Abstract
Ni-TaC composite powder has been synthesized electrochemically from the mixture of NiO, Ta2O5 and carbon in molten CaCl2-NaCl at 800°C. The reaction pathway was investigated by examination of the partially and completely reduced samples recovered from reduction of the NiO/Ta2O5/C pellets in combination with direct extraction of TaC. The results showed that the composite powder with the multicore-shell structure involving the nano-sized TaC particles located inside the micron-sized Ni matrix was formed through the electrochemical route. Ni metal acted as a catalyst to accelerate the reduction of the cathodic pellets by generation and recovery from Ni-Ta alloys. The electrochemical route in molten salt achieved the preparation of TaC and metal-based TaC composites at a relatively lower temperature.
Export citation and abstract BibTeX RIS
Due to the outstanding properties of high melting point (above 3800°C), extreme Vicker hardness (above 9.4 GPa), superior thermal and chemical stability, low vapor pressure at elevated temperature, tantalum carbide (TaC) is considered as a promising candidate in high-performance cutting tools, wear resistance parts, aerospace propulsion components, as well as a catalyst or catalyst support material for ammonia decomposition.1–5 Especially, tantalum carbide has been usually added into steels to enhance properties of the matrixes.6–7 However, there are still some challenges for the carbides serving as the reinforcement, such as the low wettability and unsatisfactory sinterability. The published studies pointed out that these insufficient features could be improved by the core-rim structure of metal matrix-carbide composites, in which the carbide powder was backed by a more ductile material8–11
The metal matrix-carbide composites were conventionally prepared by the powder metallurgy technique, in which the carbide or carbon powder was added into the metal powder, following by the processes of ball milling and vacuum-sintering. Many cemented composites have been fabricated, such as Fe-TiC,12 Ni-WC,13 Ni-TiC,14 Cu-NbC,15 Ni-(Ti, W)C,8 Ni-(Ti, Mo)C,16 as well as the TaC related cement of Ni-TaC-TiC.17 However, there are still some limitations for this method. The defects inevitably formed on the surface of the crystallized solid lead to severe aggregation of individual particles.11,18 Some other methods involving self-propagating high-temperature synthesis,19,20 combustion synthesis,21,22 spark plasma sintering23,24 and carbothermic reduction25 were also studied. As reported, it is difficult for these methods to obtain fine ceramic particles due to the coarsening of the products induced by the high working temperature.
Electrochemical reduction in molten salt is another optional route for the preparation of carbides and metal matrix-carbide cements. At present, several carbides of NbC,26 TiC,27 HfC,28 Cr3C2,29 ZrC30 and Cr2AlC31 have been produced successfully from the precursors of metallic oxides and carbon powder. Furthermore, the fabrication of cemented composites was also attempted. Zou and coworkers32 reported direct synthesis of Ti5Si3/TiC composites electrochemically from their oxides/C precursors in molten CaCl2. Tran-Nguyen et al.33 investigated electrochemical preparation of Co involved tungsten carbide cement. Sun et al.11 obtained TiC reinforced Fe based composite powder from titanium-rich slag by molten salt electrolysis, in which Fe was used as a binder to form a Fe-TiC matrix composite. In general, the operating temperature employed in the electrochemical reduction in molten salt is obviously lower than that used in other metallurgy routes. It is an important issue to obtain nano-sized carbide particles in the metal matrix.11 Nevertheless, the preparation of metal based TaC composite particles through electrochemical route in molten salt has seldom been reported, and the role of the metal matrix played in the intermediate and final products should be considered.
In this study, the Ni-TaC composite powder containing a multicore-shell structure was fabricated by electrochemical reduction in molten CaCl2-NaCl. The mechanistic insight of the pathway for reduction of the cemented composite was also investigated by comparison of the process for electrochemical preparation of pure TaC. In addition, the multi-core microstructure of the obtained composite powder was confirmed.
Experimental
Tantalum pentoxide, NiO and carbon powder were uniformly mixed at a given stoichiometric molar ratio with the addition of polyvinyl alcohol (about 5% in mass). The carbon powder used in this work presented an amorphous structure, which comprised nano-sized particles around 100 nm in size, as depicted in the previous study.26 Approximately 1 g of the mixture was pressed into a cylindrical pellet under a pressure load of 8 MPa (15 mm in diameter and 1.5 mm in thickness), followed by a sintering process at 1000°C for 3 h in an argon atmosphere. The pellet fabricated guaranteed sufficient mechanical strength for further operation. The eutectic mixture of CaCl2 and NaCl (about 400 g) contained in an alumina crucible was first dried at 350°C for 24 h, and then melt at the target temperature inside a sealed tubular stainless steel reactor. The sintered pellet was contacted to a stainless steel wire to form the cathode, and a high-density graphite rod (12 mm in diameter, 70 mm in length) was employed as the anode. All chemical reagents used in this study were of analytical grade.
