Abstract
The chemical mechanical planarization (CMP) removal rate (RR) of germanium using potassium periodate as oxidizer with fumed silica based slurries have been investigated. Static etch rate (ER) of germanium was performed as a function of solution temperature, pH and concentration of potassium periodate. Change in the enthalpy (ΔHact) and entropy (ΔSact) of activation for the Ge in the proposed oxidizer was found to be 11.029 kJ/mol and −271.06 J/mol.K, respectively. The values suggest that the dissolution of germanium in the proposed oxidizer is endothermic in nature and the etching is controlled by activation complex. The effect of slurry pH, KIO4 concentration, turntable speed and down pressure on Ge RR were studied. ER and RR of Ge was found to increase with pH. In the absence of oxidizer, polishing of germanium with 3 wt% fumed silica showed almost zero removal. With the addition of 1 wt% KIO4 + 0.1M KOH to 3 wt% fumed silica slurry, significant increase in the RR of Ge was observed for complete range of pH. Ge is oxidized to form germanium dioxide in the presence of KIO4, which on subsequent oxidation resulted in the formation of soluble species.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Chemical mechanical planarization process is an crucial step for successful integration of integrated circuits (IC) using complementary metal oxide semiconductor (CMOS) technology. Since 1947, silicon (Si) is dominating the semiconductor industry.1 However, with the continuous shrinking size of the device, there is a potential need to replace silicon with higher mobility channel materials. Germanium (Ge) is promising candidate, with lower bandgap and three times higher electron and hole mobility as that of Si, to manufacture the next generation advanced, fast and miniaturized devices in microelectronics industry.2 Ge is considered for integrating p-type metal oxide semiconductor (PMOS) channel for future metal oxide semiconductor field effect transistor (MOSFET) devices.3
Chemical mechanical planarization step is of great importance to take away the excess material from the surface during device integration to provide a flat planarized surface.4 For Ge CMP, very few research articles are available in public literature. Peddeti et al.5 reported H2O2 based colloidal silica slurry to polish germanium. Ge removal rate was found to be maximum at pH 12. Zeta potential of silica particle and Ge surface were measured as a function of pH. Strong repulsion exists between the particles and surface at pH 6 and 10. Higher silica concentration of 3 wt% silica with oxidizer was used to enhance the Ge removal rate. The importance of ionic strength of silica-based slurry containing H2O2 oxidizer with KCl, K2SO4, KNO3 and NaNO3 electrolytes was studied by Matovu et al.6 The bond strength reported for Ge-Ge bond and Ge-O bond is 37.6 kcal/mol and 86 kcal/mol, respectively. The material removal reported was due to the rupture of weaker Ge-Ge bond. Hsu et al.7 used Ge removal rate enhancers such as methylpyridine compounds, in addition to H2O2. Out of various methylpyridine derivatives, 4-methylpyridine showed highest Ge removal of 3,478 Å /min. Tooth lock removal mechanism was reported by Gupta et al.8 for Ge polishing in the absence of oxidizer. Maximum removal rate of 160 nm/min was reported for germanium with rutile titania based slurry at pH 3. Higher material removal was attributed to structural similarity between germanium dioxide and the rutile titania, which resulted in the formation of Ti-O-Ge bond. In alkaline region, pH≥11, germanium dioxide was found to change in amorphous form, which suppresses tooth lock between germanium dioxide and the rutile titania and resulted in lower removal rate. Reshma et al.9 proposed oxone as oxidizer for polishing Ge using fumed silica based slurry. In the presence and absence of 3 wt% fumed silica, higher material removal was reported in alkaline region as compared to acidic and neutral region. Maximum removal of ∼284 nm/min was reported at pH 11 with 1 wt% oxone in 3 wt% fumed silica based slurry.
The presence of sodium salts is not preferred in IC chip fabrication.10 Few literature suggests KIO4 as oxidizer to polish ruthenium and copper.11–14 H2O2 is widely used oxidizer for polishing Ge. But the hydrogen peroxide is a thermodynamically unstable compound, which decomposes to give oxygen and water. Moreover, hydrogen peroxide can reduce the pot life of the CMP slurry. Hence, there is always a need for an alternate oxidizer to overcome the above challenges.15–17 This is the first report proposing KIO4 as oxidizer to polish Ge using fumed silica based slurry.
