for the PVDF-based electrode. In fact, although at low to medium rates (from C/10 to 2C) the electrode containing PVDF displays the higher values of specific capacity (between 140 and  ), at a high C-rate the higher discharge capacity is displayed by the electrodes containing CMC (between 120 and
), at a high C-rate the higher discharge capacity is displayed by the electrodes containing CMC (between 120 and  ). However, when the entire range of investigated discharge currents is considered, it is possible to assert that the electrodes containing CMC display overall better performance. In fact, the decrease in the discharge capacity detected on going from the lowest (C/10) to the highest (5C) discharge currents is only around 20% for the CMC-based electrodes vs a value of around 60% for the PVDF-based electrodes.
). However, when the entire range of investigated discharge currents is considered, it is possible to assert that the electrodes containing CMC display overall better performance. In fact, the decrease in the discharge capacity detected on going from the lowest (C/10) to the highest (5C) discharge currents is only around 20% for the CMC-based electrodes vs a value of around 60% for the PVDF-based electrodes.
Figure 3. Rate performance and the discharge voltage profiles of  electrodes using [(a) and (b)] PVDF binder and CMC binder dried at [(c) and (d)]
electrodes using [(a) and (b)] PVDF binder and CMC binder dried at [(c) and (d)]  and [(e) and (f)]
and [(e) and (f)]  . The tests were performed at room temperature with various discharge currents ranging from C/10 (ca.
. The tests were performed at room temperature with various discharge currents ranging from C/10 (ca.  ) to 5C
) to 5C  .
.
The difference in rate performance is easily understood by observing the shape of the voltage profiles corresponding to the different C-rates. As shown in Fig. 3b, 3d and 3f, at low discharge currents (from C/10 to C/2) all electrodes display the typical plateau of  cathodes around 3.4 V vs
cathodes around 3.4 V vs  . However, when the discharge current is increased, the voltage profile of the PVDF-based electrode changes dramatically with a very large increase in the ohmic drop. As a consequence, the delivered discharge capacity of this electrode is strongly reduced. For the CMC-based electrodes, the situation is rather different. In fact, the discharge current increase modifies the voltage profile in terms of shortening the voltage plateau rather than increasing the initial ohmic drop. As a result, the discharge capacity of these latter electrodes is not strongly affected like that of the PVDF-based electrode. A small plateau appears for a C-rate higher than 3C but, anyhow, does not seem to negatively affect the performance of the electrodes.
. However, when the discharge current is increased, the voltage profile of the PVDF-based electrode changes dramatically with a very large increase in the ohmic drop. As a consequence, the delivered discharge capacity of this electrode is strongly reduced. For the CMC-based electrodes, the situation is rather different. In fact, the discharge current increase modifies the voltage profile in terms of shortening the voltage plateau rather than increasing the initial ohmic drop. As a result, the discharge capacity of these latter electrodes is not strongly affected like that of the PVDF-based electrode. A small plateau appears for a C-rate higher than 3C but, anyhow, does not seem to negatively affect the performance of the electrodes.
To summarize, the results reported in Fig. 3 indicate that the capacity fading vs the rate increase is mostly due to the fast rising ohmic drop with increasing discharge current for the PVDF-based electrode, although it is mostly associated to the limited  cation diffusivity in the CMC-based composite electrodes.26
cation diffusivity in the CMC-based composite electrodes.26
Because all the electrodes considered in this work display comparable mass loadings, the different behavior observed for CMC- and PVDF-based electrodes is to be related with the electrode preparation procedure and, in particular, with the electrode morphology (see Fig. 2). The good low rate performance of the PVDF-based electrode is due to its high porosity (low density) that allows the electrolyte to wet every single active material particle in the electrode. However, the high porosity results in a low overall electronic conductivity of the composite electrode which, in turn, results in a very large ohmic drop at high rates. In fact, the roll pressing procedure is needed for  -based electrodes containing PVDF to improve their electrochemical performance. On the other hand, the behavior of the CMC-based electrodes tested indicates that the roll pressing step is not needed when this binder is used. These composite electrodes are rather compact and do not show low overall electronic conductivity. However, at a very low rate, they show a somehow low capacity most likely due to the presence of active material particles that are not wet by the electrolyte. Nevertheless, at high and very high rates, their capacity performance is rather good by being only limited by the lithium diffusion coefficient in the active material particles. For the above-mentioned results, it is possible to state that the use of CMC as a binder allows not only the use of aqueous slurries but also a simpler and less expensive postcoating treatment of the battery electrodes.
