Abstract
Most methods for improving supercapacitor performance are based on developments of electrode materials to optimally exploit their storage mechanisms, namely electrical double layer capacitance and pseudocapacitance. In such cases, the electrolyte is supposed to be electrochemically as inert as possible so that a wide potential window can be achieved. Interestingly, in recent years, there has been a growing interest in the investigation of supercapacitors with an electrolyte that can offer redox activity. Such redox electrolytes have been shown to offer increased charge storage capacity, and possibly other benefits. There are however some confusions, for example, on the nature of contributions of the redox electrolyte to the increased storage capacity in comparison with pseudocapacitance, or by expression of the overall increased charge storage capacity as capacitance. This report intends to provide a brief but critical review on the pros and cons of the application of such redox electrolytes in supercapacitors, and to advocate development of the relevant research into a new electrochemical energy storage device in parallel with, but not the same as that of supercapacitors.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Charge storage in supercapacitors (SCs) is achieved by one or both of two main mechanisms, namely, electrical double layer (EDL) capacitance and pseudocapacitance. EDL capacitance is widely accepted to be due to electrostatic separation of electronic and ionic charges at the electrode | electrolyte interface, whilst pseudocapacitance has been broadly and ambiguously associated with fast faradaic processes which are also often termed as charge transfer or redox processes at electrode surfaces.1,2 Accordingly, SC research and development have mostly involved optimization of electrode materials to more efficiently exploit these storage mechanisms. However, in recent years, attention has been drawn to exploitation of the redox activity from the electrolyte in a bid to improve the performance of SCs. In this vein, redox active species, such as simple and complexes of transition metal ions,3,4 halide ions,5,6 quinones,7 and phenylamide,8 have been shown to be capable of undergoing fast redox reactions on the surfaces of some carbon or metal oxide based electrode materials. These reactions can then enhance the charge storage capacity of SCs. Accordingly, electrolytes which exhibit redox activity in SCs have been classified into redox additive electrolytes in which redox active species are added to enable fast electron transfer reactions at the electrode/electrolyte interface, and redox active electrolytes which are inherently able to undergo fast electron transfer reactions.9 Supercapacitors are conventionally built with a separator membrane (between the two electrodes) in which the electrolyte is added. If a redox electrolyte is added to the membrane, a so called redox additive polymer electrolyte may be prepared.9 Nonetheless, it should be pointed out that an intrinsically redox active polymer is conducting to electrons, and hence should not be used as an electrolyte.10
There are literatures providing detailed and extensive discussions on the application of redox electrolytes in SCs.9,11,12 The use of redox electrolytes has been claimed as a cheap means of improving the performance of SCs, particularly charge storage capacity,9,11 promoting the use of more environmentally friendly aqueous electrolytes,4 optimizing the low temperature performance of water based SCs,13,14 and inhibiting the corrosion of current collectors,15 just to mention a few. Still, it is neccesary to draw attention to some important issues inherent in the application of redox electrolytes in SCs. A number of these technical issues such as the existence of high charge capacities only at low (relatively impractical) voltages,16 the irreversibility of some redox species,7,9 and the effects of the migration of redox species between electrodes,17 have been addressed.