Materials and Methods
Ge disk with 99.999% purity, 1 in. diameter and 0.5 in. tall, was procured from RWMM (Rare World Metals Mint, USA). Fumed silica procured from Cabot India (CAB-O-SIL M-5) with an average particle length of 0.2–0.3 μm18 and KIO4 purchased from Pallav Chemicals & Solvents Pvt. Ltd, India were used for the slurry formulation. Prior to CMP experiments, dissolution study was conducted to determine the Ge ER and to analyze the impact of chemical action on the material removal. Etchant solution was prepared using oxidizer, KIO4, which has very low solubility of 0.018 moles/liter at 20°C.11 According to literature,11,13 addition of KOH increases the solubility of KIO4 in water. Synergetic effect of both the compounds results in the formation of a soluble dimesoperiodate complex, which is an effective oxidizer with similar properties as of KIO4.11 Unless mentioned otherwise, KOH concentration of 0.1 M was used with all the etchant solutions and slurry for the CMP experiments. Slurry pH was adjusted using HNO3. Benchtop chemical mechanical polisher, purchased from Struers, Denmark (LaboPol-5/LaboForce-3), was used for performing the polishing experiments. The detailed experimental procedure for polishing Ge is reported elsewhere.8,9,19 Zeta potential and effective diameter of the particles in the slurry with and without oxidizer were investigated over the pH using NanoBrook 90 Plus PALS zeta potential-cum-particle size analyser. Surface roughness of polished and unpolished samples were evaluated using 3D optical microscope, Bruker, Contour GT-K0, Germany. Etch rate and Ge removal rate was calculated using difference in intial and final weight of Ge. As the density and polishing area are available the weight difference can be converted to film thickness. Ge ER and RR were found by dividing film thickness by polishing time.20 Ge removal using the proposed slurry was tested for Prestonian behavior, RR(nm/min) = APv where A is Preston coefficient, P is the down pressure and v is the table speed.4
Results and Discussion
Etching experiments
Effect of KIO4 concentration on Ge ER
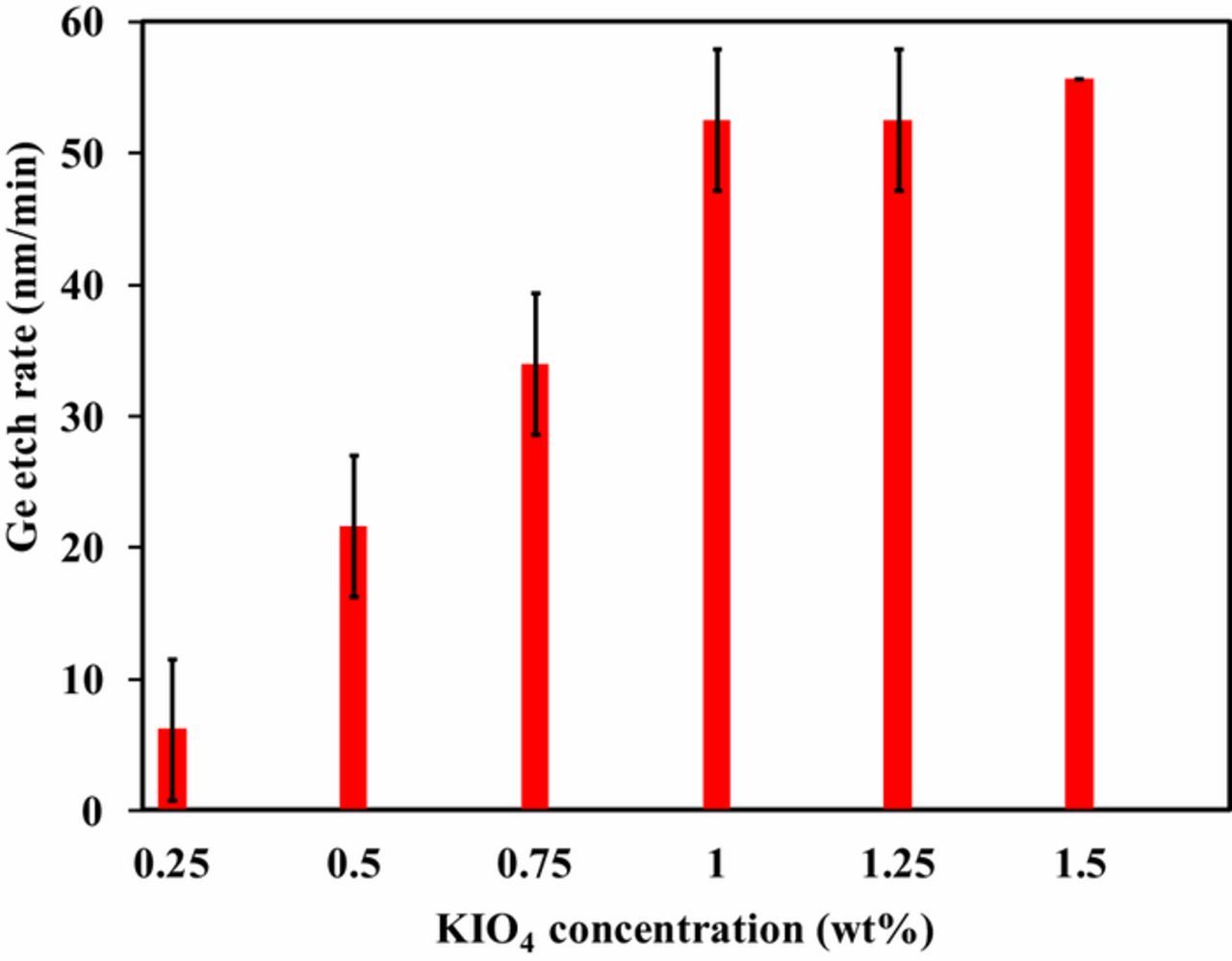
Etching experiments on Ge were performed by varying KIO4 concentration ranging between 0.25 wt% to 1.5 wt%. The pH of the solutions with different KIO4 concentration were adjusted to 11 using HNO3. It can be depicted from Figure 1 that ER significantly depends on KIO4 concentration. Ge ER was increased to ∼52 nm/min from ∼6 nm/min with KIO4 concentration increased to 1 wt% from 0.25 wt%, respectively. This might be due to increased oxidation of Ge surface with IO-4ions and also due to the formation of soluble dimesoperiodate complex.11,13 Further increase beyond 1 wt% of KIO4 concentration (1.25 wt% and 1.5wt%), as seen in Figure 1, Ge ER was found to be saturated. The observed saturation can be attributed to the limitated Ge surface available for oxidation.21 KIO4 concentration of 1 wt% with 0.1 M KOH was selected for further study, as there was no remarkable increase in Ge ER above 1 wt% KIO4 concentration. Ge ER was zero for the pH adjusted distilled water in the absence of KIO4.9
Figure 1. Effect of KIO4 concentration on Ge ER at pH 11.
Effect of pH on Ge ER
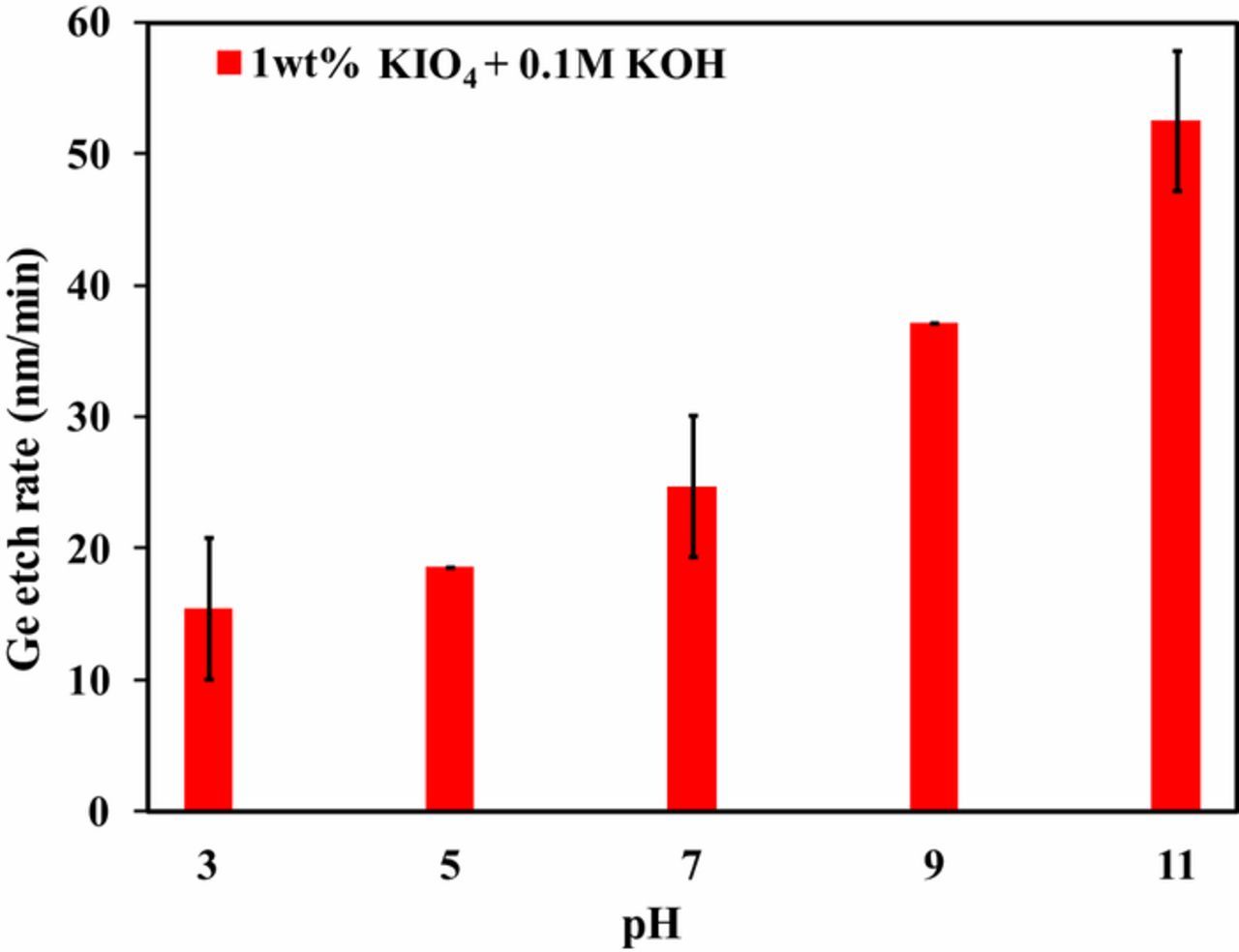
At fixed KIO4 concentration, the effect of solution pH on Ge ER was studied. KIO4 concentration of 1 wt% was used by adjusting the pH using HNO3 for this study. An increase in Ge ER with increasing solution pH can be seen in Figure 2. Ge ER was enhanced to ∼52 nm/min from ∼15 nm/min, when pH of the solution was increased to pH 11 from pH 3, respectively. Increase in ER while moving toward alkaline region might be due to the oxidation of Ge surface with IO4− anions formed from dissociation of KIO4, as shown in Eq. 1 and with *OH radicals, formed from the dissociation of KOH, as shown in Eq. 2. The observed ER is in line with the reported literature of H2O2 system.5,6
![Equation ([1])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0001.gif)
![Equation ([2])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0002.gif)
Figure 2. Effect of pH on Ge ER using 1 wt% KIO4 solution.
