Abstract
A radiation grafting graphene oxide reinforced polybenzimidazole (PBI-RGO) was fabricated and then crosslinked with a self-made crosslinker containing benzimidazole groups by a facile method to obtain crosslinked PBI/RGO (cPBI/RGO) membrane, which was further doped with phosphoric acid (PA) for high temperature proton exchange membrane (HT-PEM) applications. The introduction of benzimidazole groups in the crosslinker results in an increase in PA uptake from 222% to 303% and a reduction of tensile strength of the membrane. The crosslinking structure can effectively improve the tensile strength compared with uncrosslinked membrane at the same PA uptake. The crosslinked membrane shows higher remaining weight after immersion in Fenton's solution for 6 days and PA for 8 days than uncrosslinked membrane, indicating that it has better oxidative and chemical stability. The introduction of RGO and the crosslinking structure can also improve the proton conductivity of membrane. The proton conductivity of PA-doped cPBI/RGO membrane is 58.6 mS·cm−1 at 170°C without humidity, which is increased by 2.6 times compared with that of PA-doped PBI membrane. The crosslinking structure allows the membrane to have better stability and higher proton conductivity, thus making it suitable for HT-PEM applications.
Export citation and abstract BibTeX RIS
Phosphoric acid (PA) doped polybenzimidazole (PBI) membrane is particularly appealing for high temperature proton exchange membrane (HT-PEM) applications at temperatures up to 100°C and low relative humidity1,2 because of the high proton conductivity of PA at high temperatures3,4 and the excellent properties of PBI such as good chemical stability and mechanical strength.5,6 However, some problems need to be solved to further expand its applications, such as deterioration of mechanical properties and acid leaching. This can be achieved by adding inorganic particles7 and crosslinking.8 Inorganic particles containing hydroxyl groups on their surface can absorb PA via hydrogen bonds, resulting in an increase of proton conductivity by the "hopping mechanism".9 The high strength and thermal stability of inorganic particles can also contribute to improving the mechanical properties and thermal stability of PBI membranes.10 Commonly used inorganic particles include hygroscopic oxides (e.g., SiO2, TiO2, ZrO2, and SnO2),7,11–18 inorganic solid acids and salts (e.g., heteropolyacids, clays, phosphates, and perovskites)9,19–25 and carbon-based materials (e.g., carbon nano tube and graphene oxide (GO)).26–31 In particular, GO with a single atomic layer and oxygen-containing groups on the surface can significantly improve the mechanical properties and proton conductivity of composite membranes.32 We have previously prepared sulfonated GO via radiation grafting (denoted as RGO) and found that even 1 wt% RGO could improve the mechanical properties and proton conductivity.27 The crosslinking network structure resulting from the reaction between the -NH- groups in PBI chains and bifunctional or multifunctional halohydrocarbons33 or epoxides34 in the crosslinker can also improve the performance of composite membranes, especially the mechanical properties.35 Different crosslinkers were developed for the preparation of crosslinked PBI (cPBI),36–40 and PA-doped cPBI membranes showed better mechanical properties and proton conductivity than PA-doped linear polymer membranes. However, no attempt has yet been made to incorporate inorganic particles into the crosslinked polymer matrix for the preparation of composite membranes for HT-PEM applications.
In this study, a new crosslinker containing imidazole rings was synthesized and then dissolved in dimethyl formamide (DMF). RGO was introduced into PBI to prepare PBI/RGO composite membrane, which was then immersed in the crosslinker solution to obtain crosslinked PBI/RGO (cPBI/RGO) membrane. Compared with the traditional crosslinking method in which the crosslinker and PBI are simultaneously dissolved in DMF and crosslinking reaction occurs during the evaporation of solvent, our method appears to be more facile. Then, the cPBI/RGO membrane was further doped with PA for HT-PEM applications. The effects of the crosslinking structure with additional imidazole rings on PA uptake and retention, oxidative and chemical stability of cPBI/RGO membrane, and mechanical properties and proton conductivity of PA-doped cPBI/RGO membrane were investigated. The results show that PA-doped cPBI/RGO membrane prepared in this study has high strength, stability and proton conductivity.
Experimental
Materials
PBI containing ether bonds was synthesized by polycondensation of 3,3'-diaminobenzidine (DAB, Chemical Technology Co., Ltd., Shanghai, China) and 4,4'- dicarboxydiphenyl ether (DCDPE, Aladdin Industrial Co., Ltd., Shanghai, China) in methanesulfonic acid (MSA, Adamas Reagent Co., Ltd., Shanghai, China) at 140°C for 2 h.41 RGO with sulfonated groups (IEC: 2.8 meq g−1) was prepared by grafting reaction induced by 80 kGy of 60Co γ-ray irradiation using purified acrylic acid (AA, Lingfeng Chemical Reagent Co., Ltd., Shanghai, China) and sodium p-styrenesulfonate (SSS, Aladdin Industrial Co., Ltd., Shanghai, China) as co-monomers and copper acetate (CuAc2, Lingfeng Chemical Reagent Co., Ltd., Shanghai, China) as an inhibitor.27 Methyl 4-bromomethylbenzoate (MBMB, Adamas Reagent Co., Ltd., Shanghai, China) and phosphorus pentoxide (P2O5, Adamas Reagent Co., Ltd., Shanghai, China) were dried at 80°C under vacuum prior to use. Polyphosphoric acid (PPA, Lingfeng Chemical Reagent Co., Ltd., Shanghai, China) and DMF (Lingfeng Chemical Reagent Co., Ltd., Shanghai, China) were used as solvents without further purification. All other reagents were used as received.
Preparation of crosslinker solution
Crosslinker was synthesized in a three-necked round-bottom flask with a mechanical stirrer under nitrogen atmosphere. Specifically, 2.14 g (0.01 mol) of DAB, 16.36 g of P2O5 and 48.80 g of PPA were stirred at 150°C for at least 10 h to obtain a homogeneous mixture, and then 2.29 g (0.01 mol) of MBMB was added into the flask quickly and stirred for 1 h. During this process, the color of the mixture changed from yellow to green and the viscosity increased. After cooling to about 100°C, deionized water was added dropwise to the mixture to precipitate the product. Finally, the solid precipitate was collected by filtration, washed with 5 wt% of sodium hydroxide solution and water until pH = 7, and dried to obtain a yellow product (yield: 2.29 g). The completely dried crosslinker was dissolved in DMF and filtered, yielding a clear crosslinker solution.