Systematic experiments were carried out at 800°C, and argon was supplied continuously into the reactor to provide an inert atmosphere during the experiment. To eliminate the moisture and redox impurities from the melt, pre-electrolysis was performed under 3.1 V for 2 h. The cathodic pellet was electrochemically reduced at 3.0 V for appropriate durations, and the variation of current versus time during the reduction was recorded. Following the reduction process, the solidified salt remained in the samples was washed with tap water, and dried for characterization. The phase composition of each sample was determined by X-ray diffraction (XRD) using a Rigaku D/Max-2500PC X-ray diffractometer with Cu-Kα radiation. The morphology of the samples was examined by a JSM-6360LV scanning electron microscope (SEM), and the local elemental composition was analyzed by energy-dispersive X-ray (EDX) spectroscopy (Oxford INCA X-Max20).
Results and Discussion
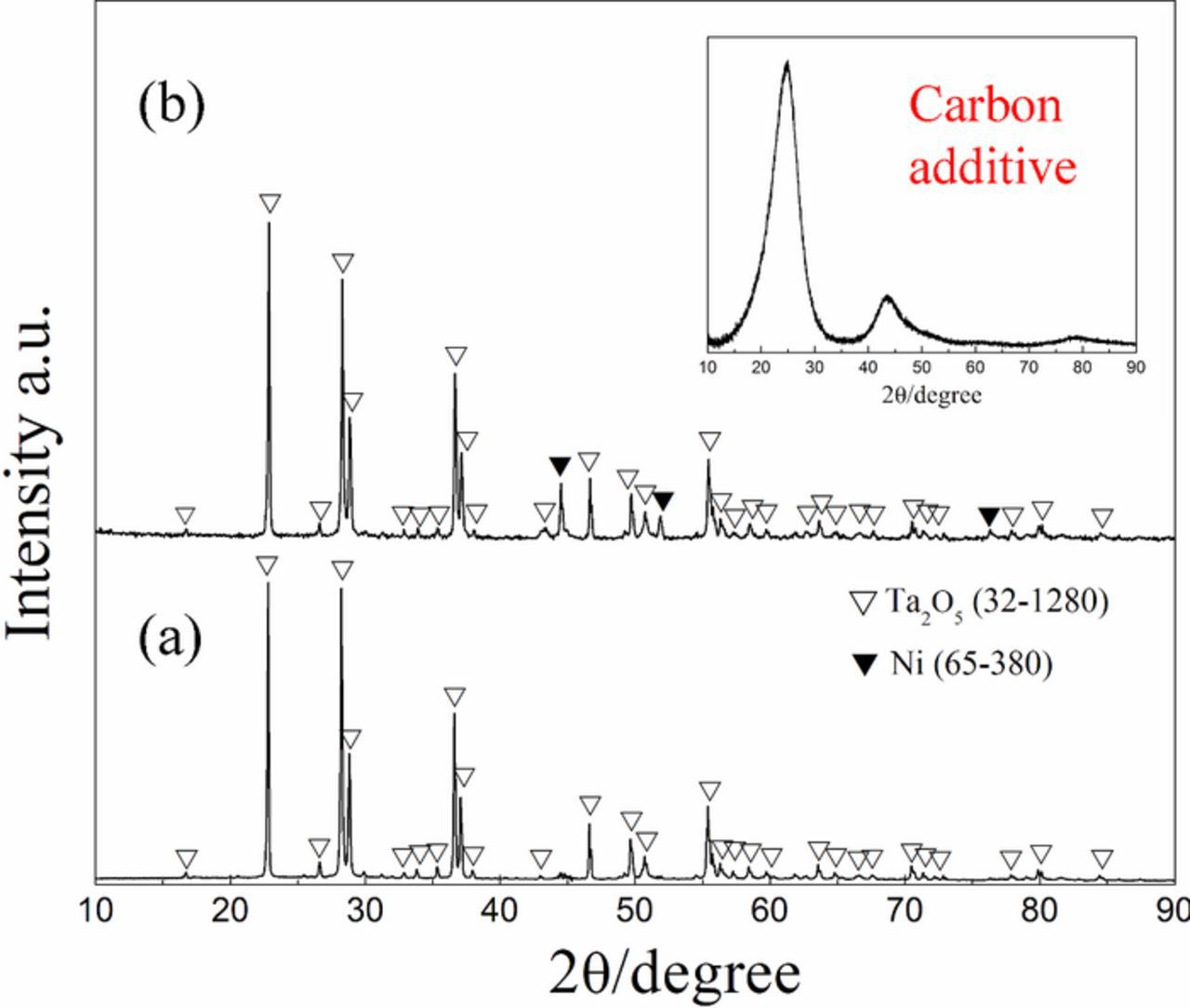
Fig. 1a shows the XRD pattern of Ta2O5 and carbon mixture sintered at 1000°C for 3 h under the inert atmosphere, in which the molar ratio of tantalum to carbon is 1 : 1. A single phase of Ta2O5 was documented without the observation of carbon due to the amorphous structure of the carbon additive used in this work, as indicated by the inserted pattern in Fig. 1. Amorphous structure of the carbon additive is available for the formation of carbides with metals.26 The weight loss of the sintered pellet is below 1%. This phenomenon also suggests the absence of any reaction between Ta2O5 and carbon. Then Ni metal appeared in the sintered pellet with the addition of NiO into the mixture which implies the chemical reduction of NiO by carbon reductant (Eq. 1). The weight loss maintains at about 8%, which is in agreement with the amount of carbon and oxygen consumed for the carbothermic reduction of NiO.