Effect of temperature on ER
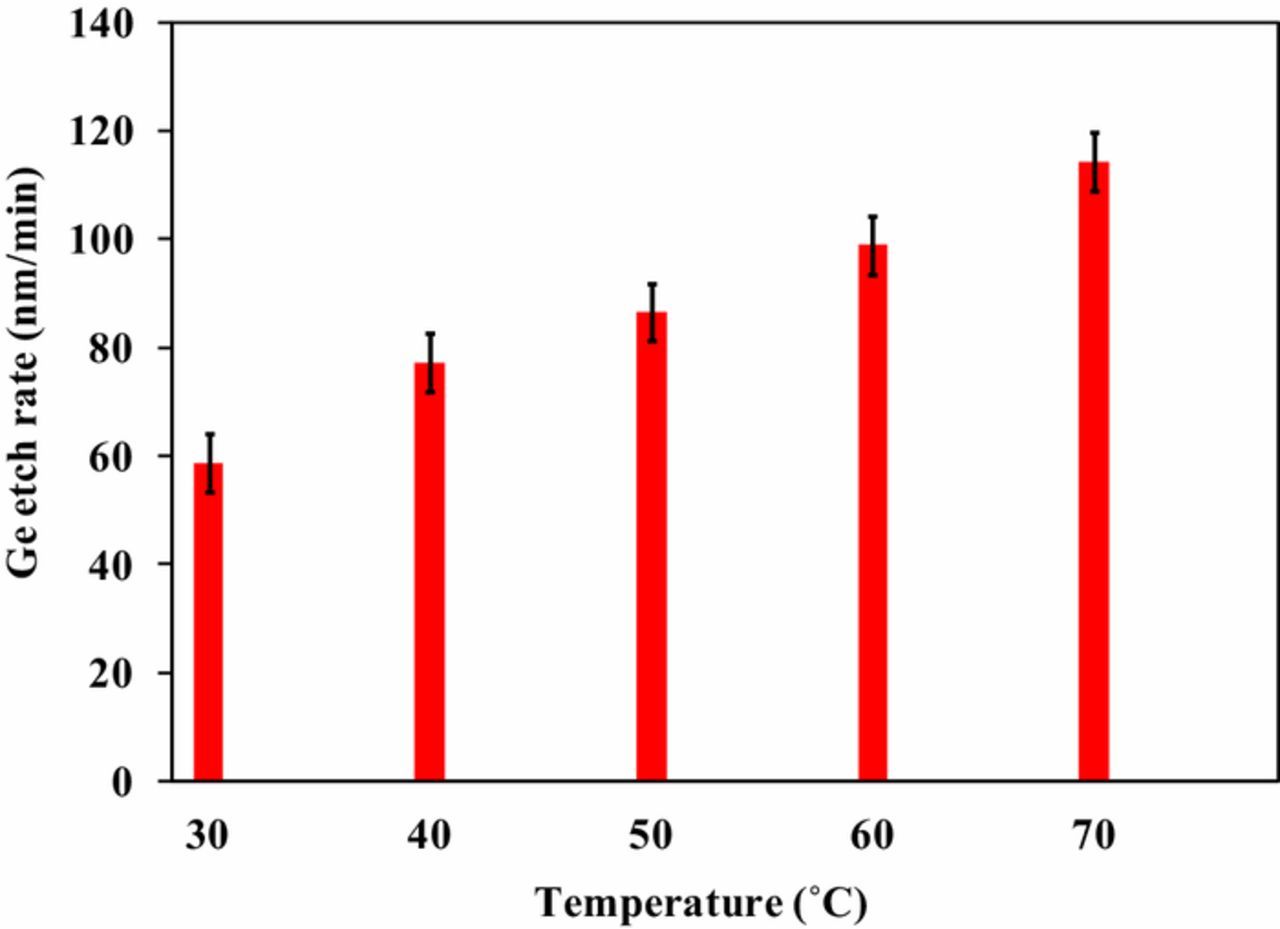
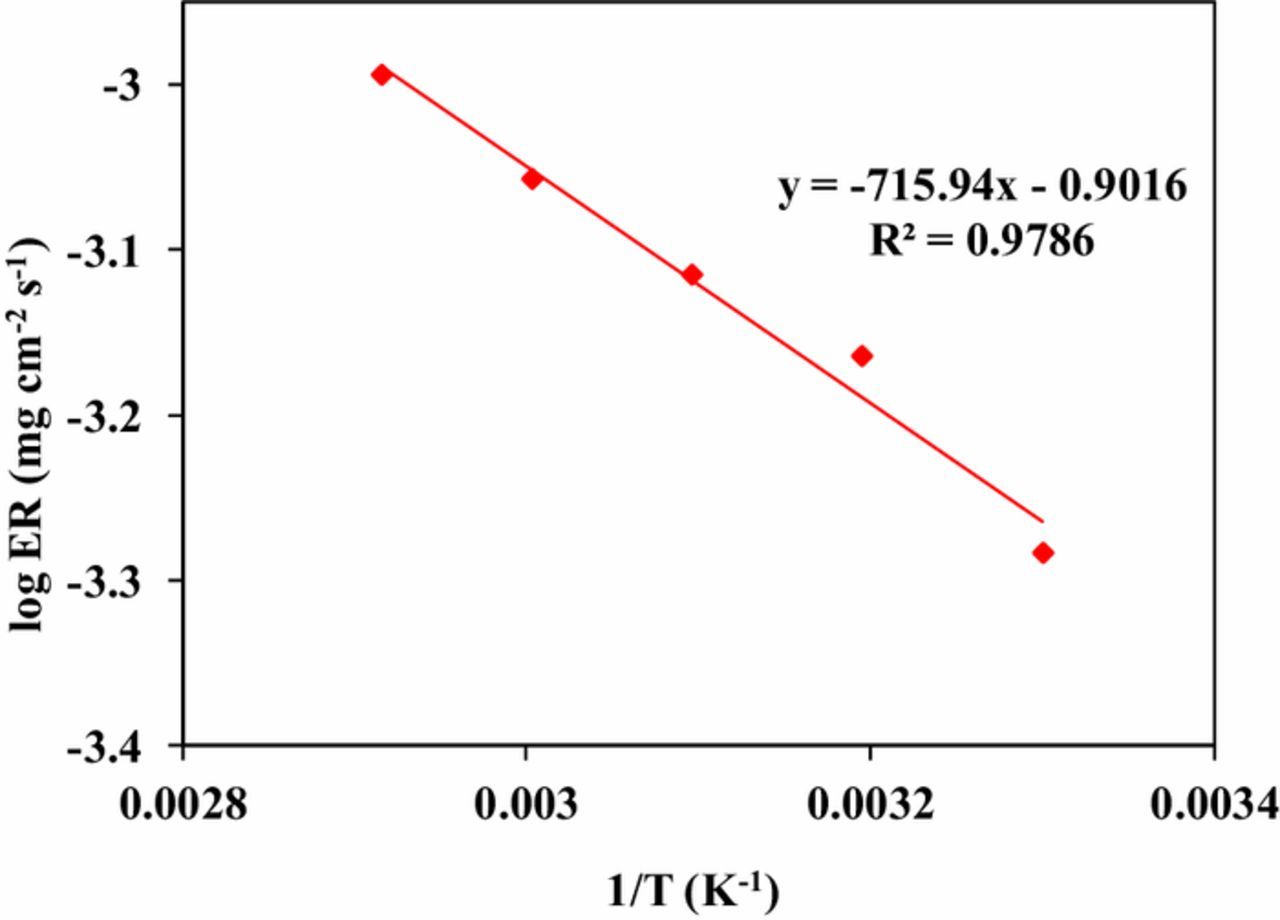
The relation between solution temperature and ER was studied in 1wt% KIO4 with 0.1 M KOH solution. The pH of etchant solution was maintained at 11. A temperature range 30°C–70°C was selected for dissolution study. The results are shown in Figure 3. The etch rate was found to increase with increase in temperature. At 30°C the ER was ∼58 nm/min, which enhanced to ∼114 nm/min at 70°C. Activation energy for Ge dissolution in potassium periodate solution was calculated using Arrhenius equation, as shown in Eq. 3. Where, A is the Arrhenius pre-exponential factor, Ea is the activation energy, R is the universal gas constant (8.314 J/mol.K) and T is the absolute temperature (K).22 Figure 4 shows the semi-logarithm plot of etch rate and 1/T. The activation energy was found to be 13.7 kJ/ mol.