The purpose of the current critique is to appraise three main issues inherent in SCs containing redox electrolytes. These are (i) the validity to use capacitance to describe the charge storage performance, (ii) the necessity to include the redox electrolyte mass for normalization of the device energy storage capacity, and (iii) the mechanisms responsible for the enhanced charge storage. In the authors' view, SCs containing redox electrolytes take advantage of different charge storage mechanisms and are therefore hybrid devices whose performance characteristics should be explained and compared as the starting point for discussion.
Hybrids of Supercapacitor and Battery: Supercapattery and Supercabattery
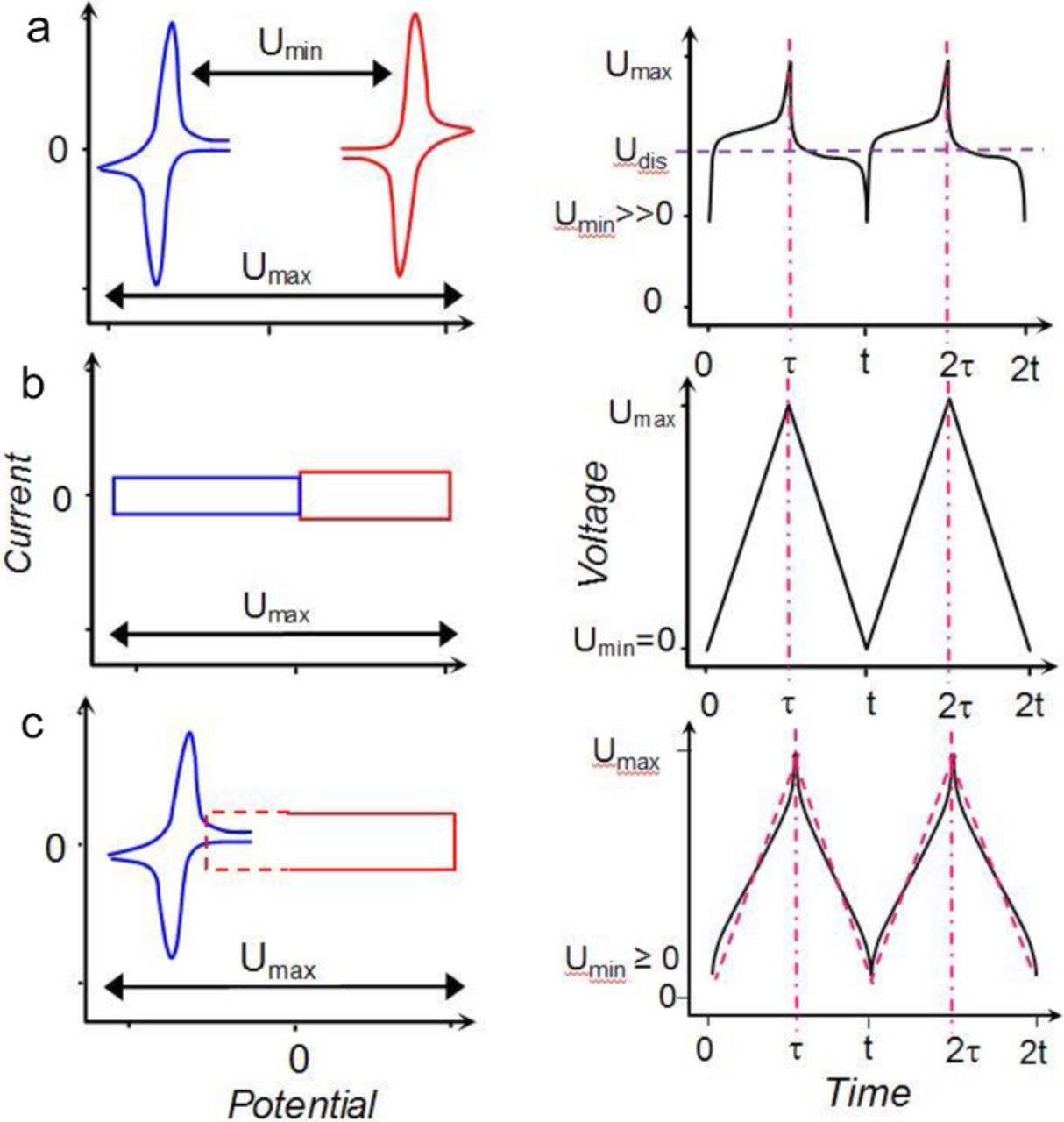
In terms of fundamental science, EDL capacitance and pseudocapacitance have been given detailed quantitative and qualitative descriptions both from a physicochemical perspective and also from an electrical device response point of view.18,19–22 Electrochemical characterizations of battery, supercapacitor and the so called supercapattery are illustrated in Figure 1 by the respective schematic cyclic voltammograms (CVs) and galvanostatic charging and discharging plots (GCDs). It should be mentioned that the concept of supercapattery has been proposed to describe hybrid systems in which the charge storage mechanisms of both the supercapacitor and battery are combined into one device, at either the electrode material or device levels. In Figure 1, the supercapattery performance is presented at the device level with one electrode displaying battery like properties and the other displaying capacitor like properties.23,24 At the electrode materials level, one or both of the electrode materials can be the nanostructured composite of an EDL material and a battery material, such as the nanocomposite of carbon nanotubes and manganese dioxide.23,24
Figure 1. Schematic illustration of the electrochemical characteristics of (a) battery, (b) supercapacitor, and (c) supercapattery24 represented by (left) the cyclic voltammograms and (right) galvanostatic charging and discharging plots. (Udis: Average discharging voltage. Other symbols are self-explanatory.)
A comparison between the CVs and GCDs in Figure 1 would show that all devices are capable of storage or release of a certain amount of charge, ΔQ, over a range of voltages, ΔU = Umax – Umin. For the CV and GCD of the supercapacitor, the following Equation 1 is applicable for derivation of the capacitance, C, of the device.18
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn1.jpg)
Note that the CV and GCD of the supercapattery in Figure 1 are sufficiently similar to those of the supercapacitor, and can be assessed in terms of capacitance as derived from Equation 1.
However, it is also possible that the battery electrode dominates over the supercapacitor electrode, and the hybrid device performs more like a battery as shown in Figure 1a, instead of Figure 1c. In such a case, application of Equation 1 can lead to a disproportionately high value of the ΔQ/ΔU ratio. If one regards this ΔQ/ΔU ratio as capacitance, misleading energy capacity can be obtained as discussed below. Instead, the energy storage capacity in a battery-like hybrid should be derived in the same way as for a battery. To differentiate between capacitor-like and battery-like hybrids, and also to aid discussion, we propose to name the former as supercapattery and the latter supercabattery.