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0001.gif)
Figure 1. XRD patterns of the (a) Ta2O5/C (Ta : C = 1 : 1); (b) NiO/Ta2O5/C (Ni : Ta : C = 1 : 1 : 3) composite pellets sintered at 800°C. The inset is the XRD pattern of the carbon additive used in this work.
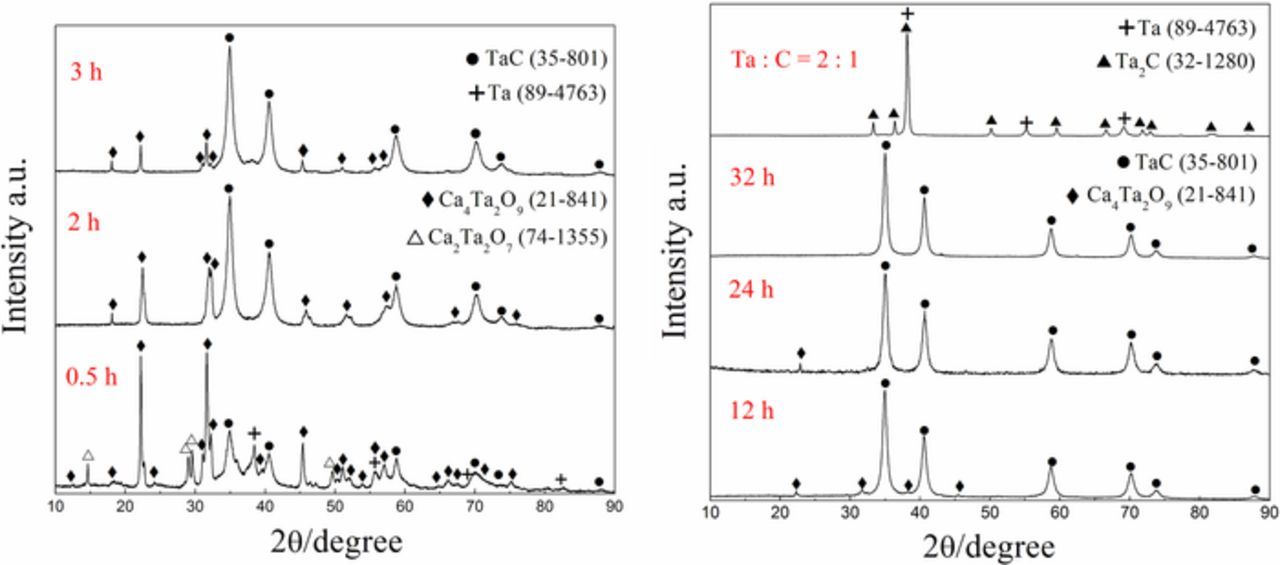
Electrochemical reduction of the Ta2O5/C composite pellet was conducted at 3.0 V for various durations to investigate the reaction pathway. As shown by Fig. 2, the phase composition of the products changes with respect to electrolysis time. A series of calcium tantalates such as CaTa4O11, Ca2Ta2O7 and Ca4Ta2O9 was generated by the participation of CaO in molten salt at the initial stage of the reduction (Eq. 2). Though appearance of CaTa4O11 is not apparent in the XRD patterns, this tantalate can be predicted present in the intermediate products base on its existence during direct electro-deoxidaion of Ta2O5.34,35
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0002.gif)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0003.gif)
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0004.gif)
Figure 2. XRD patterns of samples electrochemically reduced from the Ta2O5/C composite pellets (Ta : C = 1 : 1) under 3.0 V for various durations, as well as the pellet (Ta : C = 2 : 1) reduced at 3.0 V for 23 h.
Meanwhile, the pentavalent tantalum compounds involving Ta2O5 and calcium tantalates were electrochemically reduced to Ta (Eq. 3), which would combine carbon to form TaC (Eq. 4). So the co-existence of tantalates, Ta and TaC was confirmed in the interrupted sample for 0.5 h. Moreover, the synergetic process of electrolysis and carburization should probably occur during the reduction (Eq. 5).
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0005.gif)
With the increasing of the duration, calcium tantalates were gradually decomposed to Ta for further carbonization. The diffraction peaks of calcium tantalates can be hardly observed after 12 h of reduction. Then a single phase of TaC without obvious impurity was achieved in the sample electrolyzed for 32 h. In addition, Ta2C was formed instead of TaC when the molar ratio of Ta to carbon is 2 : 1 in the precursor. It means that every carbon atom tends to combine more tantalum atoms during the carbiding process once carbon is insufficient in the cathodic pellet.