![Equation ([3])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0003.gif)
![Equation ([4])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0004.gif)
Figure 3. Effect of solution temperature on ER at pH11.
Figure 4. Plots of log (ER) vs. 1/T for germanium in potassium periodate solution at pH11.
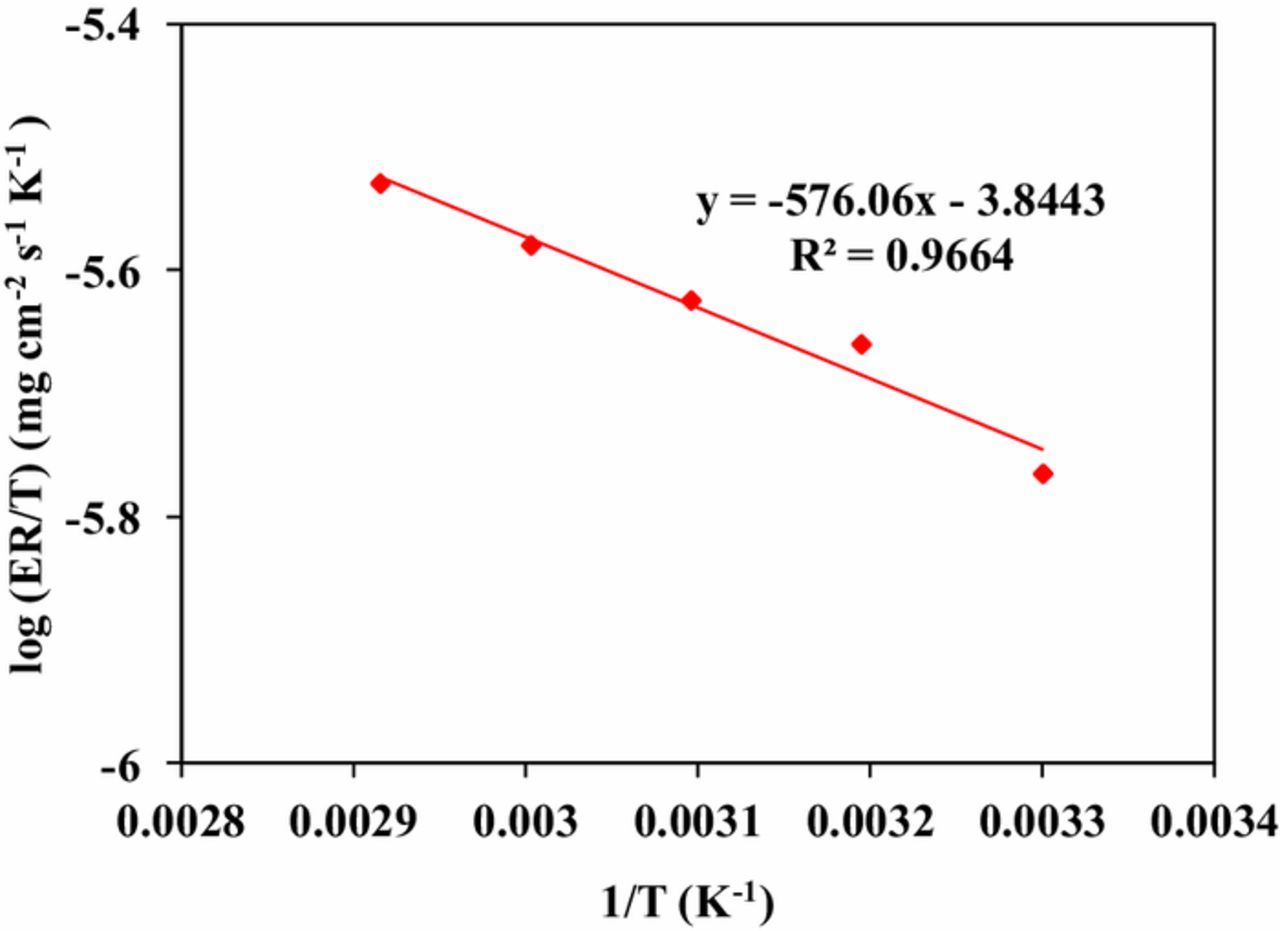
In order to determine the thermodynamic property, the transition state equation was applied to evaluate enthalpy and entropy of activation, as shown in Eq. 4. Where, ΔHact is the enthalpy of activation, ΔSact is the entropy of activation, R is the universal gas constant, h is Planck's constant (6.626176 × 10−34 J-s) and N is Avogadro's number (6.02252 × 1023 mol−1). Figure 5 shows the plot of log (ER/T) versus 1/T. It can be seen that a straight line is obtained with a slope of (-ΔHact /2.303R) and an intercept of [log (R/Nh) + (ΔSact /2.303R)]. The calculated values of ΔHact and ΔSact are 11.029 kJ/mol and −271.06 J/mol.K. The calculated value ΔHact is positive, which indicates that the dissolution process of germanium in potassium periodate solution is endothermic in nature. A negative ΔSact value shows that the dissolution of Ge in potasssium periodate solution is controlled by activation complex.23
Figure 5. Plots of log (ER/T) vs. 1/T for germanium in potassium periodate solution at pH11.