-based electrodes containing PVDF to improve their electrochemical performance. On the other hand, the behavior of the CMC-based electrodes tested indicates that the roll pressing step is not needed when this binder is used. These composite electrodes are rather compact and do not show low overall electronic conductivity. However, at a very low rate, they show a somehow low capacity most likely due to the presence of active material particles that are not wet by the electrolyte. Nevertheless, at high and very high rates, their capacity performance is rather good by being only limited by the lithium diffusion coefficient in the active material particles. For the above-mentioned results, it is possible to state that the use of CMC as a binder allows not only the use of aqueous slurries but also a simpler and less expensive postcoating treatment of the battery electrodes.
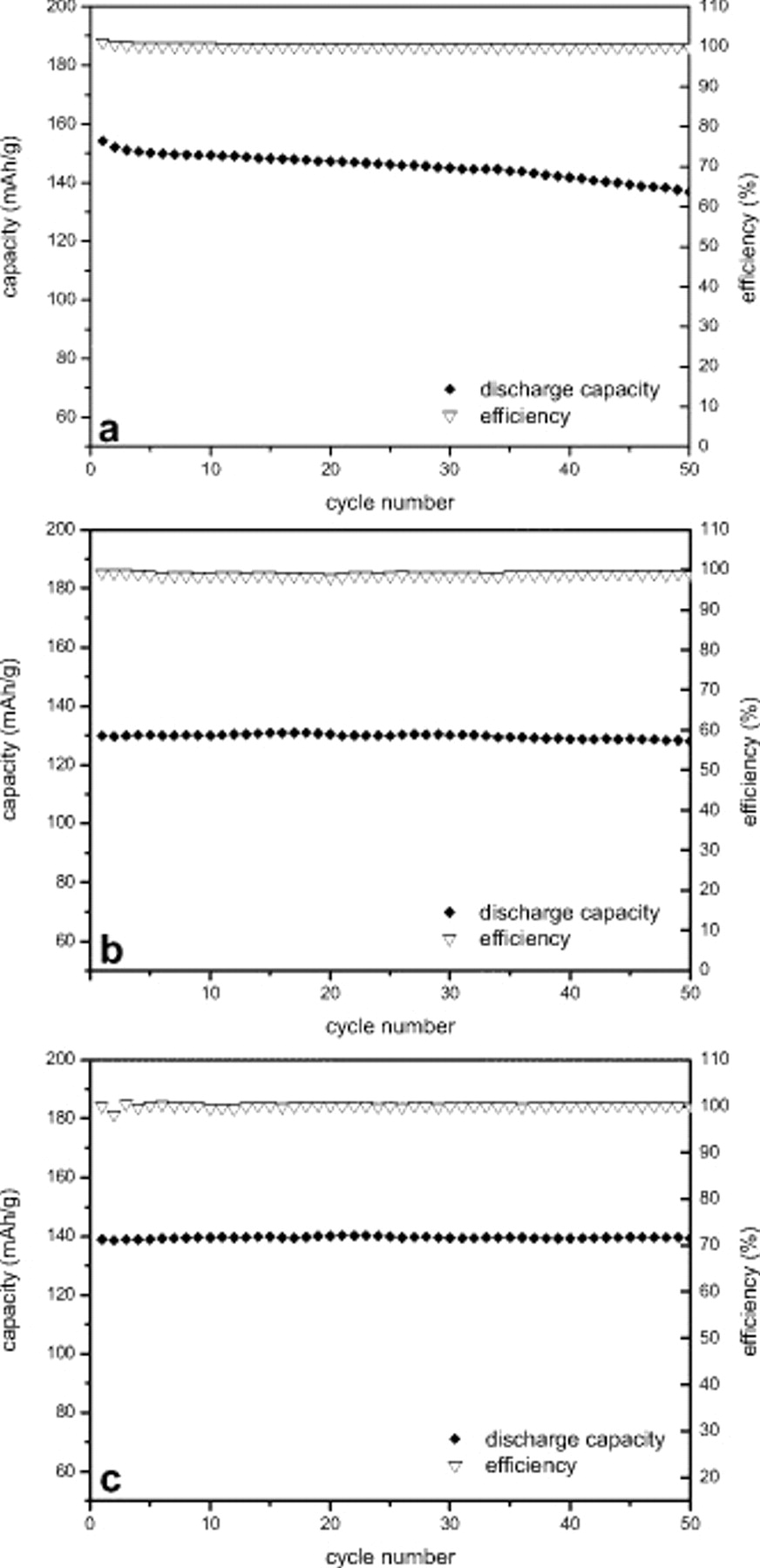
The different behavior of the electrodes containing CMC and those containing PVDF was also observed during the cycling tests. CCCV tests were carried with a current density corresponding to a 1C rate, the results of which are reported in Fig. 4. The PVDF-based electrode displayed an initial discharge capacity of  , which agrees well with the value obtained during the power rate test at the same discharge rate. However, after the 50th cycle, the delivered capacity decreased to
, which agrees well with the value obtained during the power rate test at the same discharge rate. However, after the 50th cycle, the delivered capacity decreased to  , thus corresponding to an average capacity fading of 0.2% per cycle, which is a rather high value in view of an application in a battery. The CMC-based electrodes dried at 120 and