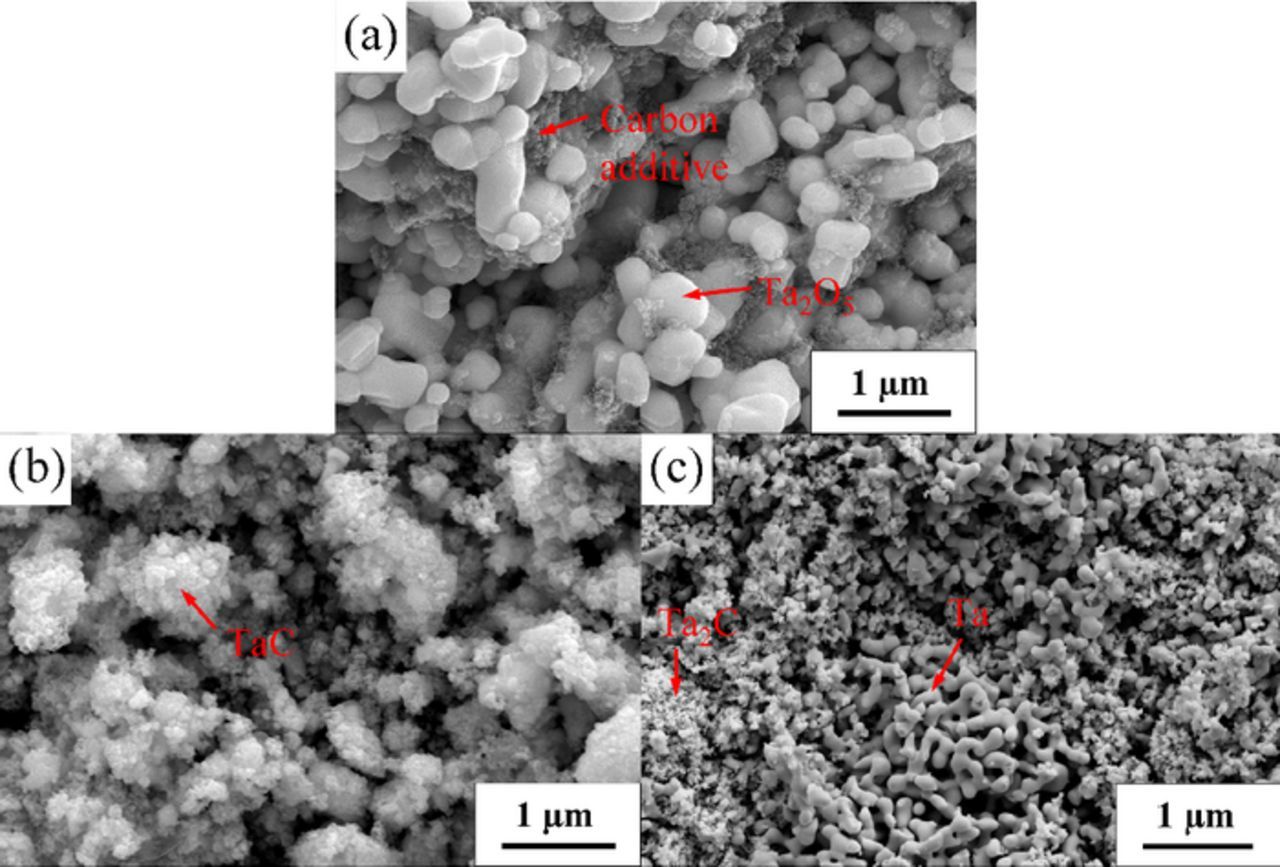
Figs. 3a–3b present the SEM images of the sintered Ta2O5/C precursor and the sample electrochemically reduced for 32 h, respectively. The results point out that the initial pellet is composed of smooth Ta2O5 particles about 500 nm with much smaller carbon particles existing around the oxide particles, whereas TaC particles are found to be less than 100 nm across the pellet obtained after reduction for 32 h. This suggests the difficult growth of the TaC particles at the relatively low temperature of 800°C. Fig. 3c shows that Ta particles with nodular shape were found adjacent to the fine Ta2C particles.
Figure 3. SEM images of the (a) Ta2O5/C pellets (Ta : C = 1 : 1) sintered at 1000°C for 3 h; samples electrolyzed from the sintered pellet with Ta to C ratio of (b) 1 : 1; (c) 2 : 1 for 32 and 23 h, respectively.