Charge Storage Capacity of Supercapacitors Containing Redox Electrolytes
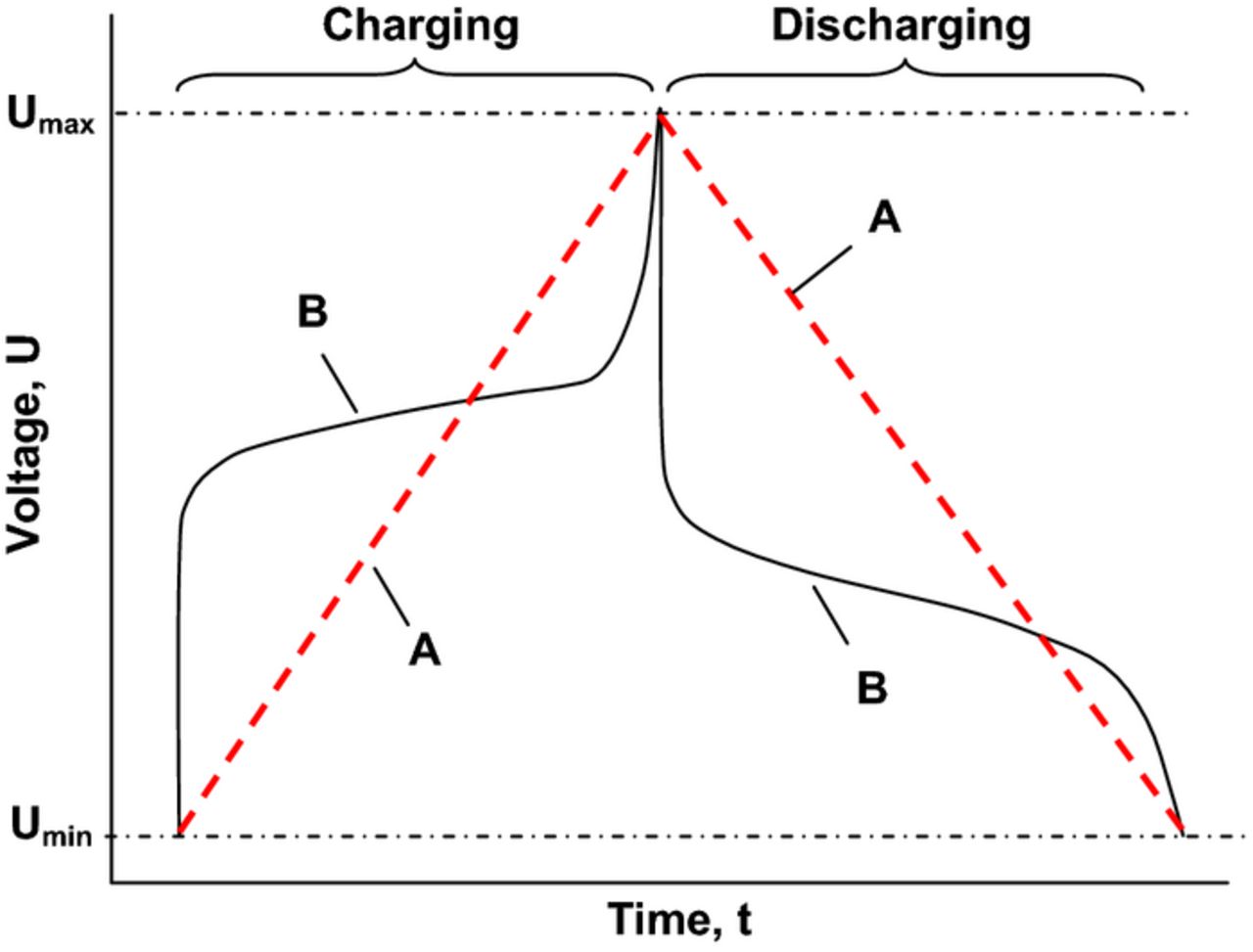
In relation with the application of Equation 1, Figure 2 gives a comparative illustration between the GCDs of a conventional SC (plot A, linear) and an SC with redox electrolytes (plot B, non-linear). Clearly, under galvanostatic conditions (i.e. constant current), these two curves have the same amount of charge, ΔQ, and the same voltage range, ΔU, for either charging or discharging, and hence the same ΔQ/ΔU ratio. However, if this ΔQ/ΔU ratio is the capacitance for the linear plot A, it cannot be the same for the non-linear plot B. In other words, for the non-linear plot B, the capacitance concept should not be applied, although the ΔQ/ΔU ratio can always be derived with the unit of F.
Figure 2. Illustrative comparison of the galvanostatic charging and discharging plots of a conventional supercapacitor (plot A, linear), and a supercapacitor containing a redox electrolyte (plot B, non-linear). Note that plot B represents very well the characteristics of some SCs containing redox electrolytes as reported in the literature.26,28,33–35 Such battery-like devices can be reasonably placed into the category of supercabattery.
The above analysis and conclusion on the linear and non-linear GCDs in Figure 2 are valid against Equation 1. However, these GCDs are important for measurement of the amount of energy, W, stored in or released from the device. Equation 2 below is the general form for the energy stored in the charging process under a constant current, I, whilst a similar equation can be written for discharging.25
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn2.jpg)
For a supercapacitor, U(t) is a linear function, and Equation 2 simplifies to Equation 3.
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn3.jpg)
For a nonlinear GCD plot, Equation 3 is invalid and one has to apply Equation 2 for calculation of the energy stored in the device.
Graphically, the amount of energy derived from Equation 2 is the area under the GCD plots in Figure 2. For supercapacitors, the area under the linear plot A is the same as that represented by Equation 3, in which C is calculated from the ΔQ/ΔU ratio. For the non-linear GCD plot B in Figure 2, the same ΔQ/ΔU ratio can be derived as from the linear plot A. Therefore, if one applies this ΔQ/ΔU ratio in Equation 3 for calculation of the amount of energy stored, the value will be different from that calculated using Equation 2 for the non-linear plot B. Specifically for Figure 2, the linear plot A gives a smaller amount of energy for the charging process than the non-linear plot B. For discharging, the non-linear plot B corresponds to a smaller amount of energy than the linear plot A. Such differences indicate that charging up the SC with redox electrolyte requires a greater amount of energy than it can discharge, which is an indication of lower energy efficiency than the conventional SC. Because the non-linear plot B in Figure 2 is a trace from the literature,26 the analysis provided here argues strongly why it is inappropriate to use the ΔQ/ΔU ratio as the capacitance. Similarly, Equation 3 should not be applied for analysis of non-linear GCDs, which is unfortunately contradictory to some literatures.
In the literature, other approaches have been proposed to by-pass the above discussed problems in analysis of non-linear GCD plots. For example, the following Equation 4 was proposed to define and calculate the ΔQ/ΔU ratio and hence the "claimed capacitance", Cclaim, for a device that exhibited non-linear GCD plots.
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn4.jpg)
The right side of Equation 4 is copied directly from the literature26 in which im (A g−1) is the mass normalized current, t the time, and U (same as V as in ref. 26) the applied potential (or voltage) with Ui and Uf being the initial and final values, respectively. Equation 4 is valid for calculation of ΔQ/ΔU from a non-linear GCD plot as that in Figure 2, but this ratio is not capacitance.18 There is no doubt that using such a "claimed capacitance" derived from Equation 4 and a non-linear GCD plot will lead to incorrect calculation of energy stored in the relevant device as discussed above.