, thus corresponding to an average capacity fading of 0.2% per cycle, which is a rather high value in view of an application in a battery. The CMC-based electrodes dried at 120 and  displayed a lower initial discharge capacity of ca. 130 and
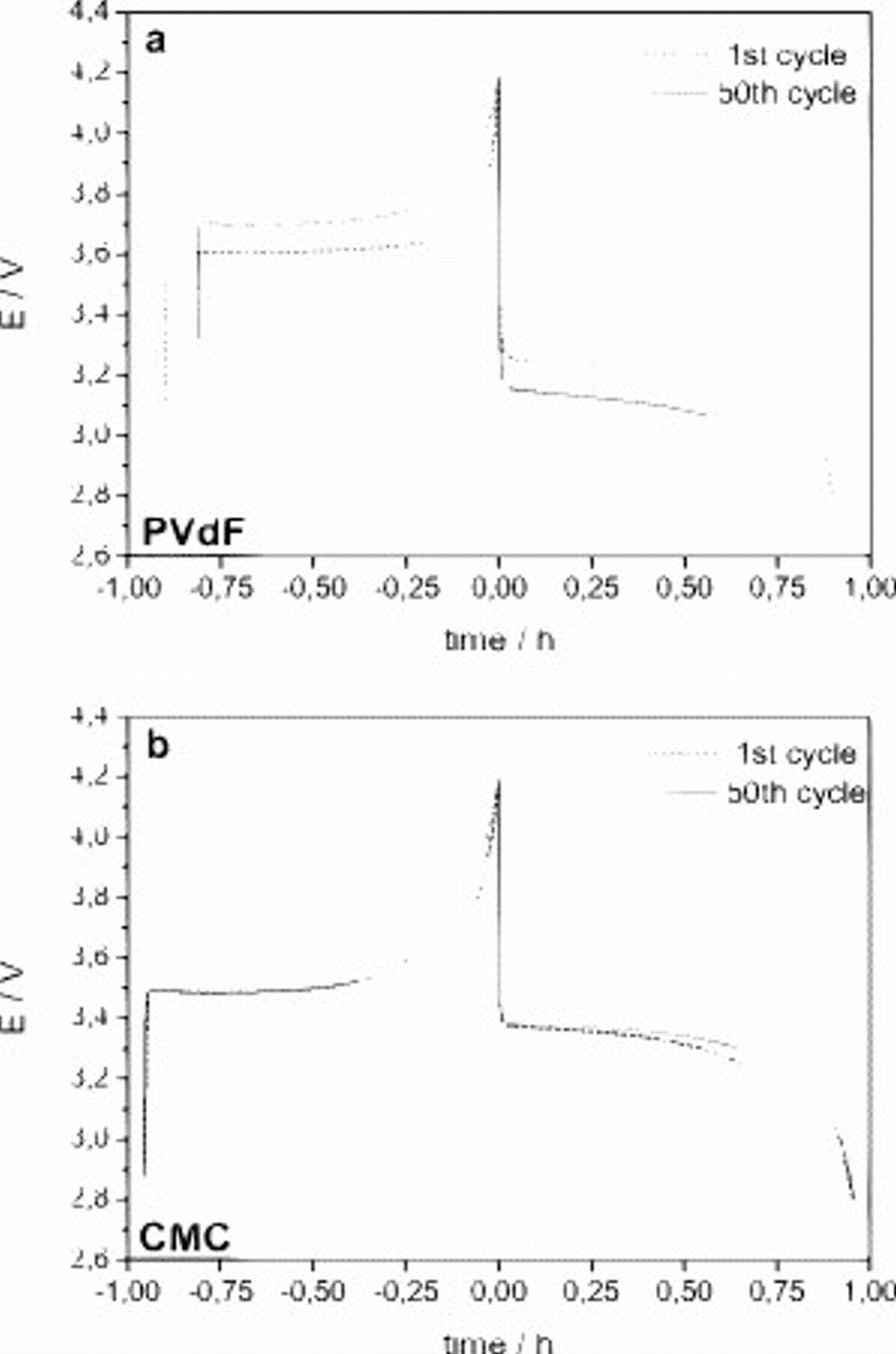
displayed a lower initial discharge capacity of ca. 130 and  , respectively, that remained unchanged. The differences in the cycle performance are well evidenced by the comparison of the voltage profiles during the 1st and the 50th cycles of the PVDF-based electrode and the CMC-based electrode dried at
, respectively, that remained unchanged. The differences in the cycle performance are well evidenced by the comparison of the voltage profiles during the 1st and the 50th cycles of the PVDF-based electrode and the CMC-based electrode dried at  (Fig. 5). In the PVDF-based electrode, the cooperative increase in the ohmic drop and the shortening of the voltage plateau during cycling resulted in a sharp decrease in the discharge capacity (Fig. 5a). On the contrary, for the CMC-based electrodes, the voltage profiles at the 1st and the 50th cycles are practically identical (Fig. 5b and 5c). These results clearly show that
(Fig. 5). In the PVDF-based electrode, the cooperative increase in the ohmic drop and the shortening of the voltage plateau during cycling resulted in a sharp decrease in the discharge capacity (Fig. 5a). On the contrary, for the CMC-based electrodes, the voltage profiles at the 1st and the 50th cycles are practically identical (Fig. 5b and 5c). These results clearly show that  electrodes containing CMC as a binder are able to display high specific capacity also at a high C-rate with a very good capacity retention. For that, they can certainly be considered as promising electrode materials. In particular, the electrode dried at
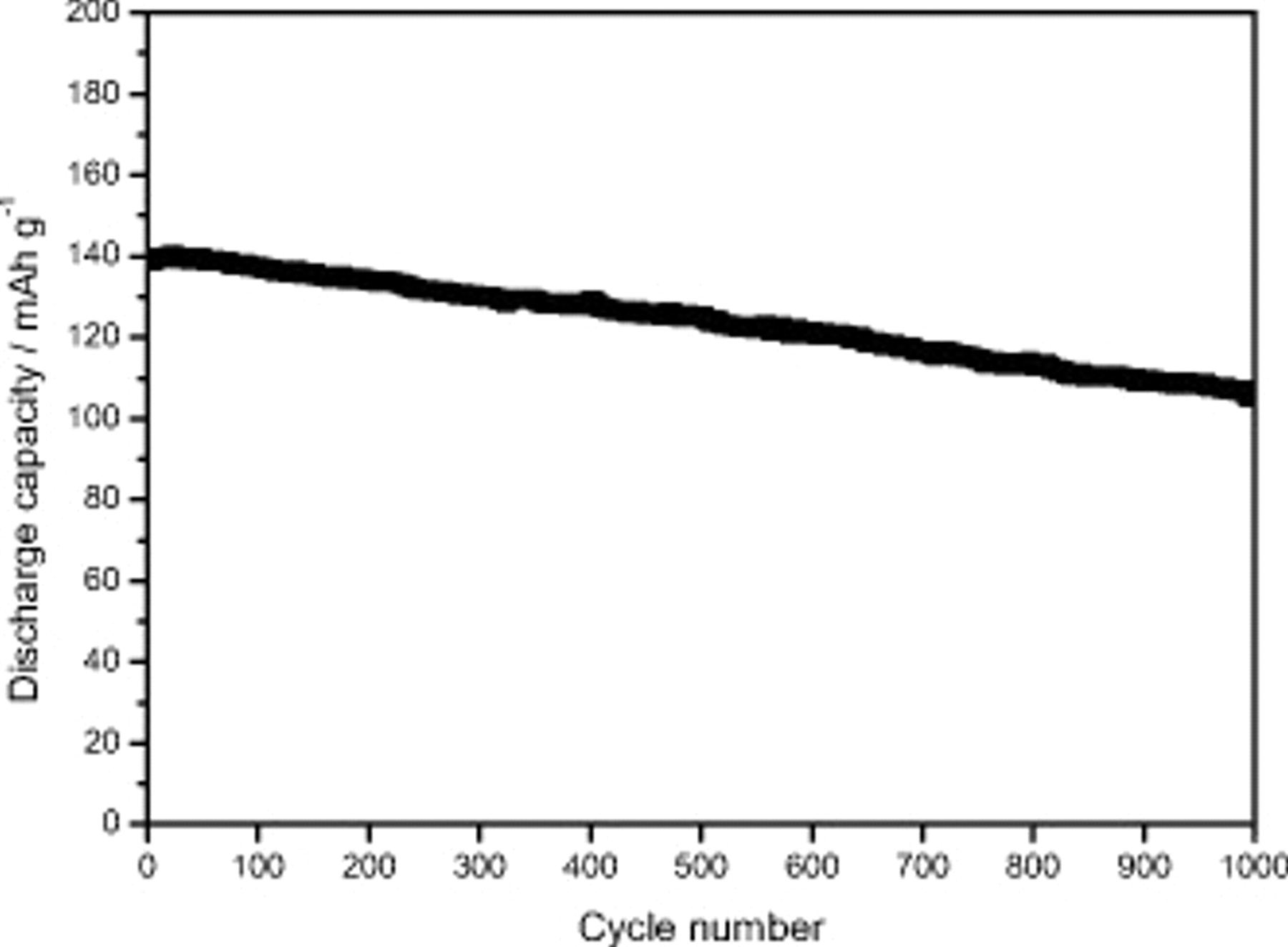