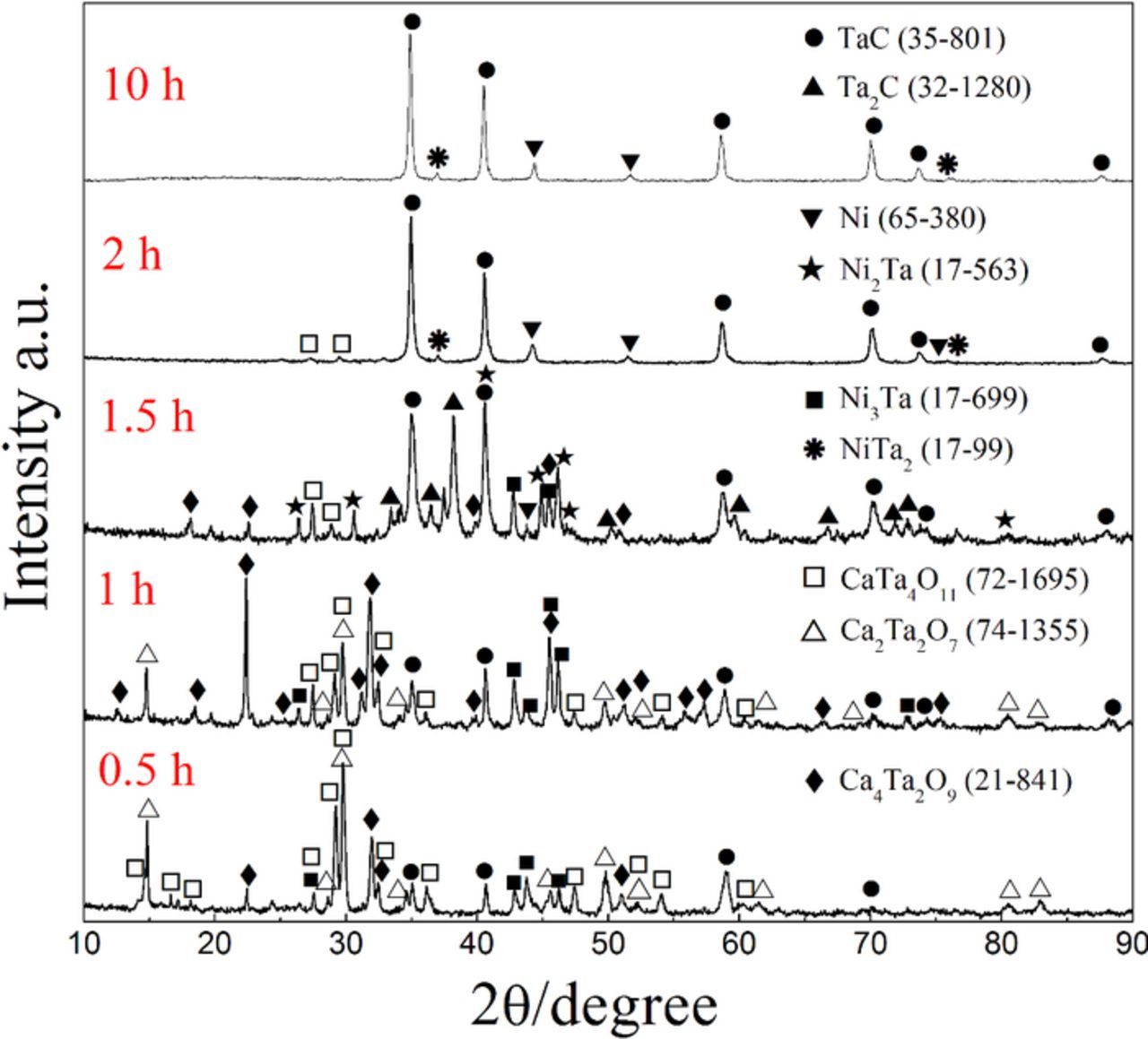
Subsequently, the cathodic polarisation of the precursor with the addition of Ni (Ni : Ta : C = 1 : 1 : 3, molar ratio) was carried out versus the graphite anode for various durations. According to Fig. 4, the reduction process can be divided into two main stages. In the first stage, the formation and electrolytic decomposition of calcium tantalates are inevitably experienced, which are similar to those during the reduction of the Ta2O5/C composite pellet. The appearance of CaTa4O11 has also been proved. The second stage is characterized as the generation and decomposition of Ni-Ta alloys. It can be seen that Ni3Ta started to appear besides TaC once the reduction of pentavalent tantalum compounds occurred (Eq. 6), and its amount increased with the reaction time. Then Ni2Ta and NiTa2 were formed with the gradual recovery of Ta element (Eqs. 7–8). Subsequently, TaC was gradually formed at the expense of the Ni-Ta alloys (Eq. 9). Finally, a Ni-TaC composite was synthesized after 10 h of reduction.
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0006.gif)
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0007.gif)
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0008.gif)
![Equation ([9])](https://content.cld.iop.org/journals/1945-7111/163/3/E49/revision1/d0009.gif)
Figure 4. XRD patterns of samples electrochemically reduced from the Ta2O5/NiO/C composite pellets (Ni : Ta : C = 1 : 1 : 3) under 3.0 V for various durations,
Based on the results provided above, the positive effect of Ni for the generation of the Ni-TaC composite should be considered. The Ni metal in the precursor firstly introduces an excellent conductive phase to accelerate the electro-deoxidation of the pentavalent tantalum compounds. Moreover, the participation of Ni into the reactions deserves more attention for the reduction. The Ni metal residing in the cathodic pellet would react with Ta to create Ni-Ta alloys immediately when it was recovered from the pentavalent tantalum compounds. At the beginning, more Ni atoms were incorporated to become Ni3Ta because of the sufficient existence of Ni in the pellet. Then the continuous generation of tantalum lead to the combination of extra Ta atoms to produce Ni2Ta and NiTa2. So a single phase of Ta can hardly be detected besides several kinds of Ni-Ta alloys throughout the reduction. At last, the Ni atoms in the Ni-Ta alloys were substituted completely by carbon atoms with the individual phase of Ni left in the product. The participation and recovery of Ni should accelerate the reduction of the pentavalent tantalum compounds. This phenomenon was evidenced with comparison to the process with the absence of NiO in the precursor. The time spent on the transformation from the Ta2O5/NiO/C mixture to the Ni-TaC composite is 10 h. It is much more efficient than the process for direct electro-reduction of the Ta2O5 /C mixture. Correspondingly, the current efficiency shows an obvious enhancement from 7.8% to 20.6%.