Another interesting attempt was reported to conceal the above mentioned mistakes on deriving the "claimed capacitance" from non-linear GCD plots. This was presented by correlating the charge capacity with the unit of F/g.26 The consequence was that specific capacitance values as high as 4700 F/g were claimed and used to calculate the energy stored according to Equation 3, leading to a truly meaningless specific energy value of 163 Wh/kg.26 In fact, based on the published GCD plot26 (traced as plot B in Figure 2) and Equation 2, it can be derived that the amounts of energy incurred during charging and discharging were about 48 Wh/kg and 31 Wh/kg, respectively, leading to an energy efficiency ca. 65%. In other words, because of the incorrect use of Equation 3, the reported specific energy (163 Wh/kg) is about 5.3 times overestimated of the actual value for discharging.
In fact, the generally high "claimed capacitance" values ascribed to some SCs with redox electrolytes are a result of the incorrect use of the capacitance concept and its relation with the ΔQ/ΔU ratio under well-defined conditions for description of battery-like performances. It must have been very tempting to report large ΔQ/ΔU ratios as capacitance values against properly measured literature data, which is a historically known18 and recently re-emphasized25 source of great confusion.
Nevertheless, in spite of the above mentioned misleading claims of capacitance values in some literature, SCs with redox electrolytes are still promising electrochemical energy storage devices with good performance metrics such as significantly increased charge storage capacity and uncompromised cycle stability.11,16,26–29 Research findings from these devices should however be analyzed with appropriate experimental and theoretical tools, and compared with that from batteries or other battery-like hybrid devices. In most cases, assessing the overall performance of SCs with redox electrolytes should not be based on capacitance or pseudocapacitance.
Performance Metrics of Supercapacitors Containing Redox Electrolytes
It has been shown that the use of redox electrolytes can increase the charge storage capacity of SCs, but reporting the performance by considering the contribution of the redox electrolyte as capacitance can be misleading in most cases, if not all. Further, it is commonplace in the literature that the increased charge capacity is only normalized against the mass of electrode materials, but the mass of the redox species in the electrolyte is ignored, which may also be misleading. In order for the comparisons of charge storage capacity and/or energy content to be done fairly and properly, it is important to also consider the electrolyte additive as being an active part of the device. In a recent report, the redox electrolyte was indeed considered as an active part of the SC, but the increased charge storage capacity was still evaluated incorrectly as capacitance.27 The consideration of the mass of redox species becomes even more important if SCs containing redox additives are to be scaled up (for instance in stacked cells) for practical applications. In such devices, omission of the redox additives as active materials could lead to overestimations of the specific charge and energy storage capacity of the device.
Inclusion of the mass of redox electrolyte into analysis of any mass normalized property of the SC device is not yet standardized, but may be practiced through the following considerations. It is obvious that only a proportion of the redox species in the electrolyte will participate in the electrode charging and discharging processes. However, it should not be taken as an excuse to only consider the proportion contributing to the increased charge capacity. This is because without those redox species in the bulk electrolyte, it may not be possible to load these species into the porous structure of the electrode. The total mass of redox species can be easily derived from the volume and concentration of the redox species in the electrolyte. Obviously, if the redox species is ionic, the mass of the charge balancing counter ions should also be considered.
On the other hand, even though the redox species may only be involved in one of the two electrodes, the electrolyte is always shared between the positive and negative electrodes. Further, unlike electrode capacitance which can be different between the positive and negative electrodes, the amount of charge passing through the SC is always the same for each of the two electrodes. Therefore, it should be acceptable if a half of the total mass of redox species in the electrolyte is included in assessing the specific charge capacity of one electrode.
The above considerations should be valid for a redox additive electrolyte in which the redox species are additional to an inert electrolyte and contribute little to the conduction of ionic current. However, the situation would become more complex for a redox active electrolyte in which the redox species may also function as the ionic charge carriers. In such a case, if the formula mass of the redox species or ion is comparable to that of the ion in a conventional and inert electrolyte, it may not be necessary to consider the mass of the redox electrolyte. On the other hand, if the formula mass of the redox species is very large, consideration may become necessary.
Mechanisms of Charge Storage in Supercapacitors Containing Redox Electrolytes
The reactions of various redox active species on the surfaces of activated carbon (AC), functionalized carbon, or materials that display pseudocapacitance are the basis for the applications of redox electrolytes in SCs. Here, redox species refer to those capable of undergoing electrochemical reactions with good charging and discharging performances. In general, the charge storage mechanisms in SCs with redox electrolytes could be described with respect to whether the SC without the redox electrolyte displays either (or both) of the two charge storage mechanisms of SCs, i.e. EDL capacitance and pseudocapacitance. The mechanism of charge storage enhanced by introducing a redox electrolyte into the supercapacitor is complex and may be explained by the illustration in Figure 3.