Preparation of crosslinked membrane
PBI-RGO composite membrane was prepared by the solution-casting method. RGO powder was added into DMF in an ultrasonic bath for 4 h to obtain a homogeneously dispersed solution, which was then mixed with 3 wt% of PBI solution under stirring. The RGO content was 1 wt% relative to PBI. Then, the PBI-RGO (DMF) solution was cast onto a clean glass plate at 80°C for 12 h, and the obtained membrane was immersed in the crosslinker solution at 80°C for 3 h. Finally, the cPBI/RGO membrane was washed several times with water to remove residual solvent, and then dried overnight at 80°C under vacuum. Pure PBI and cPBI membranes prepared by the same procedure served as the control. The thickness of all membranes was around 50–60 μm before crosslinking and 60–70 μm after crosslinking, respectively.
Characterization and measurements
The representative functional groups in crosslinker were analyzed using a Nicolet 6700 Fourier transform infrared (FT-IR) spectrometer in the range of 400–4000 cm−1 at a normal resolution of 4 cm−1. The solubility of crosslinked and uncrosslinked membranes was evaluated by immersion in DMF for 24 h at 80°C.
All membranes were immersed in an 85 wt% PA aqueous solution at 80°C or 120°C for 48 h, wiped repeatedly with filter paper, and dried at 120°C to obtain PA-doped membranes, which were weighed and denoted as WPA. Subsequently, they were washed thoroughly with 5 wt% of sodium hydroxide aqueous solution and water, and dried to a constant weight in a vacuum oven at 120°C to obtain dry membranes, which were then weighed and denoted as WD. The PA uptake was calculated from the following equation:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/165/11/F914/revision1/d0001.gif)
The tensile properties of PA-doped membranes were measured by an MTS E43 universal testing machine at room temperature. All samples were cut into small dumbbell-shaped pieces with a width of 4 mm and measured independently at a rate of 10 mm·min−1 for at least five times.
The chemical and oxidative stabilities of membranes were measured by the remaining weight of membranes after immersion in PA at 80°C and Fenton's solution (3 wt% H2O2 and 4 ppm Fe2+) at 68°C for several days.42 The membranes were washed and then dried to a constant weight at 120°C. The remaining weight was calculated by the following equation:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/165/11/F914/revision1/d0002.gif)
where W0 and W1 are the net weight of initial and final membranes, respectively.
Acid retention test was performed by immersing PA-doped membranes in distilled water for about 6 h and recording the weight of dry membranes every 1 h (the membranes were dried in an oven at 80°C and weighted). Then, the acid retention rate was calculated by the following equation:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/165/11/F914/revision1/d0003.gif)
where W0 and WPA are the initial weight of the PA-doped membranes and PA in the membranes, and Wi is the weight of the dry membranes after leaching for i hours.