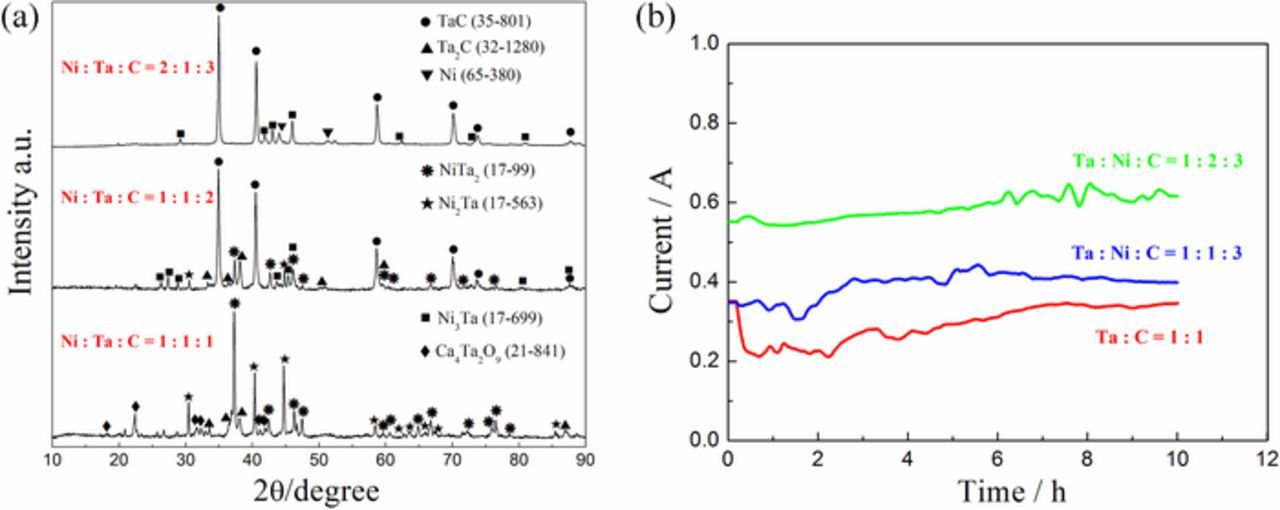
The influence of Ni and carbon on the reduction process as well as the final products was further investigated by varying the respective ratio of the elements in the precursor. As can be seen from Fig. 5a, the electrolytic reduction of the pellet is incomplete and the carbonization of Ta is not obvious except for the presence of some Ta2C if the cathode is lack of carbon (Ni :Ta : C = 1 : 1 : 1), because most of the carbon powder acted as reducing agent during the sintering process. The complete disintegration of calcium tantalates was achieved when the ratio was enhanced to 1 : 1 : 2, but some Ni-Ta alloys were also detectable because of the deficient of carbon during the carbonization. In addition, the amount of Ni in the precursor was increased to 2 : 1 : 3, and the product predominantly contained Ni and TaC with small amount of Ta2C and Ni3Ta.
Figure 5. (a) XRD patterns of the samples electrochemically reduced from the Ta2O5/NiO/C composite pellets with various molar ratios; (b) current versus time curves recorded during the reduction of the composite pellets with various molar ratios.
Fig. 5b displays a set of current versus time curves recorded during the reduction of the pellets with various molar ratios. It illustrates that the current increased with the amount of Ni in the cathodic pellets. This phenomenon should be attributed to two possible reasons. The first reason is the introduction of Ni as an excellent conductive phase throughout the pellet, providing active area for the reduction. The other reason is the driving force originated from the chemical reactions between Ni and Ta, which facilitates the electrolytic reduction of the cathodic pellet. Generally, the current efficiency drops apparently (13.8%) once more Ni is present in the precursor.