Polishing experiments
Effect of pH on Ge RR
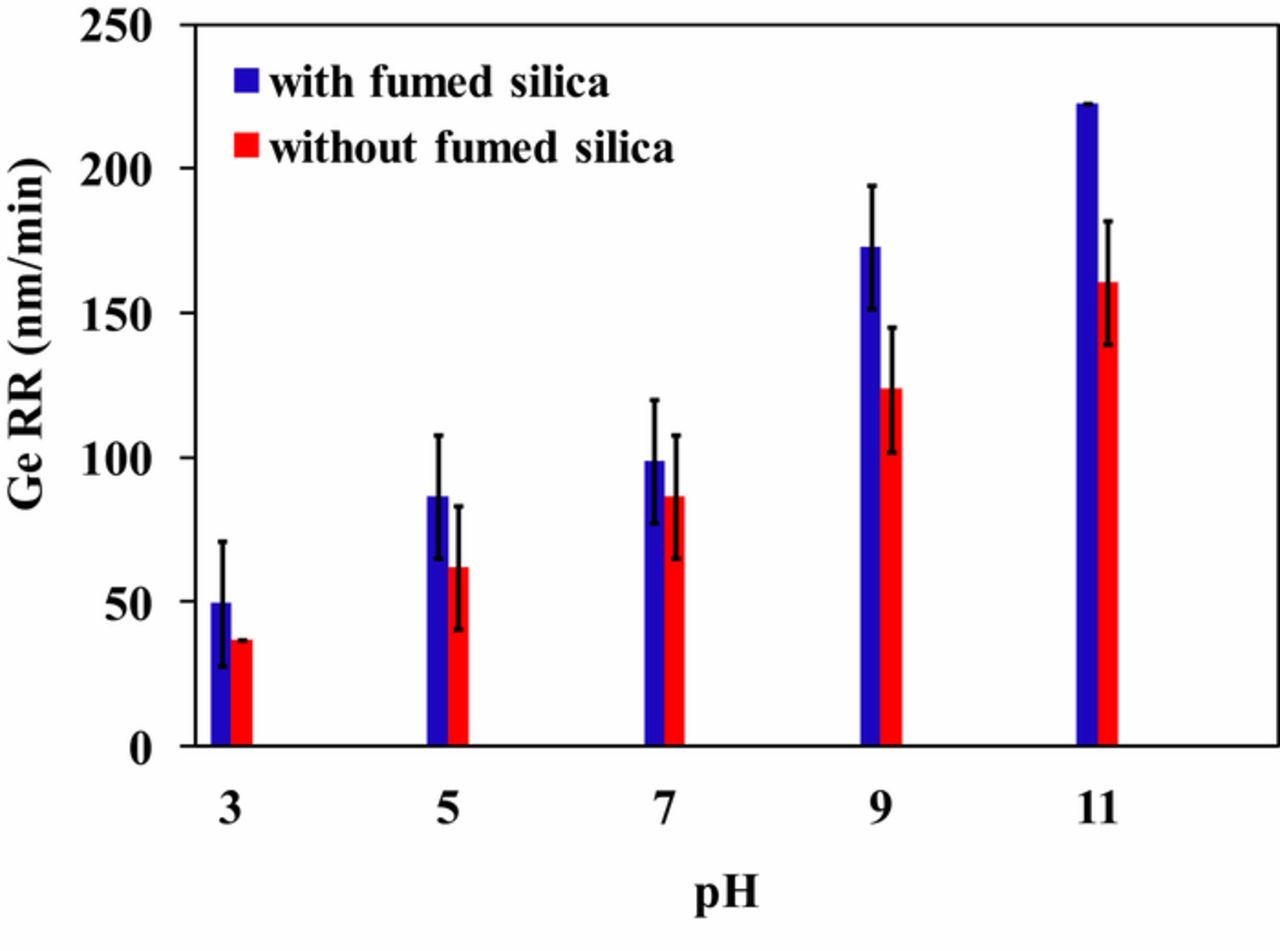
Polishing experiments were perfomed on Ge in the presence and absence of 3 wt% fumed silica using 1 wt% KIO4 + 0.1 M KOH solution. Figure 6 illustrates the effect of pH on Ge RR. In the absence of fumed silica, Ge RR enhances from ∼37 nm/min to ∼160 nm/min when the pH of the slurry was varied from 3 to 11, respectively. Whereas, in the presence of 3 wt% fumed silica with 1 wt% KIO4 + 0.1 M KOH slurry, Ge removal rate was found to be ∼49 nm/min at pH 3 and ∼222 nm/min at pH 11. The main cathodic reactions are the reduction of IO-412–14 to form IO-3, as shown in Eq. 5. Increased RR of germanium with pH could be attributed to the availability of *OH radicals, IO-4and IO-3 anions and resulted in the oxidation of Ge surface to form germanium dioxide (GeO2).
![Equation ([5])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0005.gif)
Figure 6. Effect of pH on Ge RR with and without 3 wt% fumed silica.
Effect of KIO4 concentration on Ge RR
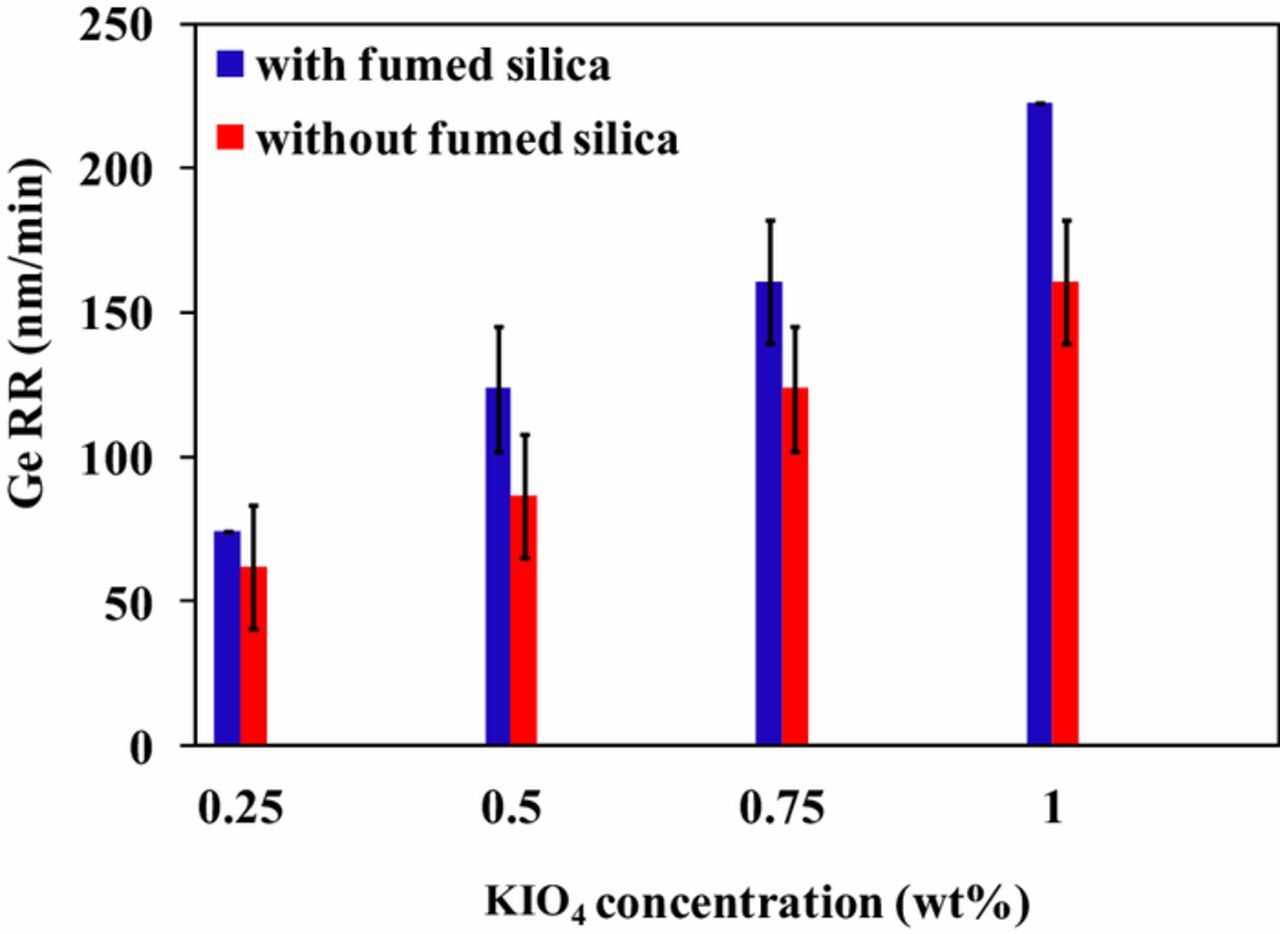
Figure 7 shows Ge RR at constant pH in presence and absence of 3 wt% fumed silica. For this study the pH was maintained at 11. Ge RR increased with increase in KIO4 concentration from 0.25 wt% to 1 wt%. Ge RR was found to be zero with 3 wt% fumed silica over the entire range of pH. In the absence of abrasive, the increase in oxidizer concentration from 0.25 wt% to 1 wt% enhances Ge RR from ∼61 nm/min to ∼160 nm/min. Whereas with addition of 3 wt% fumed silica in polishing slurry, Ge RR was found to increase from ∼74 nm/min to ∼222 nm/min with KIO4 concentration increasing from 0.25 wt% to 1 wt%, respectively. The enhanced RR of Ge with increasing KIO4 concentration might be due to the increased oxidation of Ge with IO-4 and IO-3anions, as shown in Eqs. 6 and 7. As the concentration of KIO4 increases, Ge surface oxidizes rapidly to form GeO2 with subsequent formation of soluble species. With the addition of abrasive, Ge RR is relatively higher, which might be attributed to the combined effect of free abrasive polishing and chemical etching.5
![Equation ([6])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0006.gif)
![Equation ([7])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0007.gif)
Figure 7. Effect of KIO4 concentration on Ge RR with and without 3 wt% fumed silica at pH 11.