The proton conductivity of membranes was measured by four-point probe method by the AC impedance spectroscopy with a PARSTAT 2273 electrochemical analyzer (AMETEK, Inc., America) at a frequency of 106 to 103 Hz at 100–170°C under anhydrous conditions. The in-plane proton conductivity (σ, S·cm−1) was determined by σ = L/RS, where L is the interval between the two electrodes, R is the resistance of the membrane obtained from the electrochemical impedance spectroscopy, and S is the sectional area of the membrane. In addition, water absorbing compounds such as anhydrous calcium chloride and calcium oxide were added to absorb water around the sample. The test would begin when the humidity tester showed that the RH (relative humidity) was nearly 0%.
Results and Discussion
Crosslinker preparation
Benzimidazole-containing crosslinker (I) was synthesized by direct benzimidazolization in PPA, as shown in Scheme 1. The obtained crosslinker can react with itself, resulting in the formation of multimer such as compound II and III. Thus, the product ought to be a kind of mixture, and every component has the crosslinking capability due to the presence of at least two bromomethyl groups.

Scheme 1. Synthesis of the crosslinker with benzimidazole groups.
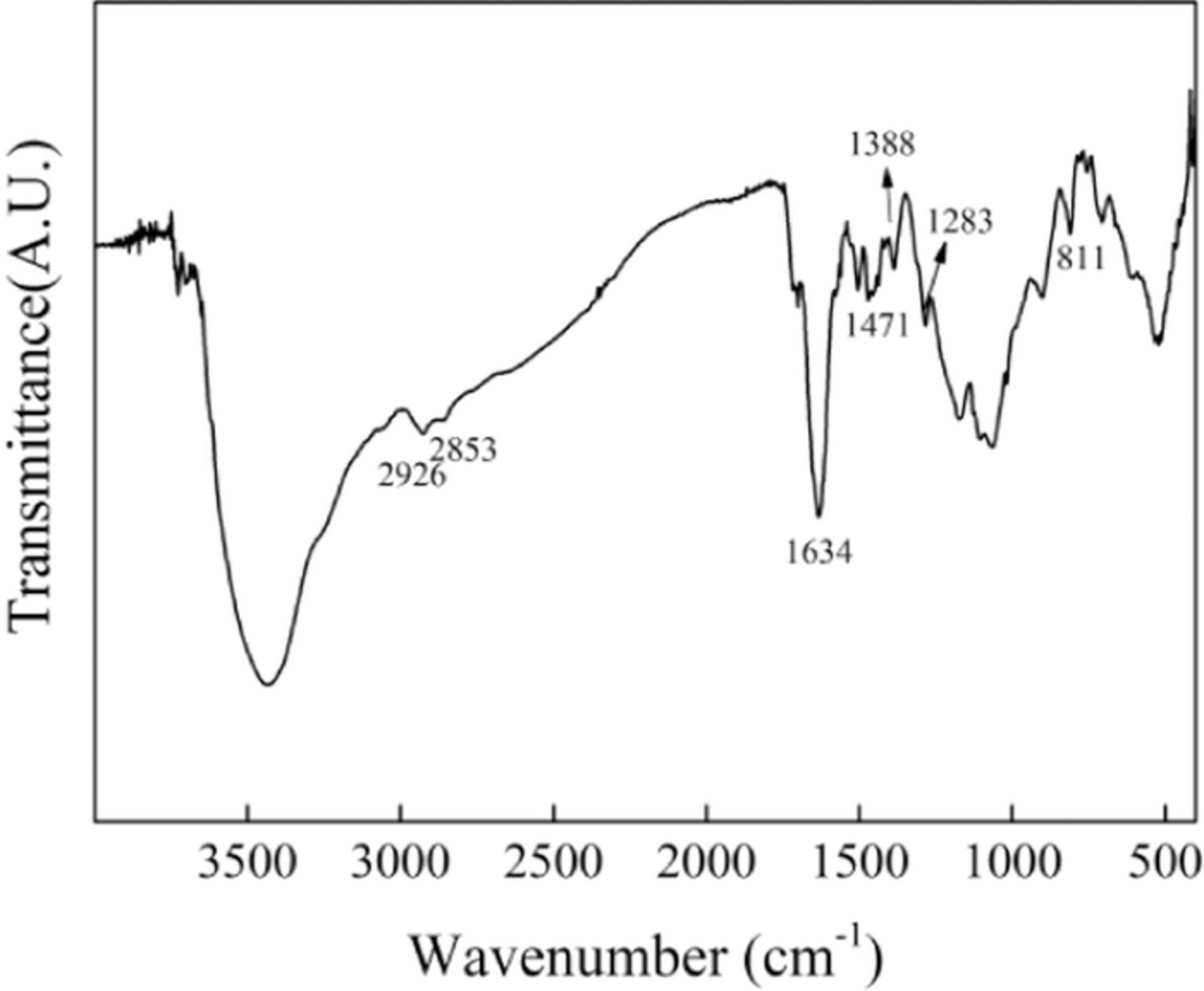
The FT-IR spectrum of the crosslinker is shown in Fig. 1. The peaks at 1388 and 1634 cm−1 can be assigned to the vibration of C-N and C=N bonds, respectively;43 while those at 811, 1283 and 1471 cm−1 can be attributed to the heterocyclic-ring vibration, imidazole ring breathing and in-plane ring vibration of 2,5-disubstituted benzimidazole, respectively,44 indicating the presence of benzimidazole groups. Accordingly, the peaks at 2926 and 2853 cm−1 are attributed to the existence of methylene structure. Therefore, the crosslinker contains benzimidazole and methylene structure. However, the vibration of C-Br is too weak to be easily observed in the FT-IR spectrum and then needs to be further proved by another method.
Figure 1. FT-IR spectrum of the crosslinker.
Solubility
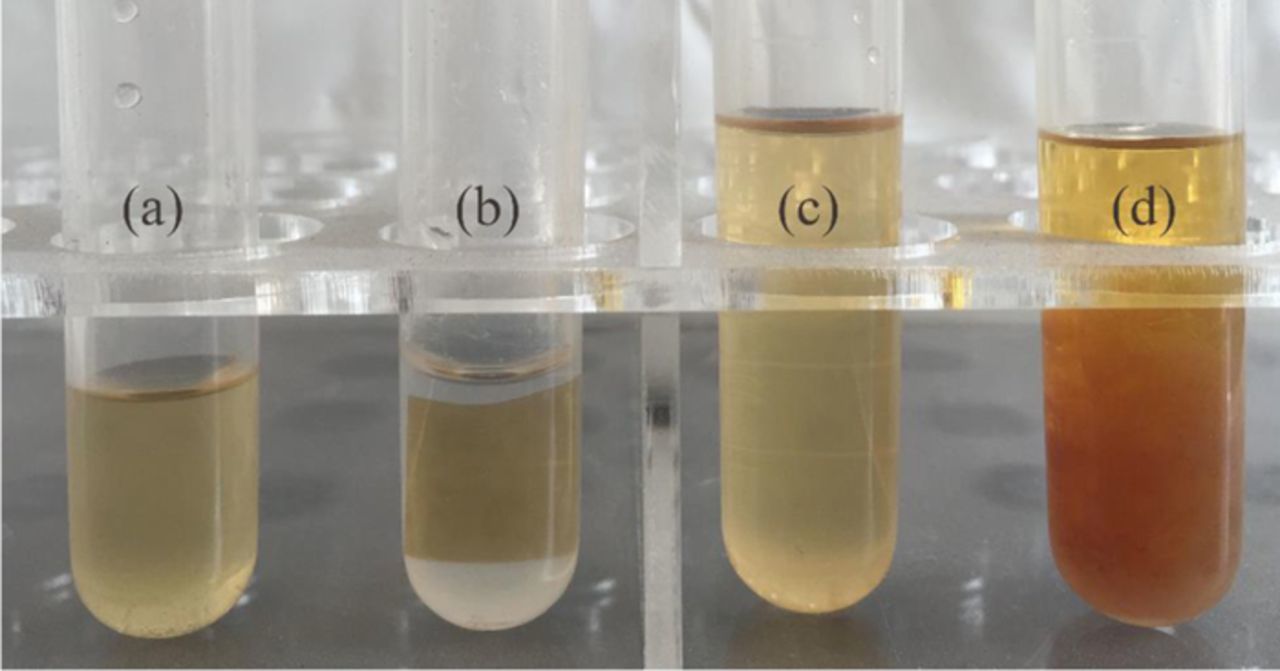
The solubility of the membranes was investigated to determine the formation of crosslinking network in the membranes. Figs. 2a and 2b show the solubility of pure PBI and cPBI membranes. After immersion in DMF at 80°C for 24 h, pure PBI membrane is completely dissolved, while cPBI membrane still keeps the original shape without dissolution in DMF as indicated by colorless and transparent solution.
Figure 2. The solubility of pure PBI (a) and cPBI (b) membrane in DMF and PBI solution in 4,4'-dibromomethyl diphenyl ether DMF solution (c) and self-made crosslinker DMF solution (d).
Several drops of 3 wt% PBI solution were added into two crosslinker DMF solutions as shown in Figs. 2c and 2d. It is clearly observed that the 4,4'-dibromomethyl diphenyl ether (synthesized in a previous work44) solution is still clear, but precipitate is clearly observed in the self-made crosslinker solution, indicating that the traditional evaporation method for the preparation of crosslinked membranes is not appropriate for crosslinking PBI membranes in this study. Thus, the facile immersing method was adopted.