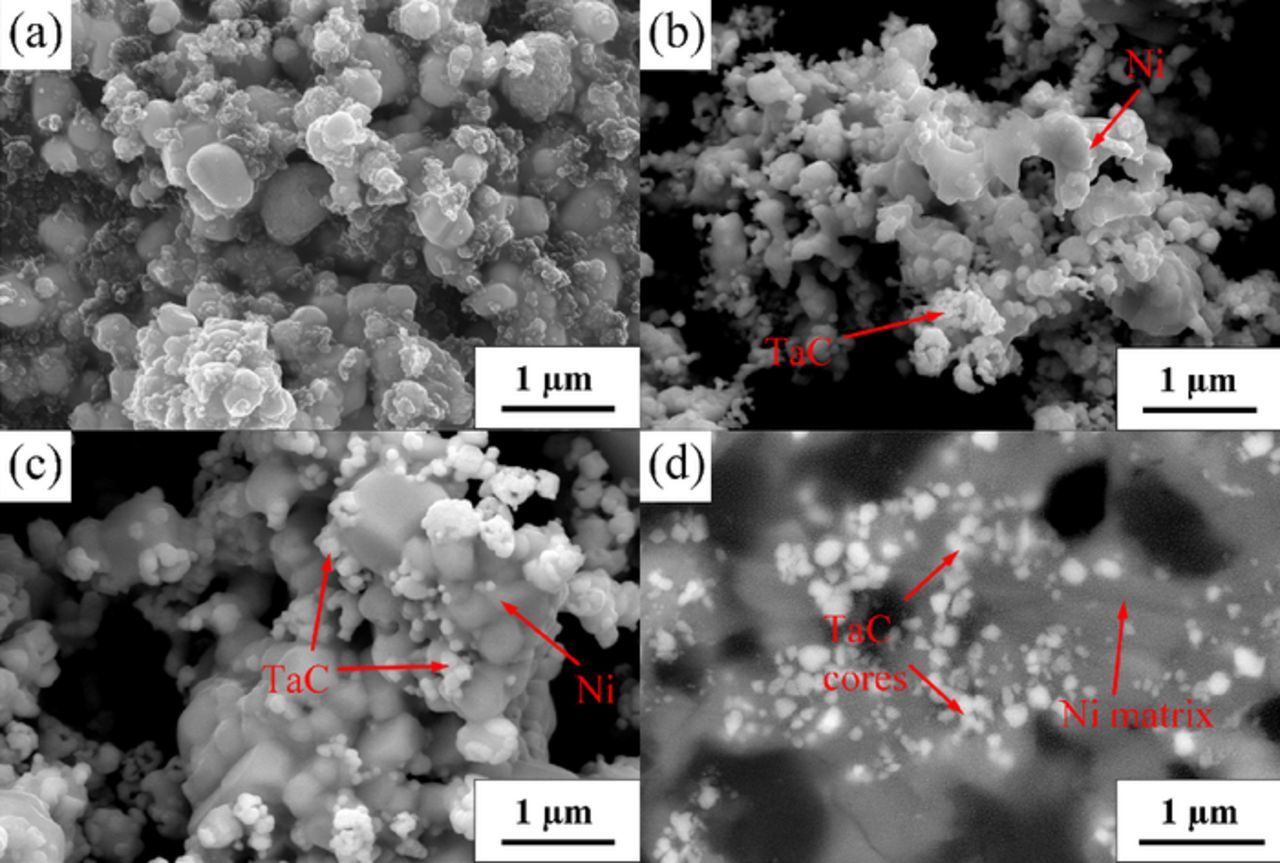
Fig. 6a gives the morphology of the pellet with the inclusion of NiO (Ni : Ta : C = 1 : 1 : 3) sintered at 1000°C. The structure seems to be more compact than that of the Ta2O5/C sintered pellet due to the chemical reduction of NiO in the sintering process, though it is difficult to distinguish Ni in the SEM image. TaC and Ni were found to mix together in the pellet electrolyzed for 10 h. As shown in Fig. 6b, the carbide particles are similar to those obtained from the Ta2O5/C precursor, and the Ni particles present irregular shape.
Figure 6. SEM images of the (a) NiO/Ta2O5/C pellets (Ni : Ta : C = 1 : 1 : 3) sintered at 1000°C for 3 h; samples electrolyzed from the Ta2O5/NiO/C composite pellets with the molar ratio of (b) Ni : Ta : C = 1 : 1 : 3; (c) Ni : Ta : C = 2 : 1 : 3 under 3.0 V for 10 h; (d) Back scattered SEM image for the cross section of the multicore Ni-TaC particles.