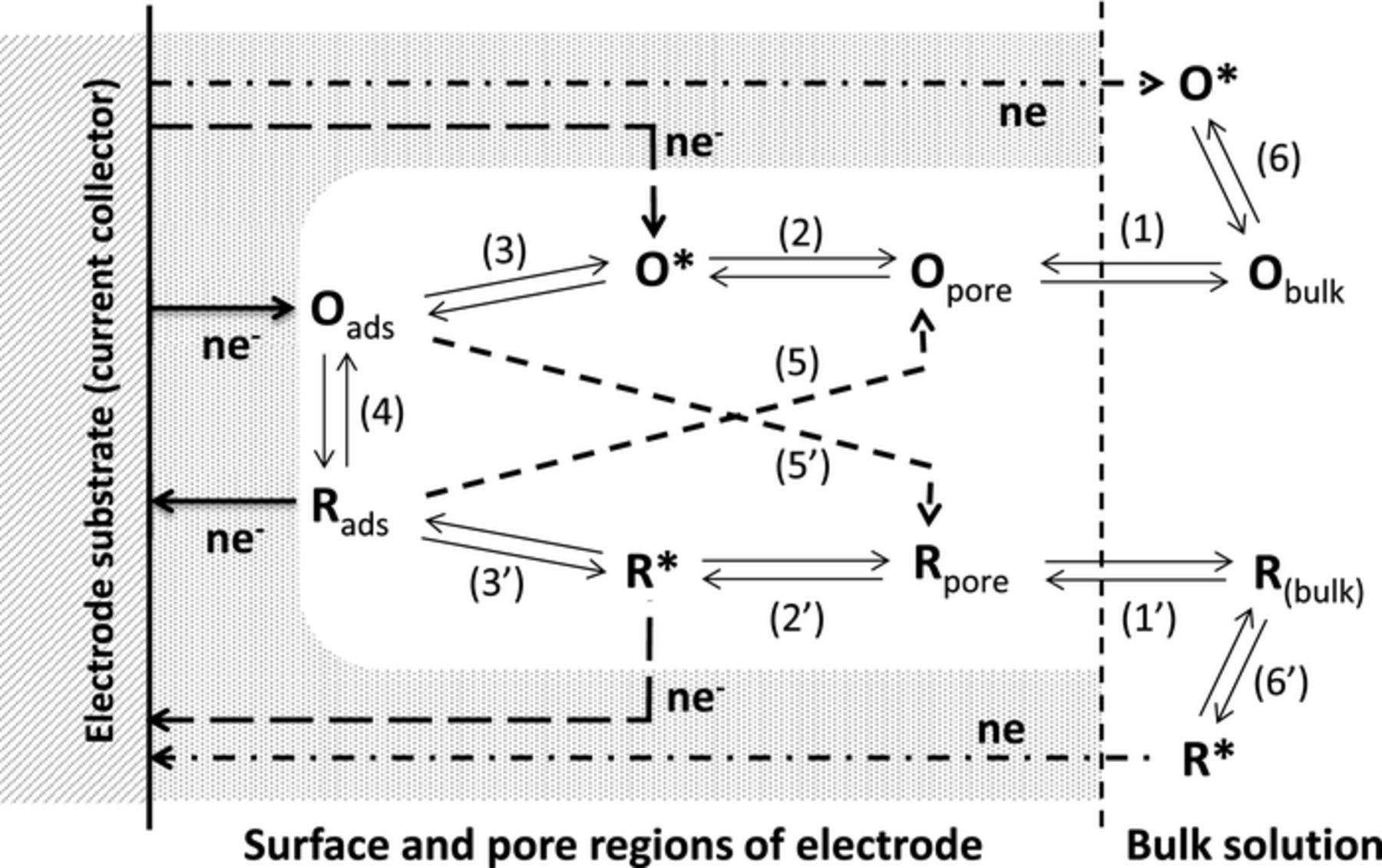
Figure 3. Illustration of charge storage mechanisms in the porous carbon electrode of a supercapacitor with a redox electrolyte.
In Figure 3, O and R are the oxidized and reduced states of the redox species in the electrolyte. To participate in the charge storage process, the redox species in the bulk solution (either Obulk or Rbulk) need to firstly enter the pores of the electrode, accompanied likely by process (1) or (1') which represents the equilibria of de-solvation and solvation of the species when entering or exiting the pores. Before electron transfer reactions, redox species in the pore (Opore and Rpore) must reach the transition state (O* or R*) via (2) or (2') as in a conventional electrode reaction. Processes (3) and (3') show the conversion from the transition states to the adsorbed states (Oads and Rads) which may not be present in a conventional electrode reaction, but is a necessity for application in supercapacitors. Electron transfer via (4) causes the conversion between the adsorbed redox species on the internal and external surfaces of the electrode, which is directly responsible for the enhanced charge storage capacity.
Note that once in the transition state, the redox species (O* and R*) may also undergo electron transfer reaction via (5) or (5') without invoking adsorption. Further, it is possible that upon electron transfer, the adsorbed species (Oads or Rads) is converted via (5) or (5') to a soluble product (Opore or Rpore) that can diffuse through the pore and enter the bulk electrolyte. In both cases, if the electron transfer reaction corresponds to the charging process, the soluble product means that charge storage would be short-lived or not occur because the product of the charging process may diffuse into the bulk electrolyte, and is not fully recoverable electrochemically. On the other hand, the electron transfer reaction can take place not only inside the pores, but also on the outer surface of the electrode via (6) or (6'). However, because the outer surface area is usually much smaller than the internal surface area of a porous electrode, the contribution from either (6) or (6') should be insignificant.
The above analysis and discussion warrant some conclusions. To enable a meaningful application of redox electrolytes in supercapacitors, the electrode material must be sufficiently porous and be able to retain the redox species, particularly the product from the charging reaction, inside the pores of the electrode. Adsorption of the redox species on the internal and external surfaces of the porous electrode is a good way toward retention. However, there are other retention mechanisms, such as entrapment of the redox species in the pores of the electrode, or simply deposition of only the product from the charging reaction.