PA uptake and retention
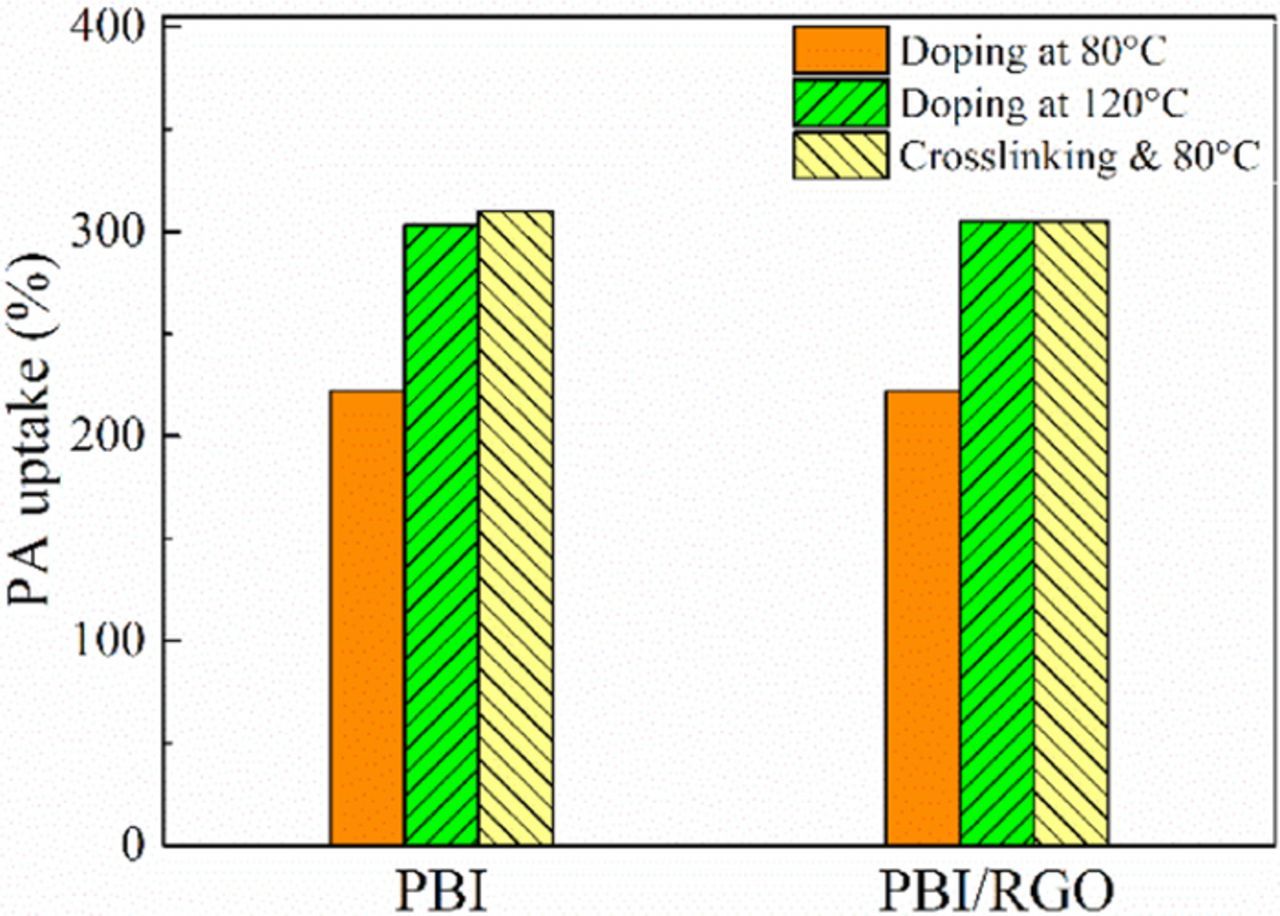
It is essential for PA-doped membranes to have high PA uptake and low leaching because of the need of sufficiently high proton conductivity and stability during operation. The PA uptake of PBI, PBI/RGO, cPBI and cPBI/RGO membranes at 80°C and that of PBI and PBI/RGO membranes at 120°C are shown in Fig. 3. As the temperature increases from 80°C to 120°C, the PA uptake of PBI membrane is increased by about 36.4% from 222% to 303% (i.e. the PA doping level (PADL) is increased from 9.1 to 12.4), indicating that increasing temperature can effectively promote the absorption of PA. Interestingly, the PA uptake of cPBI membranes doped at 80°C is similar to that of PBI membrane doped at 120°C, but much higher than that of PBI membrane doped at 80°C. This may be related to the special structure of the crosslinker, which can endow cPBI with imidazole groups in the side chain and thus increase the active sites of the polymer chain to improve the PA uptake. In addition, it is easily observed that the PA uptake of membrane keeps steady after adding RGO into PBI or cPBI, which is similar to our previous work.27
Figure 3. PA uptake of PBI, PBI/RGO, cPBI and cPBI/RGO membranes at 80°C and that of PBI and PBI/RGO membranes at 120°C.
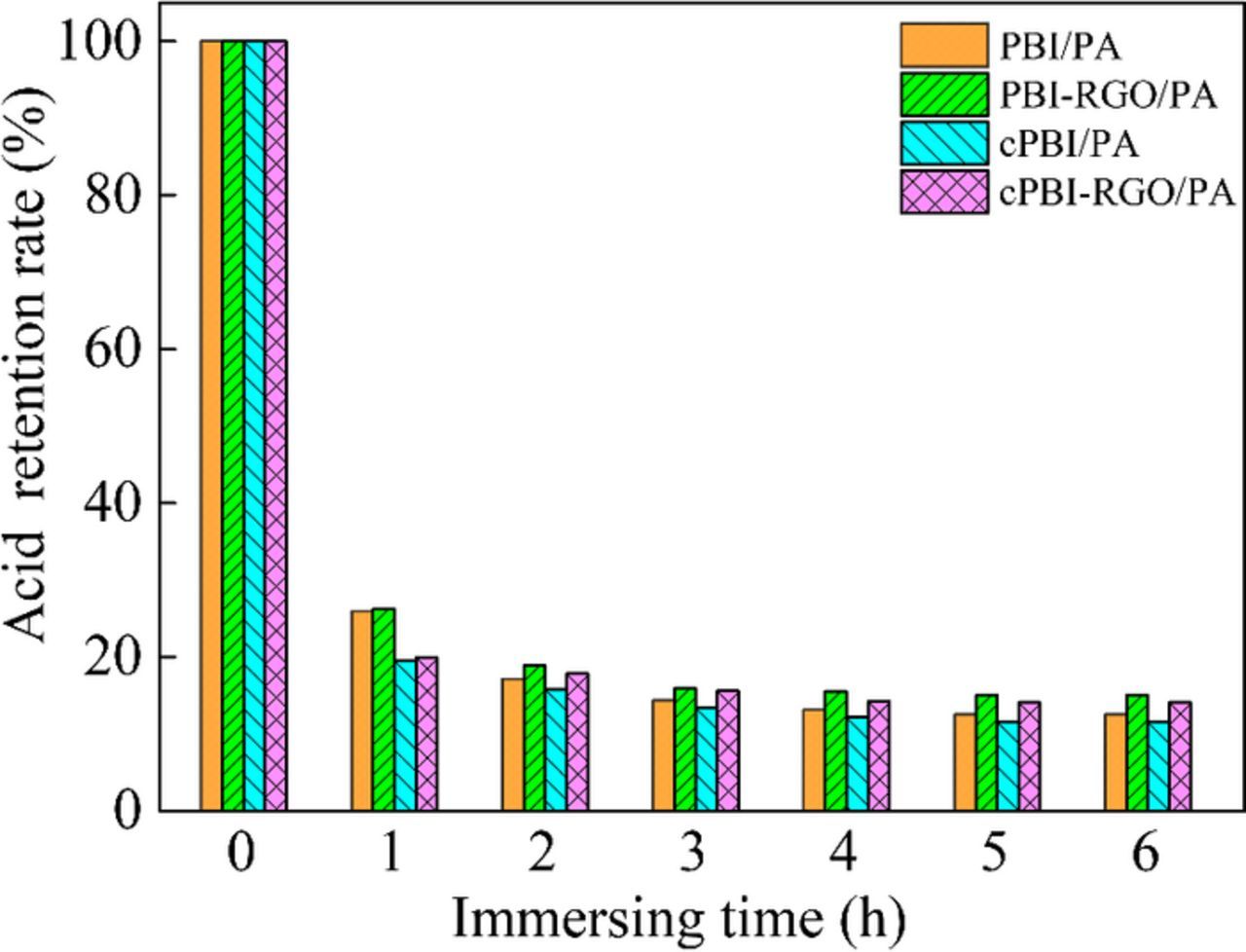
The PA loss during the operation of fuel cells will inevitably lead to deterioration of membrane performance, which can be overcome by adding inorganic particles.2 The time dependence of acid retention rate is shown in Fig. 4. The addition of RGO into the membranes results in an increase in acid retention rate due to hydrogen bonding interactions between the oxygen-containing groups and PA molecules as well as the inhibition of the layer structure. The acid retention rate of crosslinked membranes is lower than that of uncrosslinked ones before immersion in water, as the high PA uptake makes it easier for PA loss. However, after immersion in water for 3 h, the acid retention rate of cPBI/RGO membrane is maintained at a steady level of about 14%, while that of PBI/RGO membrane is about 15%, indicating similar bonded PA content before and after crosslinking.
Figure 4. The time dependence of acid retention rate of PA-doped PBI, cPBI, PBI/RGO and cPBI/RGO membranes.