electrodes containing CMC as a binder are able to display high specific capacity also at a high C-rate with a very good capacity retention. For that, they can certainly be considered as promising electrode materials. In particular, the electrode dried at  showed an exceptional combination of performance with a very high capacity retention during the 50 cycle charge–discharge test at 1C and a discharge capacity only slightly affected by the discharge rate. For that, the long-term cycling stability of this CMC-based electrode was subjected to further investigation, the result of which is reported in Fig. 6. The performance shown in this test was certainly very impressive because the cell was able to complete more than 1000 full charge–discharge cycles at the 1C rate. The CMC-based electrodes were realized with a lab-scale coater without any postcoating processing in addition to simple drying in air and then in oven. The CMC-based electrodes displayed an initial capacity of
showed an exceptional combination of performance with a very high capacity retention during the 50 cycle charge–discharge test at 1C and a discharge capacity only slightly affected by the discharge rate. For that, the long-term cycling stability of this CMC-based electrode was subjected to further investigation, the result of which is reported in Fig. 6. The performance shown in this test was certainly very impressive because the cell was able to complete more than 1000 full charge–discharge cycles at the 1C rate. The CMC-based electrodes were realized with a lab-scale coater without any postcoating processing in addition to simple drying in air and then in oven. The CMC-based electrodes displayed an initial capacity of  that decreased to