There is another important aspect worth mentioning. A redox reaction is determined by the thermodynamic stability of the redox species with respect to the electrode potential and the supporting electrolyte. When involving simple or complex metal ions, redox reactions may be identified from the Pourbaix diagram.16 However, thermodynamic data do not usually consider the influence of the environment in the pores on the redox reaction, such as de-solvation and adsorption. Therefore, the thermodynamically predicted electrode potential for a redox reaction may be altered when the reaction occurs inside the porous structure of the electrode. Depending on the surface properties of the electrode material, redox species could either be electro-adsorbed in the electrode material (porous carbon electrodes),6,7 or interact with the reactive edge sites and functional groups on the surface of the carbon material for example carbonyl groups (surface functionalized carbon materials).3,4,26 Also, if the electrode material displays pseudocapacitance or non-capacitive activity, the electron transfer reactions occurring in the lattice of the electrode could be shuttled by the redox species of the electrolyte.14,30
In order for the SC with a redox electrolyte to display satisfactory stability in repeated charging-discharging cycles, it is important for the redox specie to show fast and reversible electrochemical activity in the supporting electrolyte. It could be ensured by matching the concentration of the redox species to the supporting electrolyte. For example, the charge storage obtained from the redox activity of the Fe(CN)63−/Fe(CN)64− couple in KOH, has been shown to be highly dependent on the concentrations of both the added K3Fe(CN)6 and K4Fe(CN)6, and the supporting electrolyte KOH.13,30 The physicochemical properties of the redox species (e.g. ion-solvent interactions) and their interrelationship to charge storage properties (e.g. adsorption/desorption) have also been described.9 Furthermore, the effect of the substituent location of the hydroxyl groups on the electrochemical performance of redox electrolytes of dihydrobenzenes has been explained.16 As expected, the wettability of the electrolyte on the electrode surface is very important in ensuring effective electro-sorption and transport of the redox species from the electrolytes. The roles of the pores of the carbon electrode in promoting the charge storage of the redox species has also been highlighted, because the various redox species from the electrolyte require optimal pore sizes to ensure effective electro- or chemisorption. For instance, when comparing two different AC electrodes, favorable charge storage behavior of the AC ∣ iodide interface was observed on that with a higher total surface area (1470 vs. 676 m2/g) and larger average pore size (1.36 vs. 1.06 nm).5,6 In a separate work, AC samples with larger pores (2–3 nm) were claimed to exhibit much higher specific capacitance in a mixed KI and H2SO4 solution,9 although it is not yet clear what may happen if these two parameters are further varied. Likewise, the nanostructure of the carbon materials which determines important characteristics such as the location of active sites, has been linked to the charge storage capabilities offered by the redox species.3 As in all electrochemical devices, improved electron and ion transport in electrode materials, and reduced charge transfer resistance at electrode/electrolyte interfaces are also desirable.31 Practically, such property improvements could be achieved from specially prepared (e.g. doping) carbon materials6 or making the carbon into a monolithic binder-free coating on the electrode.29,31 Thus, the mechanisms of charge storage accrued from redox electrolytes in SCs could be described based on the following three broad categories in relation with the retention of the redox species, particularly via electro- or chemisorption.
Charge storage enhanced by electro-sorption of anionic species on the electrode surface
Charge storage capacity can be enhanced by adjusting the pore volume and size distribution, surface area and surface functional groups in the electrode materials that can assist electro-sorption of redox species. This mechanism has been identified to change the properties of an activated carbon based positive electrode by giving it charge storage capacity that are highly voltage dependent. Typical examples include "(+) AC ∣ KI", (+) AC ∣ Br−-ionic liquid, and "(+) AC ∣ hydroquinone-H2SO4".5–7,29 (Here and below, AC stands for activated carbon.)
With M representing an alkali metal, the "(+) AC ∣ MI" interface was claimed to display a high "capacitance" value or, more accurately, the ΔQ/ΔU ratio of over 1000 F/g. This was linked to the formation and subsequent redox reactions of the polyiodide anions, In−, at the positive electrode whose characteristics were strongly potential dependent.5,6 Specially, much of the charge was stored in a narrow potential range, ΔE = 0.17 V, leading to the high ΔQ/ΔU ratio, or the "claimed capacitance". Nevertheless, the mechanism for the enhanced charge capacity is worthy of investigation. The unique aspect of electrochemical oxidation of iodide and other halide anions is that the reactant, I−, and the product, In−, are both negatively charged. This means that both can function as the charge balancing anions in the EDL, and hence electrostatically retained in the positively charged porous electrode. Thus, the increased charge capacity is a result of electro-sorption of the anionic reactant and product in the porous AC positive electrode.
When this positive electrode was incorporated into a prototype two electrode device, "(+) AC ∣ MI ∣ AC (–)", the negative electrode showed the expected EDL behavior in a wide negative potential window, which dominated the device behavior to be satisfactorily capacitive.6 Accordingly, the ΔQ/ΔU ratio measured from this device can be considered as capacitance. Interestingly, the device capacitance was found to be 203 F/g for NaI and 220 F/g for RbI. Because the same AC negative electrode was used in both cases, the larger device capacitance indicates a greater ΔQ/ΔU ratio of the positive AC electrode in the RbI electrolyte, considering that Cdevice = C+C–/(C+ + C–) = C–/(1 + C–/C+). This is understandable because Rb+ is much larger than Na+ in size, and thus has a smaller polarization power. This makes the I− and In− ions more likely to be attracted to the positive electrode surface than to the cation in the RbI electrolyte in comparison with what may occur in the NaI electrolyte.