In general, each -C=N- bond in the imidazole group can bond with one PA molecule by ion bond and several PA molecules via hydrogen bond. When immersed in water, the membrane will easily lose the free PA molecules and the molecules bonded by hydrogen bond and leave one PA molecule bonded by ion bond, and thus each imidazole group is bonded with at most one PA molecule in theory after acid retention test. The content of bonded PA molecules of each imidazole group in PBI and cPBI is 0.57 (9.1 × 0.125/2) and 0.71 (12.4 × 0.115/2), respectively, both of which are lower than the theoretical value (i.e. 1). There may be two reasons. First, one PA molecule can react with two imidazole groups due to the similar acidity of H2PO4− and imidazolium. Second, the interaction between different chains in the polymer and steric hindrances among PA molecules can enable only some imidazole groups to have reactive activity and be bonded with PA via ion bond. Moreover, the bonded PA molecules of each imidazole group in cPBI are more than that in PBI, indicating the presence of more active imidazole groups in cPBI membrane, which is in agreement with PA uptake results.
Oxidative and chemical stability
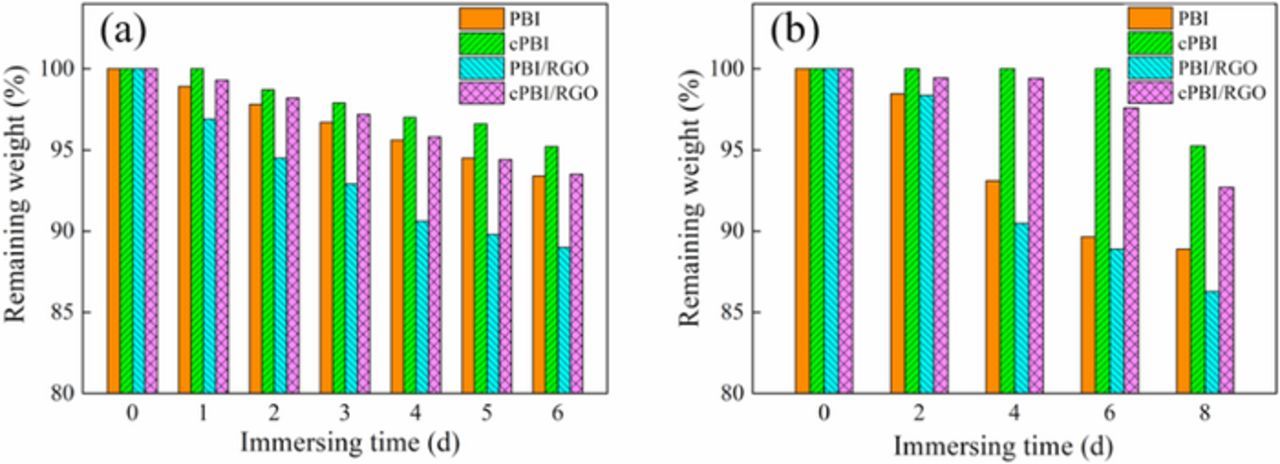
The stability of membranes has a significant impact on their service life. In this work, the resistance of membranes to oxygen free radicals and PA was evaluated, as shown in Figs. 5a and 5b. Fig. 5a shows that the remaining weight of PBI and cPBI membranes immersed in Fenton's solution for 6 days is 93.4% and 95.2%, respectively, indicating an improvement of oxidative stability. This is because the compact network structure formed by crosslinking can effectively prevent free radicals from diffusing into polymer chains, and more chain scission events are needed to break down the molecular weight. The addition of RGO results in a decrease in the residual weight of membranes due to the oxidative degradation of oxygen-containing groups, such as ether bonds, ketone and sulfoxide groups in RGO.45 Assuming that RGO can be completely decomposed, the theoretical weight loss of PBI/RGO membrane should be 1.0 wt% lower than that of pure PBI membrane. The observed difference between theoretical and actual weight loss (4.3 wt%) is likely due to the increase of the contact area between the polymer matrix and the Fenton's solution after the decomposition of oxygen-containing groups, leading to further decomposition of the matrix. In addition, free radicals may be produced in the decomposition process of some organic groups in RGO, which can accelerate the degradation of PBI.