that decreased to  after 1000 cycles. This corresponded with a cycling retention of 75% and an average capacity fading of only 0.025% per cycle. This value can certainly be considered very high and, to the best of our knowledge, has never been shown for
after 1000 cycles. This corresponded with a cycling retention of 75% and an average capacity fading of only 0.025% per cycle. This value can certainly be considered very high and, to the best of our knowledge, has never been shown for  electrodes based on CMC as a binder. The best PVDF-based electrodes display a higher capacity retention, but these electrodes are always subject to postpreparation treatments. Taking into account the performance, the preparation, and the characteristics of the investigated CMC-based electrodes, it is possible to assume that a further optimization of the electrode preparation and the use of postpreparation treatment could allow the CMC-based electrode to reach a performance comparable with that of the best PVDF electrode.
electrodes based on CMC as a binder. The best PVDF-based electrodes display a higher capacity retention, but these electrodes are always subject to postpreparation treatments. Taking into account the performance, the preparation, and the characteristics of the investigated CMC-based electrodes, it is possible to assume that a further optimization of the electrode preparation and the use of postpreparation treatment could allow the CMC-based electrode to reach a performance comparable with that of the best PVDF electrode.
Figure 4. Cycling performance of  electrodes using (a) PVDF binder and CMC binder dried at (b) 120 and (c)
electrodes using (a) PVDF binder and CMC binder dried at (b) 120 and (c)  . The discharge capacity and the charge/discharge efficiency were recorded at room temperature and 1C rate.
. The discharge capacity and the charge/discharge efficiency were recorded at room temperature and 1C rate.