Effect of pH on zeta potential
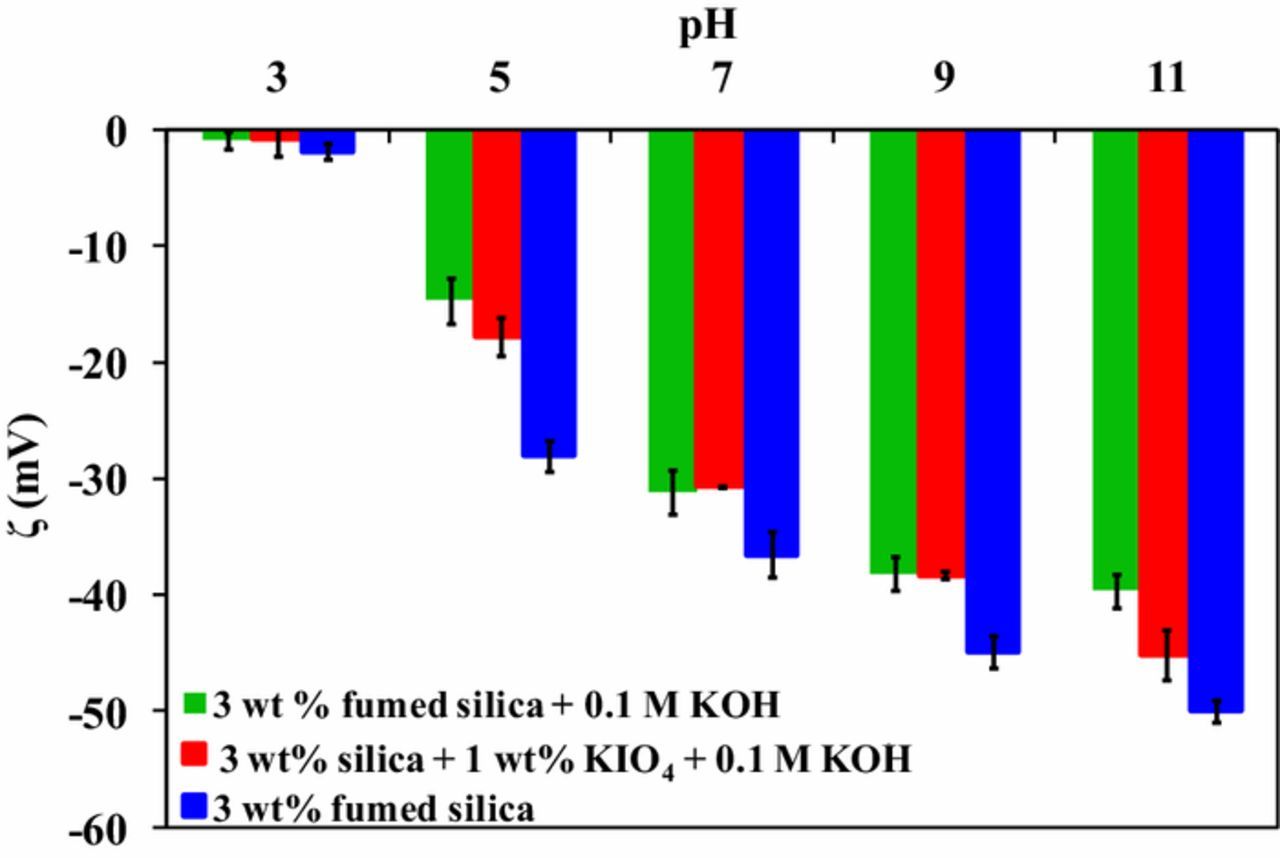
The effect of pH on zeta potential (ζ) of fumed silica, fumed silica with KOH and fumed silica with KOH + KIO4 is shown in Figure 8. It can be clearly seen that fumed silica particles are negatively charged over entire pH range in the presence and absence of KIO4 and KOH. There is no significant variation in the zeta potential value with the addition of the KOH and KIO4 to the fumed silica. The isoelectric point (IEP) of Ge surface in DI water is between pH 4 and 4.5.5,6 Thus, at pH 3 Ge surface is positively charged and fumed SiO2 particles are negatively charged (Figure 8) leading to electrostatic attraction between them. Whereas, for the slurry pH between 5 and 11, both Ge surface and SiO2 particles are negatively charged leading to repulsion between them. Thus, the observed removal beyond pH 5 cannot be attributed alone to electrostatic interactions. There might be some other reason for removal observed in this study.
Figure 8. Zeta potential of various slurries as a function pH.