Figure 5. The time dependence of the remaining weight ratio of PBI, cPBI, PBI/RGO and cPBI/RGO membranes in Fenton's reagent (a) and PA at 80°C (b).
The synthesized PBI has a wide molecular weight distribution, and thus the linear PBI with a low molecular weight may be dissolved in PA, resulting in 88.9% of remaining weight for PBI membrane after immersion at 80°C for 8 days, as shown in Fig. 5b. However, the three-dimensional network structure can be formed after crosslinking, resulting in as high as 95.2% of remaining weight for cPBI membrane. The RGO added to the matrix can be dispersed along with the decomposition of the matrix and leave some gaps, resulting in an increase in the contact area between the matrix and PA, and a reduction in the chemical stability. In general, the cPBI/RGO membrane shows a remaining weight of 92.7% after immersion in PA for 8 days and 93.5% after immersion in Fenton's solution for 6 days, indicating excellent chemical and oxidative stability.
Mechanical properties
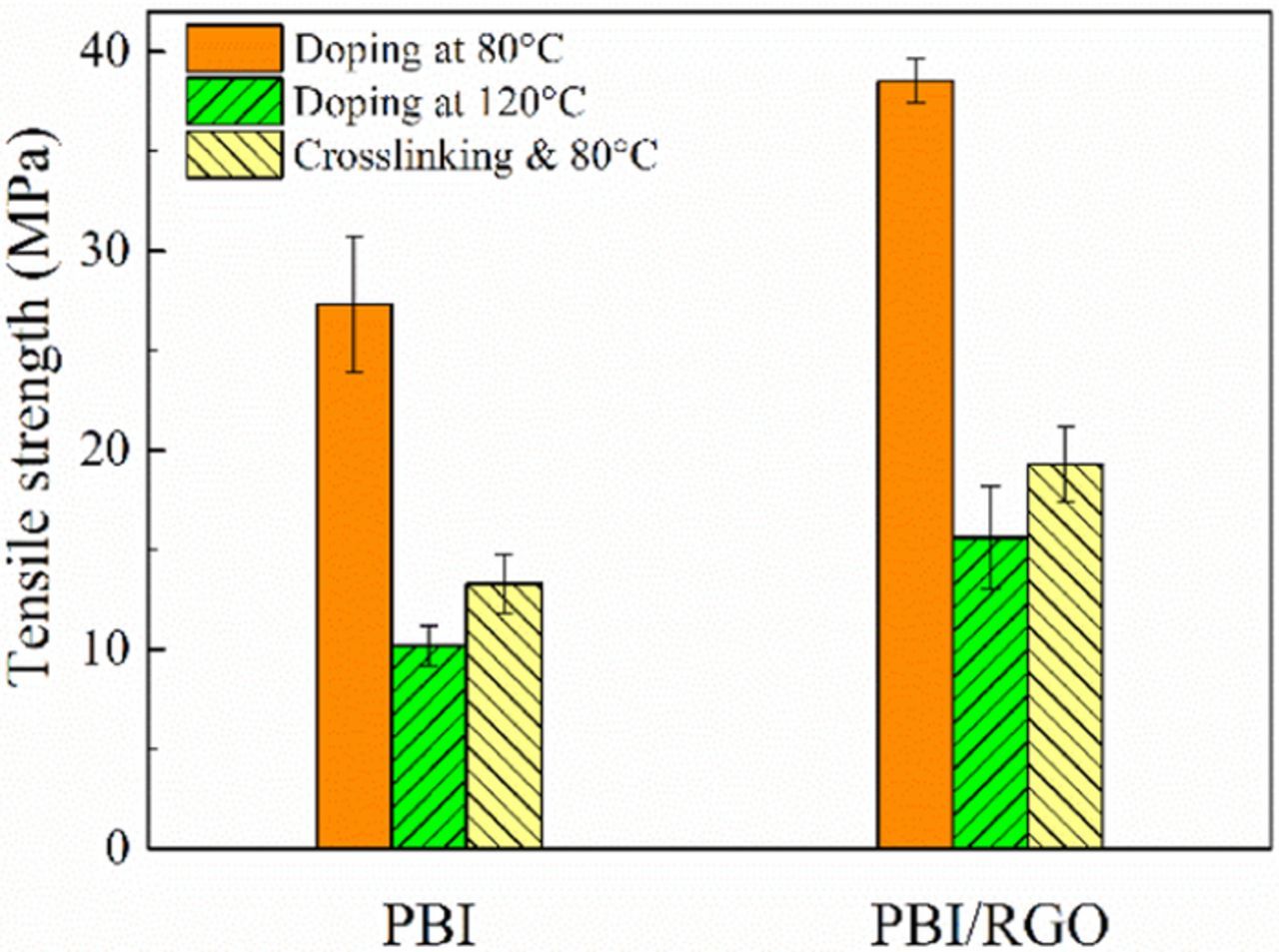
The service life of membranes can also be affected by their mechanical properties, especially for those with a high PA uptake. It is obviously observed in Fig. 6 that the addition of RGO results in an about 40% increase in tensile strength of composite membrane because of the good dispersibility and high strength of RGO, which is consistent with the previous work.27 Although the tensile strength of cPBI/RGO/305% PA membrane (cPBI/RGO membrane containing 305% PA) is much lower than that of PBI/RGO/222% PA membrane because of the intense plasticizing effect of PA,46 it is increased by 23.7% compared with that of PBI/RGO/305% PA membrane (the uncrosslinked membrane doped with PA at 120°C) at the same PA uptake, indicating that crosslinking can improve the mechanical properties of membranes.
Figure 6. Tensile strength of PBI/222% PA, PBI/303% PA, cPBI/310% PA, PBI/RGO/222% PA, PBI/RGO/305% PA and cPBI/RGO/305% PA membranes.
Table I shows the tensile strength of PA-doped cPBI/RGO membrane and various PA-doped PBI membranes reported in previous studies.11,20,35,36,40,47–51 It shows that PA-doped cPBI/RGO membrane has higher tensile strength (19.3 MPa) than most modified PBI membranes, indicating that the self-made crosslinker has a favorable crosslinking effect.