Figure 5. Voltage profiles of the 1st and 50th cycles of  electrodes using (a) PVDF binder and (b) CMC binder dried at
electrodes using (a) PVDF binder and (b) CMC binder dried at  . The tests were performed at room temperature and 1C rate.
. The tests were performed at room temperature and 1C rate.
Figure 6. Long-term cycle performance of a CMC-based  electrode dried at
electrode dried at  . The tests were performed at room temperature and 1C rate.
. The tests were performed at room temperature and 1C rate.
Considering these results, the use of CMC as a binder for  -based electrodes certainly appears as a promising contribution to the realization of greener and cost-effective lithium-ion batteries. The introduction of CMC would allow the processing of the electrodes in aqueous slurries with a significant reduction or even without the need of postprocessing (roll-pressing). Moreover, batteries employing CMC-based electrodes would offer a much easier end-of-life disposability because of the absence of fluorine in the binder. In addition to the greener characteristics in terms of material, processing, and disposability–recyclability, the CMC binder would also result in a lower cost for the battery.
-based electrodes certainly appears as a promising contribution to the realization of greener and cost-effective lithium-ion batteries. The introduction of CMC would allow the processing of the electrodes in aqueous slurries with a significant reduction or even without the need of postprocessing (roll-pressing). Moreover, batteries employing CMC-based electrodes would offer a much easier end-of-life disposability because of the absence of fluorine in the binder. In addition to the greener characteristics in terms of material, processing, and disposability–recyclability, the CMC binder would also result in a lower cost for the battery.
Work is now in progress to further investigate the characteristics and performance of the CMC-based electrodes. The influence of the operating temperature on the electrode performance as well as the use of a different electrolyte, e.g., ionic liquids, is also under investigation.
Conclusion
The introduction of CMC as a binder for  -based electrodes appears as a viable and extremely promising solution to improve the overall electrode preparation and, in general, for the development of greener, cheaper, and safer batteries.
-based electrodes appears as a viable and extremely promising solution to improve the overall electrode preparation and, in general, for the development of greener, cheaper, and safer batteries.
First of all, the introduction of CMC as a binder allows the use of aqueous slurries. Even if carbon-coated  is known to suffer from humidity, a short processing time in aqueous slurries followed by an appropriate drying procedure prevents its structural modification. On the other hand, the use of aqueous slurries represents a great advantage for the electrode preparation because it avoids the use of volatile organic compounds.
is known to suffer from humidity, a short processing time in aqueous slurries followed by an appropriate drying procedure prevents its structural modification. On the other hand, the use of aqueous slurries represents a great advantage for the electrode preparation because it avoids the use of volatile organic compounds.
Moreover, the use of CMC allows the preparation of an electrode with higher compactness with respect to the electrode containing PVDF. This would allow for a significant reduction or even the elimination of the postcoating treatment (roll-pressing) during the manufacture of the battery electrodes, with a consequent benefit in terms of costs and production time. This cost reduction is amplified when considering that CMC is a much cheaper material with very easy disposability at the end of the battery life, and no particular care needs to be taken during the coated electrode drying step.
Finally, we showed that all these advantages in terms of electrode preparation are not negatively counterbalanced by the performance of the electrodes. As reported, CMC-based electrodes dried at  are able to display an extremely high performance for 1000 cycles. As a matter of fact, the initial capacity of
are able to display an extremely high performance for 1000 cycles. As a matter of fact, the initial capacity of  was seen to only slightly decrease to
was seen to only slightly decrease to  after 1000 cycles. This corresponded to a capacity cycling retention of 75% and an overall capacity fading of only 0.025% per cycle. This value can certainly be considered very high and, to the best of our knowledge, has been never shown for LFP electrodes based on CMC as a binder. All the results shown in this paper indicate that CMC-based LFP electrodes for Li-ion batteries are worthy of further investigation for industrial scale-up.
after 1000 cycles. This corresponded to a capacity cycling retention of 75% and an overall capacity fading of only 0.025% per cycle. This value can certainly be considered very high and, to the best of our knowledge, has been never shown for LFP electrodes based on CMC as a binder. All the results shown in this paper indicate that CMC-based LFP electrodes for Li-ion batteries are worthy of further investigation for industrial scale-up.
Acknowledgments
The authors thank the European Commission within the FP6 STREP Projects ILLIBATT (contract no. NMP3-CT-2006-033181) for the financial support.
Westfälische Wilhelms University of Münster assisted in meeting the publication costs of this article.