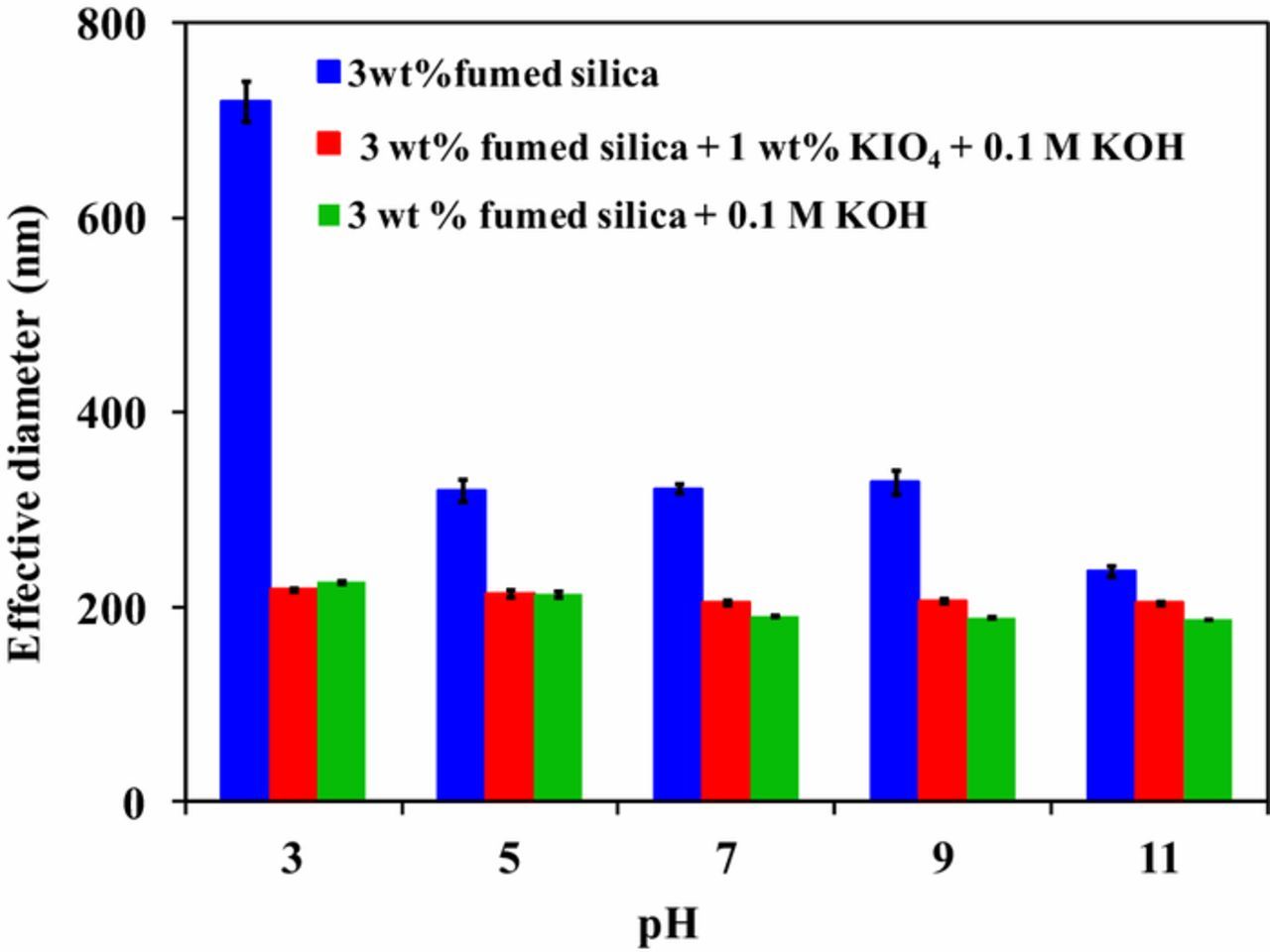
Effect of pH on effective diameter
The effective diameter of fumed silica particles in the presence and absence of KIO4 and KOH is shown in Figure 9. For the entire pH range, the effective diameter was found to be larger for 3 wt% fumed silica slurry as compared with slurry containing KIO4 and KOH or either of them. Larger effective diameter of fumed silica particles is due to its presence in agglomerated form. It can be clearly seen that effective diameter is reduced with the addition of KOH for entire pH range. This reduction in effective diameter can be attributed to KOH, which increases the solubilty of KIO4 and acts as a dispersing agent. The oxidizer, KIO4, serves as a stabilizing agent for the slurry, by refraining the particles to get agglomerate. Literature suggests that the total contact area of active abrasive particles between wafer and pad affects material removal.24 An increase in concentration of abrasive particle, enhances the total contact area resulting in higher removal till the saturation limit. While increasing the pH toward the alkaline region in presence of KIO4 and KOH, agglomerated fumed silica particles gets dispersed providing more total contact area between Ge surface and the abrasive particle and resulting in higher removal rate.
Figure 9. Effective diameter as a function pH.
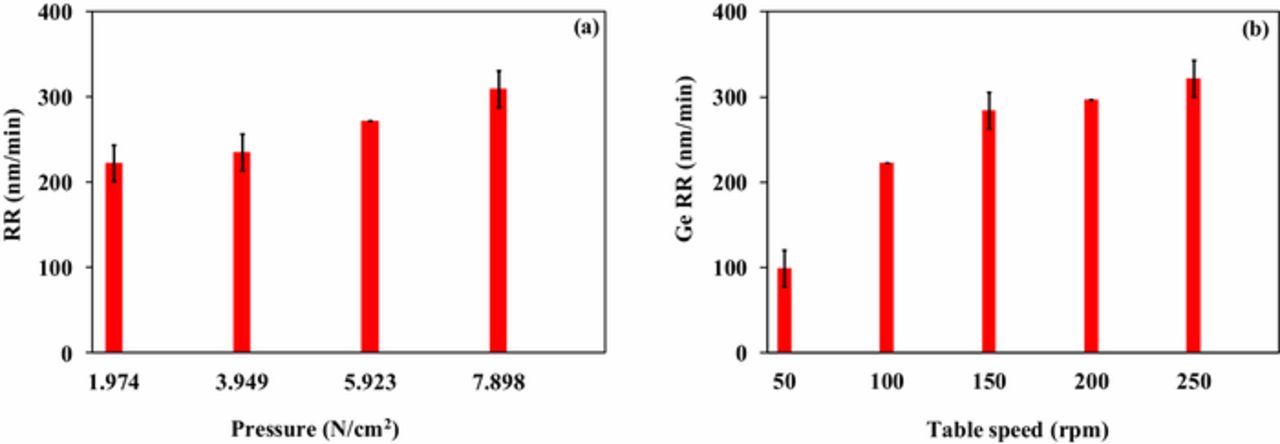
Effect of pressure on Ge RR
Figure 10a shows the effect of pressure on Ge RR using 3 wt% fumed silica containing 1 wt% KIO4 + 0.1 M KOH at pH 11. It was observed that with increase in pressure from 1.974 N/cm2 to 7.898 N/cm2 Ge RR enhances from ∼222 nm/min to ∼309 nm/min, respectively. In CMP, planarization is achieved when higher points on the surface were subjected to more pressure when compared with the lower points.25 With increase in downpressure on Ge surface, the effective local pressure increases resulting in higher contact area between germanium surface and abrasive.26 At zero pressure, Ge RR has a non-zero intercept following non-Prestonian behavior. This might be attributed to the formation of oxide film, which acts as a rate limiting step.27 Non-prestonian behavior of material removal rate with pressure was distinctly reported for ruthenium/germanium CMP in literature.8,9,28
Figure 10. Effect of (a) pressure; (b) table speed on Ge RR using 3 wt% fumed silica with 1 wt% KIO4 at pH 11.
Effect of table speed on Ge RR
Figure 10b shows the effect of table speed on Ge RR using 3 wt% fumed silica + 1 wt% KIO4 + 0.1M KOH slurry at pH 11. From Figure 10b, it is clear that as the platen rotational speed is increased from 50 rpm to 250 rpm, Ge RR increases from ∼98 nm/min to ∼321 nm/min. Higher RR is likely due to the rapid formation of GeO2 film on the Ge surface27 and the synergetic effect of oxidation and fast action of abrasive with high table speed.
Surface morphology
Surface roughness (Ra) of Ge coupons were evaluated at pH 3, pH 7 and pH 11. Before polishing, the surface roughness was found to be 0.72 ± 0.03 μm. Ge coupon polished using 3 wt% fumed silica + 1 wt% KIO4 + 0.1 M KOH slurry at pH 3 showed no significant change in surface roughness. While the germanium coupon polished using the proposed slurry at pH 7 and pH 11, shown surface roughness of 0.66 ± 0.05 μm and 0.41 ± 0.05 μm, respectively. With increase in the pH value of the slurry, Ge surface roughness was found to be decrease. Ge coupons polished with 3 wt% fumed silica + 1 wt% KIO4 + 0.1M KOH at pH 11, shows a surface roughness reduction of ∼42%.