In line with this understanding, the ΔQ/ΔU ratio (or the "claimed capacitance") of the positive AC porous electrode was observed to be 300 F/g in LiI, 492 F/g in NaI, 1078 F/g in KI, up to 2272 in RbI, and 373 F/g in CsI. Here, the exceptional behavior of CsI was explained by the structure of the polyiodide ions in association with different cations. The linear structures of the polyiodide anions, In−, with Li+, Na+, K+, or Rb+ as the counter-ion were shown to be the reason for their easy electro-sorption. However, the non-linear or curved structure of the polyiodide anion with Ce+ was claimed to have a great effect on its mobility.6
In general, 2-electrode tests of SCs containing 1.0 M of these iodides reveals capacitance values of 178 F/g for LiI, 203 F/g for NaI, 220 F/g for RbI, and 234 F/g for KI and CsI.6 Of these redox active iodide salts, KI and NaI are considered more favorable from environmental considerations.4,16 Further, Raman spectroscopy was used to analyze the charge storage mechanism in the carbon/iodide interface. The findings show that the charging and discharging processes involve the recombination of I3−/I−, I2/I−, I2/I3− redox couples toward I5−. Such redox chemistry which led to the increased charge storage capacity in a narrow potential range, together with possible C-I−n interactions, has thus a marked effect on the overall charge storage capacity of SCs.16
In addition to aqueous electrolytes, an ionic liquid was used to accommodate the redox chemistry of bromide ion at an AC electrode.29 The organic salt, 1-ethyl-3-methylimidazolium bromide that was dissolved in 1-ethyl-3-methylimidazolium tetrafluoroborate, was highly stable, offering over 10,000 charging-discharging cycles. Interestingly, although addition of the bromide ion in the ionic liquid almost doubled the charge storage capacity of the cell, it also caused the discharge rate capability of the cells to decline faster.
Charge storage enhanced by chemisorption of transition metal cations
In principle, cations are not favorable for electro-sorption on a positive electrode (and the same is true for anions and a negative electrode). However, it is possible to functionalize the electrode surface with cation affinitive groups that in turn can chemically adsorb cations. It is common knowledge that carbon atoms at the so called edge sites are more reactive than those inside the solid carbon. Specifically, these edge sites can readily be oxidized (and also stabilized) to form various oxygen containing groups, such as –OH and -COOH, that can be fully or partially deprotonated and hence negatively charged. In addition, even without deprotonation, the very electronegative oxygen (and also nitrogen, sulfur and halogen) atoms can still offer some affinity to metal cations. These surface oxy-groups can then be utilized to chemically adsorb metal cations and enable surface redox reactions. Examples of this mechanism include "(+) AC ∣ CuSO4-FeSO4-H2SO4",3 "(+) functionalized carbon surface ∣ VOSO4",4 and "(+) functionalized carbon surface ∣ CuCl2-HNO3",26 and these are further discussed below.
Practically, the increased charge storage capacity of AC electrodes was demonstrated in the aqueous solutions of mixed FeSO4, CuSO4 and H2SO4.3 It was observed that the charge storage was greatly dependent on the porosity of the AC electrode and also the concentration of the Fe2+ and Cu2+ ions. This can be explained by the accessibility of the Fe2+ and Cu2+ ions to the oxy-groups on the internal surfaces of the porous AC electrode. When the additive in the solution was the Cu2+ ion only, the reversibility of the electrode reaction was poor, owing to the irreversible deposition of Cu on the carbon matrix. However, upon the addition of Fe2+, the re-oxidation and desorption of Cu on the porous carbon matrix was enhanced by the formation of the adsorbed complex according to reactions 5 and 6, thus increasing the cyclability of the charging and discharging processes.3
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn5.jpg)
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn6.jpg)
In reactions 5, the forward reaction corresponds to charging, and the reverse reaction for discharging.3 Along these lines, it was shown that when CuCl2 was used as the redox additive in a solution of HNO3, a great increase in charge storage was achieved even at low concentrations of CuCl2. This study also revealed good charging-discharging reversibility at high current loads. The large charge storage capacity observed in the mixture of CuCl2 and HNO3 was explained by the adsorption of Cu2+ on the surface carbonyl groups to form a reversible surface layer of CuCl2 on the surface of the porous carbon microspheres.26 Also an optimal potential range was selected in order to ensure a reversible electro-sorption and chemisorption of the Cu2+ ion, and to also prevent the irreversible deposition of Cu. In fact, the increase in charge storage capacity by the Cu2+/Cu+ couple, which was achieved through an addition of Fe2+ to enable the formation of the Fe2+/Fe3+ redox couple, had benefited from the conjoint (synergistic) reaction of the carbonyl functional group on the carbon surface and the highly electroactive Cl− in the CuCl2 redox additive.
Another recent work has described the role of surface functionality in enhancing charge storage via the faradaic reactions on the surface.4 In this investigation, the transformation of the redox vanadyl at the negative carbon electrode | VOSO4 interface was shown to be supported by the carbon functionality. That is that the VO2+ ions from the bulk of the electrolyte can be transported to the electrode | electrolyte interface where there is a proton-exchange with the oxy-functional groups. Furthermore, there could be a transfer of electrons from VO2+ to the electrode along the –COOVO+ bond between the oxy-group and VO2+ ion. Unfortunately, the VO2+ can diffuse back to the bulk electrolyte. The AC electrode in the VOSO4 solution displayed a ΔQ/ΔE ratio or "claimed capacitance" value of ca. 670 F/g. (ΔE is the potential range.) It was also used as the negative electrode in the so called redox conjugated supercapacitor, "(+) AC ∣ 1.0 M KI ∣∣ 1 M VOSO4 ∣ AC (–)" where the separator (denoted by ∣∣) was a Nafion membrane.4
Charge storage enhanced by electron transfer between the redox electrolyte and the electrode material
When the electrode material is redox active, either capacitor-like (i.e. pseudocapacitive) or battery-like, such as MnO2,8 NiO,14 conducting polymers e.g. polyaniline,27 Co-Al layered double hydroxide,30 and chalcogenides e.g. CuS,32 there can be direct electron transfer between the electrode material and the redox species in the electrolyte. This mechanism has been generally described to be an enhancement in the charge storage capacity because the redox reactions in the electrolyte may help electron transfer in the redox active electrode. The relevant literature attributed the enhancement to pseudocapacitance, but it merely increased the charge stored at the interface. (In principle, pseudocapacitive is redox active, but redox active is not necessarily pseudocapacitive.) A typical example is "(+) NiO ∣ Fe(CN)63−-Fe(CN)64−-KOH"14 in which the material on the electrode and the redox species in the electrolyte can simultaneously undergo the redox reactions below at the electrode | electrolyte interface.