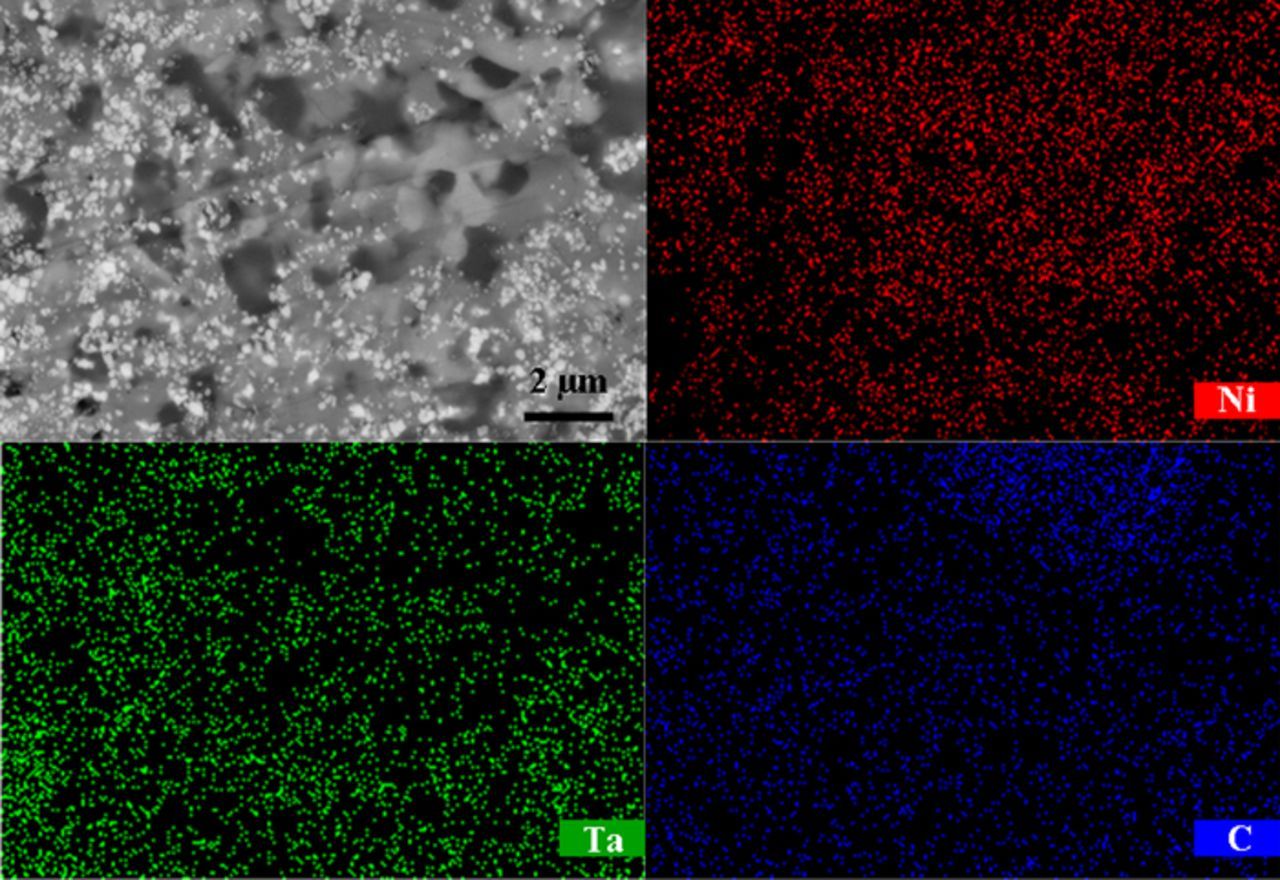
The morphology of the product retrieved from the precursor with the molar ratio of 1 : 2 : 3 (Ni : Ta : C) is visible in Fig. 6c. It reveals that the matrix particles grew to regular shaped particles if there were sufficient Ni metal in the pellet, which interconnected together to become bulky particles. Meanwhile, there are also some TaC particles existing around the bulky particles. To further probe the microstructure inside the particles, they were ground by abrasive paper to prepare a smooth surface. Fig. 6d conveys the back-scattered SEM image for the cross section of the bulky particles. It shows that many fine TaC particles were embedded in the Ni matrix to form a composite with the multicore-shell structure. This result could be evidenced by Fig. 7, in which another back-scattered SEM image of the broader region in combination with the elemental mappings taken with EDX spectroscopy over the imaging area are presented. A considerable number of carbide particles were observed as the cores in the Ni matrix, and the distribution is approximately homogeneous. Moreover, it is reasonable to predict that most of the carbide particles were located in the matrix in comparison with the dissociative carbide particles according Figs. 6c–6d and Fig. 7. The fine carbide cores can act as a reinforcing phase inside the Ni matrix to enhance the wear resistance as well as the wettability of the composite material.
Figure 7. Back scattered SEM image for the cross section of the multicore Ni-TaC particles and EDX elemental mapping across the SEM image.
Conclusions
Ni-TaC composite powder with a multicore-shell structure has been successfully prepared by electrochemical reduction of the NiO/Ta2O5/C mixture in molten CaCl2-NaCl at 800°C. The structure of the composite powder was examined in detail. It characterized as the generally homogeneous nano-sized TaC particles inside the micron-sized Ni shell. The reduction pathway was studied by interrupting the experiments for various durations, as well as comparing with the process for direct extraction of TaC from the mixed Ta2O5/C oxides. A series of tantalates was formed and subsequently disintegrated with the proceeding of the reduction. The Ni metal resident in the precursor reacted with the Ta generated from pentavalent tantalum compounds to form Ni-Ta alloys. The participation and recovery of Ni metal in the process accelerated the reduction of the pentavalent tantalum compounds. Ta atoms would combine less carbon atoms to form Ta2C if the carbon additive is insufficient in the precursor. Furthermore, the working temperature for the synthesis of TaC and metal-based TaC composite was effectively lowered down by electrochemical reduction in molten salt.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (grant No. 51404057 and 50874026) for financial support.