Removal mechanism
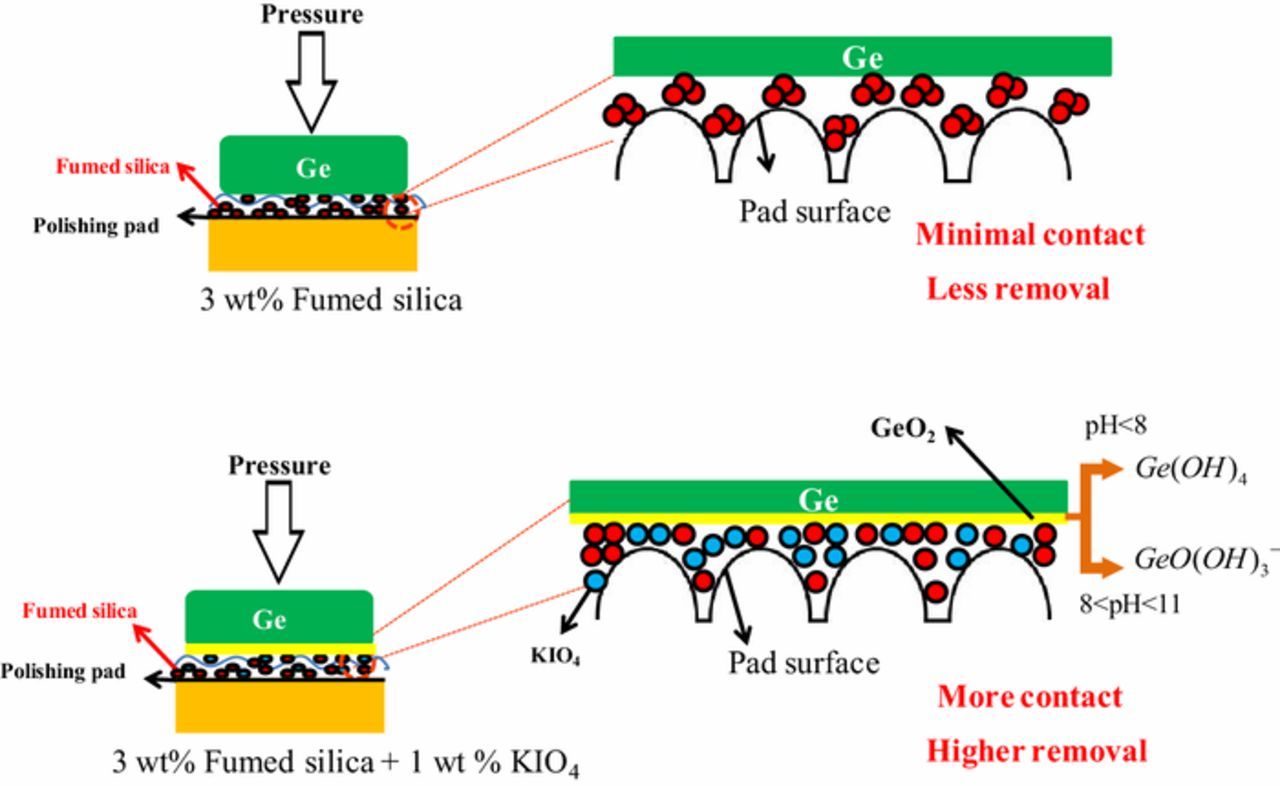
Fumed silica particles are negatively charged over the entire pH range for all the three slurries i.e., 3 wt% fumed silica, 3 wt% fumed silica + 0.1 M KOH and 3 wt% fumed silica + 1 wt% KIO4 + 0.1 M KOH. According to the literature,5,6 Ge surface have isoelectric point (IEP) between pH 4 and 4.5 in pH adjusted DI water. At pH 3, Ge surface is positively charged and SiO2 particles are negatively charged leading to electrostatic attraction among them and from pH 5 onwards both Ge surface and SiO2 particles are negatively charged leading to repulsion between them. In alkaline region, Ge polishes at higher rate with higher concentration silica based abrasives, where electrostatic repulsion exists between the SiO2 particles and Ge surface.5 Apart from electrostatic interactions, there might be some other reason behind for the higher material removal in the alkaline region.
The increase in material removal with abrasive concentration in the solid-solid contact mode of CMP was reported by Luo24 with a qualitative explanation about active and inactive abrasives. The material removal increases with more number of active abrasive between the wafer and pad surface, which in turn increases the total contact area among them, till saturation occurs. Ge polished with 3 wt% fumed silica has larger effective diameter, which is due to its presence in agglomerated form contributing to less total surface area. With the addition of 0.1 M KOH, which acts as dispersing agent, the abrasive particles are dispersed with pH, providing more total surface area for interaction. Potassium periodate works as a stabilizer and refrains particles to agglomerate. The interaction between agglomerated / dispersed fumed silica particles and Ge surface, which in turn affects the total contact between the particles and the Ge surface is schematically shown in Figure 11.
Figure 11. Interaction between aggromerated and dispersed fumed silica particles in presence and absence of KIO4 for Ge removal.
Potassium periodate dissociates to form potassium (K+) cation and periodate (IO−4) anion, as shown in Eq. 1. The main cathodic reactions for polishing Ge in KIO4 solution is the reduction of IO−4to IO−3 anions,11–14 as shown in Eq. 5. The IO−4 and IO−3 anions oxidizes Ge to form germanium dioxide, according to Eqs. 6 and 7. Literature suggest that the GeO2 undergoes hydration in the aqueous environment and form germanium hydroxide complex, GeO(OH)−3 and GeO2(OH)2 −2.28 However, the formation of those species strongly depends on the pH value. It can be explained that in the acidic region (pH<8), the reaction proceeds as shown in Eq. 8 to form of germanic acid, Ge(OH)4, which is not easily soluble in water resulting in lesser RR. While in the weak alkaline pH range between 8 and 11, the concentration of *OH radicals increases, which enhances the oxidation of germanium and hydration of GeO2 to form a more soluble dissolution product GeO(OH)−3 as shown in Eq. 9. In the highly alkaline region (pH>11), the most soluble species, GeO2(OH)2-2 is prevalent in aqueous solution as shown in Eq. 10.5,6
![Equation ([8])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0008.gif)
![Equation ([9])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0009.gif)
![Equation ([10])](https://content.cld.iop.org/journals/2162-8777/8/5/P3085/revision1/d0010.gif)
Conclusions
CMP slurry for polishing Ge disk using 3 wt% fumed silica + 1 wt% KIO4 with 0.1 M KOH was proposed. With increase in temperature ER was found to increase, the activation energy was found to be 13.7 kJ/ mol. The enthalpy and entropy of activation are 11.029 kJ/mol and −271.06 J/mol.K, respectively, which suggest that the dissolution of germanium is endothermic process and the dissolution is controlled by means of activation complex. For the same potassium periodate concentration and pH, there was an appreciable increase in the Ge MRR over Ge ER, due to the synergic effect of free abrasive polishing and chemical etching. The effective diameter of fumed silica abrasive is larger for 3 wt% fumed silica slurry than 3 wt% fumed silica + 0.1 M KOH and 3 wt% fumed silica + 1 wt% KIO4 + 0.1 M KOH slurry for complete range of pH. At pH 11, maximum RR of ∼222 nm/min was obtained with 3 wt% fumed silica + 1 wt% KIO4 + 0.1 M KOH slurry. The higher removal in alkaline region could be due to the presence of *OH radicals with increasing pH and also due to increased oxidation of Ge with IO-4 and IO-3anions. Moreover, with increase in the pH, agglomerated particles gets dispersed, leading to result more contact between Ge coupon and SiO2 particles. Subsequently, Ge oxidizes to form germanium dioxide followed by formation of rapidly soluble Ge hydroxide species. Surface roughness was found to be reduced by ∼42% at pH 11. Ge removal with potassium periodate follows non-Prestonian behavior.
Acknowledgments
The authors thank Department of Science and Technology - Science and Engineering Research Board (DST-SERB) for providing fund for CMP polisher machine with Sanction Order No. "SB/FTP/ETA-351/2013" dated 17/07/2014 and the project has been funded by the Institution of Engineers (India), 8 Gokhale Road, Kolkatta 700020 under R&D Grant-in-Aid scheme. Also, we are thankful to the Department of Civil Engineering, IIT Kanpur for carrying out particle size and zeta potential analysis and Advanced Centre for Material Science (ACMS), IIT Kanpur for providing the optical microscope facility.
ORCID
R. Manivannan 0000-0001-5584-2609
S. Noyel Victoria 0000-0002-4705-7675