Table I. Properties of PA-doped cPBI/RGO membrane and various PA-doped PBI membranes.
| PEMs | PADL | TSa (MPa) | Tb (°C) | σ(mS·cm−1) | Performance indicator | Ref. |
|---|---|---|---|---|---|---|
| cPBI/RGO | 12.5 | 19.3 | 170 | 58.6 | 1.130 | Present work |
| Modified PBI | 20.4 | 1.8 | 160 | 200.0 | 0.360 | 47 |
| PBI/SiO2 | 3.0 | 51.9 | 160 | 0.0362 | 0.00187 | 11 |
| PBI/clay | 8.9 | 3.9 | 180 | 50.0 | 0.195 | 20 |
| PBI/ZPPc | 2.7 | 6.0 | 180 | 140.0 | 0.840 | 48 |
| cPBI | 14.3 | 6.2 | 180 | 66.0 | 0.409 | 35 |
| cPBI | 14.6 | 16.5 | 200 | 17.0 | 0.281 | 36 |
| cPBI | 7.5 | 5.2 | 150 | 125.0 | 0.650 | 40 |
| cPBI | 13.6 | 4.8 | 180 | 130.0 | 0.624 | 49 |
| cPBI | 10.2 | 10.2 | 180 | 61.0 | 0.622 | 50 |
| cPPBId | 9.7 | 10.2 | 200 | 45.5 | 0.464 | 51 |
aTS means tensile strength. bT means temperature for proton conductivity measurement. cZPP means Zirconium Pyrophosphate. dcPPBI means crosslinked porous PBI.
Proton conductivity
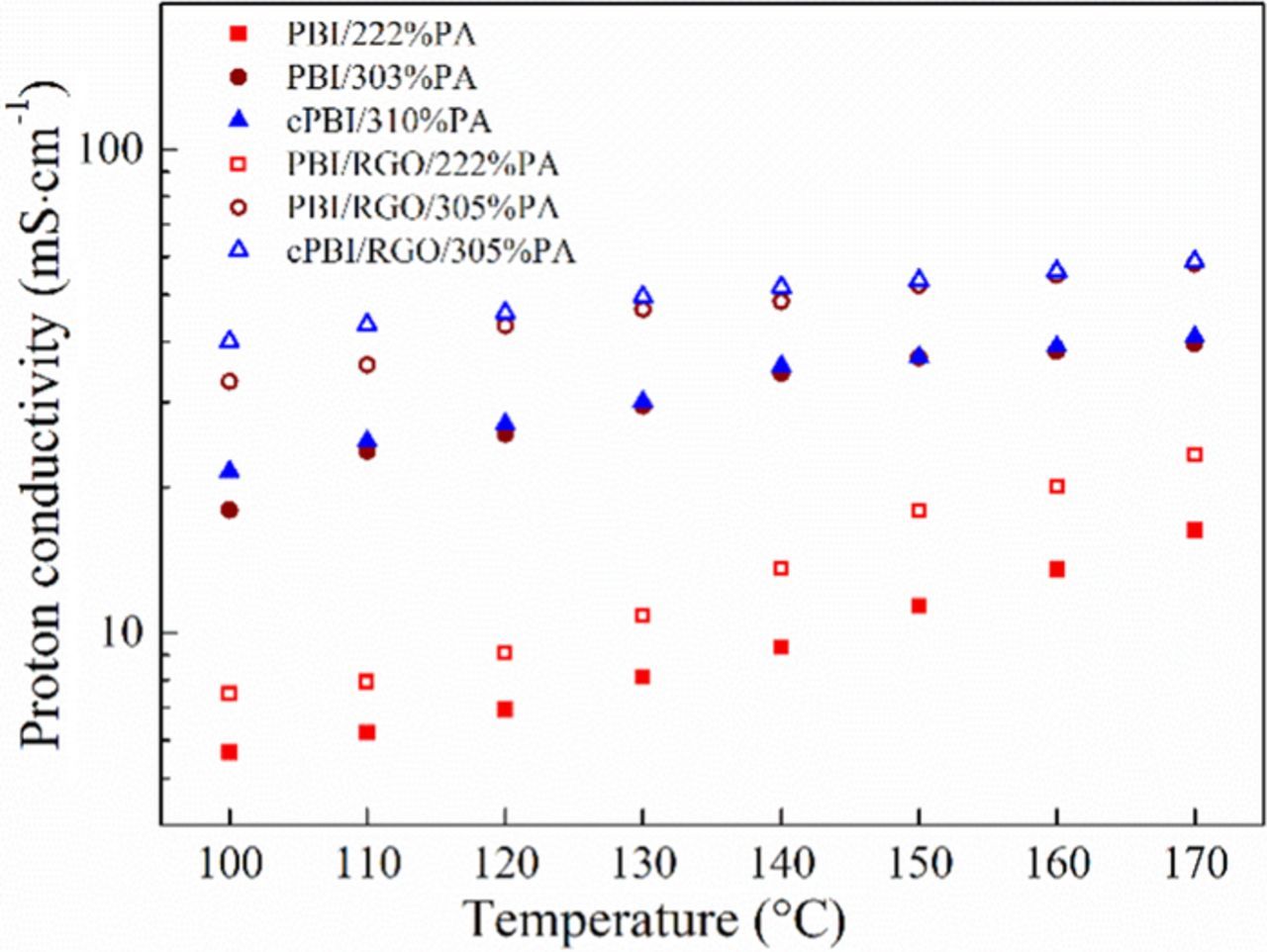
Proton conductivity is a critical index for evaluating the performance of PEMs. The temperature dependence of the proton conductivity of PA-doped PBI and modified PBI membranes at 0% RH is shown in Fig. 7. Similarly, the addition of RGO results in an about 40% increase in proton conductivity of all membranes, mainly because the sulfonic acid groups and oxygen-containing groups can interact with the -NH- groups in benzimidazole rings and absorbed PA to conduct protons via the hopping mechanism.27 After crosslinking, the PA uptake of membranes increases as described in section PA uptake and retention, resulting in a great improvement of proton conductivity. For example, the proton conductivity of cPBI/RGO/305% PA membrane is 58.6 mS·cm−1 at 170°C without humidity, which is increased by 2.6 times compared with that of PA-doped PBI membrane.
Figure 7. Proton conductivity of PA-doped PBI, cPBI, PBI/RGO and cPBI/RGO membranes.