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn7.jpg)
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn8.jpg)
In general, the charge storage capacity of a porous and redox active electrode material in a redox electrolyte can be described by the simple expression in Equation 9 below,
![Equation ([9])](https://content.cld.iop.org/journals/1945-7111/162/5/A5054/revision1/jes_162_5_A5054eqn9.jpg)
where Qt is the total charge stored in the electrode, Qdl represents the charge stored in the EDL, Qelectrode and Qelectrolyte correspond to charge stored due to redox reactions in the solid electrode and in the electrolyte, respectively.
Various experimental investigations have generally shown that depending on the electrode material (and the electrode potential), Qt could be significantly enhanced by Qelectrolyte. In principle, when measured over a given potential range, ΔE, the Qdl/ΔE ratio is independent of the applied electrode potential, E. Except for pseudocapacitive electrodes, the Qelectrode/ΔE and Qelectrolyte/ΔE ratios are firstly determined by the oxidation (or reduction) potential of the redox species as a function of the experimental conditions, including pH, composition and temperature of electrolyte and also the electrode material. More importantly, the Qelectrode/ΔE and Qelectrolyte/ΔE ratios change significantly with the applied potential E. Furthermore, if the electrode material is not redox active, Qelectrolyte is usually greater than Qdl, but the transfer of Qelectrolyte occurs in a very narrow potential width around the current peak potential, Ep on the CV. In this case, Qt is dominated by Qelectrolyte and behaves like that in a battery.5,7 However, on a redox active electrode, Qelectrode is significant compared with Qelectrolyte and Qdl. If Qelectrode and Qelectrolyte are transferred independently from each other, but occur in the same potential range, the CVs would show features (e.g. current peaks) that could be independently indexed as relating to the electrode and the electrolyte. In other words it could be assumed that both charge storage mechanisms occur concurrently.14,28,32 A more interesting mechanism would be that the transfers of Qelectrode and Qelectrolyte are coupled, i.e. there is direct electron transfer between the redox species in the electrode and in the electrolyte. There are literature claims of coupled transfer of Qelectrode and Qelectrolyte, but the reported CVs seem not to agree with the claims.14,30 It is also worth mentioning that some reports on this topic have associated the Q/ΔE ratio to pseudocapacitance, although the published CVs and GCDs actually present typical battery-like features.
To avoid further confusion, it is proposed that due to the contribution to charge storage from the redox electrolyte being very different from the capacitance of the electrode, SCs containing redox electrolytes should be considered and explained in distinctive terms from conventional SCs. Additionally, further work should be done on trying to elucidate the relationships between the physicochemical observations and electrical response of these devices, rather than describing non-capacitance behavior by capacitance or pseudocapacitance.
Conclusions
Combination of appropriate redox electrolytes and porous electrodes has been shown to be an effective means for enhancing the charge and possibly energy storage capacities of supercapacitors. This effect depends strongly on whether the redox species, particularly the product from the charging reactions, can be attached to the internal and external surfaces of the porous electrode, for example, via various forms of adsorption and entrapment. Other factors also affect the performance, including the electrode material properties, applied potential, electrolyte pH, and temperature. In most reported cases, the redox electrolyte contributes to the increased charge capacity of the device in a battery-like manner which corresponds to peak shaped cyclic voltammograms and non-linear galvanostatic charge-discharge plots. In other words, the "charge to voltage ratio" of a supercapacitor with redox electrolytes (or the charge to potential ratio of an electrode in a redox electrolyte) is significantly dependent on the applied voltage. However, this fact has been ignored by many authors who simply regarded the charge to voltage ratio (or the charge to potential ratio) as capacitance. In doing so, misleading energy capacity values are often reported in the related literature. A reconsideration of the specific energy according to a non-linear galvanostatic charging-discharging plot reported in a high profile publication has revealed the claimed specific energy to be 5.3 times overestimated. Therefore, the concept of capacitance should not be used to describe the overall electrical response exhibited by many of these devices. Instead, performance evaluation and comparison between supercapacitors with and without added redox electrolyte should be based on energy capacity and power capability, and should also consider the mass of the added redox species in addition to the mass of the electrode materials. Disregarding the misinterpretation of capacitance, the literature on redox electrolytes in supercapacitors still contains valuable and important findings from different approaches to the enhanced charge storage capacity. Electro-sorption of ionic species on the electrode, such as the I−/I3− and Br−/Br3− couples on a positive electrode, is simple and often effective, whilst chemisorption depends on proper electrode design and selection of the redox species. The interesting concept of coupling electron transfer reactions in the solid coating on the electrode with that in the liquid electrolyte at the electrode | electrolyte interface is also proposed and demonstrated for enhancing and improving charge storage. These innovative approaches and significant outcomes make the combination of redox electrolytes with supercapacitors a promising direction for development of new electrochemical energy storage technologies. Obviously, the immediate effort should be to study the correlation between the properties of the redox electrolytes and the porous structures and surface functionalities of the electrode so that better understanding of the charge storage mechanisms can be achieved and used to guide research for further improvement.
Acknowledgments
The authors thank the Ningbo Municipal Government for financial support via the 3315 Plan, and also the International Doctoral Innovation Centre of the University of Nottingham Ningbo China for awarding a PhD Scholarship to B. A.