The proton conductivity of crosslinked membranes is higher than that of uncrosslinked ones at the same PA uptake, which is particularly pronounced at low temperatures. This is because the rigid structure of the crosslinker can keep neighboring main chains away from each other, resulting in more bulk PA in the free volume and new continuous proton conducting channels,52 as shown in Scheme 2. However, as the temperature increases, the difference in proton conductivity between crosslinked and uncrosslinked membranes is reduced, because the increase of proton conducting activity of PA at high temperatures reduces the contribution from new channels for proton conductivity.
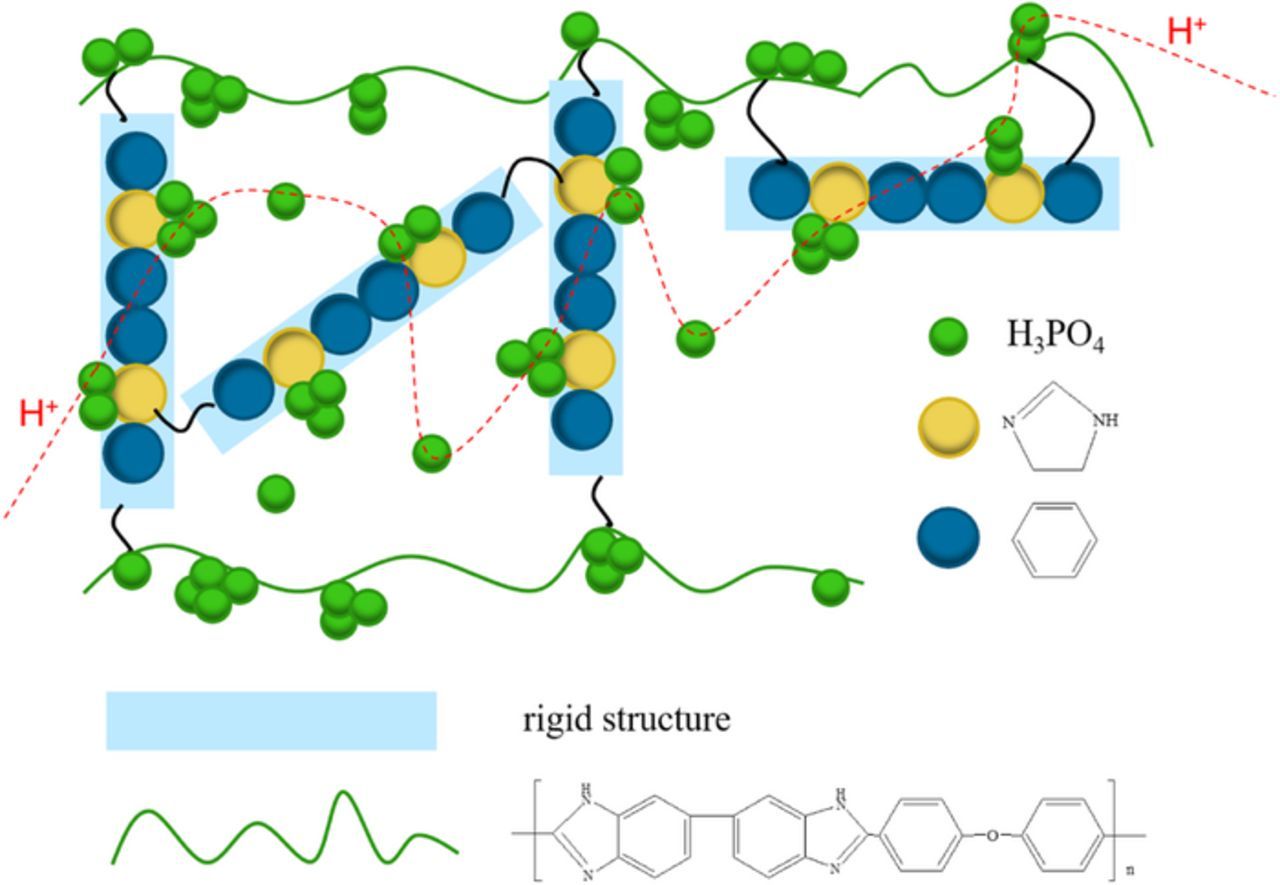
Scheme 2. Structure diagram of crosslinked membrane and the new proton path.
A "performance indicator" is proposed in this study in order to describe the overall performance of proton conductivity and tensile strength, which is similar to the parameter given by Joseph et al.53 It is defined as performance indicator = proton conductivity (mS·cm−1) × tensile strength (MPa) × 10−3. Obviously, membranes with a performance indicator below 0.1 have rather poor proton conductivity or tensile strength, while those with a performance indicator above 1 can be considered as potentially good membranes. Table I shows the proton conductivity and performance indicator of PA-doped cPBI/RGO and various PA-doped modified PBI membranes reported in previous studies.11,20,35,36,40,47–51 It shows that the performance indicators of most PA-doped inorganic particles modified PBI and cPBI composite membranes (around 0.6) are lower than that of PA-doped cPBI/RGO membrane (1.13). Accordingly, the proton conductivity of PA-doped cPBI/RGO membrane is much higher than the required proton conductivity (10 mS·cm−1), and thus it can have promising applications in HT-PEM.
Conclusions
In this study, a crosslinker with benzimidazole groups was synthesized by benzimidazolization in PPA and then dissolved in DMF to obtain the crosslinker solution, and cPBI/RGO membrane was prepared simply by immersing the PBI/RGO membrane into the crosslinker solution. The introduction of the crosslinking structure results in an increase of PA uptake from 222% to 303%, because the special structure of crosslinker endows the cPBI with imidazole groups in the side chain and thus increases the active sites of the polymer chain. In addition, the crosslinked membranes show higher remaining weight after immersion in Fenton's solution for 6 days and PA for 8 days than uncrosslinked membranes, indicating that they have better oxidative and chemical stability. Also, the introduction of RGO and crosslinking structure enables PA-doped membranes to have higher proton conductivity. The cPBI/RGO/305% PA membrane shows a proton conductivity of 58.6 mS·cm−1 at 170°C without humidity, which is increased by 2.6 times compared with that of PA-doped PBI membrane. In addition, it has a tensile strength of 19.3 MPa. In conclusion, these cPBI/RGO membranes have the potential to be used for HT-PEM applications.
Acknowledgments
This research is financially supported by the National Natural Science Foundation of China (21764011), the Foundation from Qinghai Science and Technology Department (2017-HZ-803), Thousand Talents Program of Qinghai Province, Kunlun Scholar Award Program of Qinghai Province and Shanghai Aerospace Science and Technology Innovation Foundation.
ORCID
Shiai Xu 0000-0002-6011-8911