Abstract
We studied the effects of O2− ions on the coordination structure of Si ions and the electrodeposition process of Si films in molten fluorides using SiO2 powder (as a source of Si) and Li2O (as a source of O2−). High-temperature Raman spectroscopic data revealed that the dissolution of SiO2 in molten KF, LiF-KF, and LiF-NaF-KF without Li2O was proceeded by the formation of silicate ions, with the [Si2O5]2− ion acting as the dominant species. On the other hand, when 3.0 mol% Li2O was added to molten LiF-KF and LiF-NaF-KF, the intensity of Raman band due to [SiO3F]3− ion was increased compared to that without Li2O system, which indicated that the O2− ions could cause breakage of Si-O-Si bonds of SiO2 or [Si2O5]2− ions. Furthermore, the reduction currents, attributed to the reduction of Si ions, increased significantly by the addition of Li2O; moreover, the thickness and current efficiency of the electrodeposited polycrystalline Si layer prepared by potentiostatic electrolysis was improved. These results indicated that the O2− ions can change the coordination structure of Si ions in molten fluorides and that the design of the molten salts bath is a key technology for fabricating high-quality Si layers with high current efficiency.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
A major current focus in silicon solar cells is how to fabricate Si films by environmentally friendly and inexpensive processes. The molten salt electrochemical process is a key technology for fabricating polycrystalline Si thin films in an environmentally friendly, simple, and inexpensive manner. In this process, the morphology, crystallinity, and texture of electrodeposited Si can be easily controlled by changing melt conditions and electrochemical parameters such as applied potentials and current densities.
Since the 1970s, the electrodeposition of Si by a high-temperature molten salt electrochemical process has been studied in various molten fluorides such as LiF-KF1–4 and LiF-NaF-KF5,6 containing K2SiF6 systems. Subsequently, other fluoride systems such as NaF-KF7 and NaF-AlF38 have also been reported. Since the 2000s, using a chloride system, the direct electrochemical reduction of bulk SiO29–11 and SiO2 nanoparticles12–15 in molten CaCl2 at 1123 K has been studied. Recently, the electrodeposition of Si from SiCl4 in molten KF-KCl at 923 K has been reported.16
In our previous study, we examined the electrodeposition of Si in molten LiF-NaF-KF containing SiO2 powder at 873 K.17 We obtained dense and adherent polycrystalline Si films, whose thickness was 1–2 μm, by potentiostatic electrolysis. Compared to the molten chloride system, one of the benefits of the molten fluoride system is its high solubility for SiO2 at a low temperature. Although the solubility process of SiO2 is a very important step for the electrodeposition of Si films, only a few studies have examined the coordination structure of Si ions in molten fluorides by in-situ measurements. In particular, because certain studies have proposed that the solubility of SiO2 was enhanced by adding O2− ions in a chloride system,14,15 it is necessary to understand the effects of O2− ions on the coordination structure of Si ions and electrodeposition process of Si in molten fluoride.
In this study, we examined the dissolution behavior of SiO2 powder in molten fluorides with and without a Li2O system using spectroscopic techniques. We used Li2O as a source of O2− ions. First, we selected molten KF as a simple electrolyte. In order to reveal the interaction between KF and SiO2, the phase diagram of the KF-SiO2 binary system was described by thermogravimetry and differential thermal analysis (TG-DTA). Moreover, using high-temperature Raman spectroscopy, which is a useful technique for determining the coordination structure of silicate ions,18 we examined the coordination structure of Si ions in eutectic KF-SiO2. The differences between the coordination structures of Si ions in molten LiF-KF and LiF-NaF-KF with and without Li2O system are then discussed. Furthermore, we performed the electrodeposition of Si by potentiostatic electrolysis in molten LiF-NaF-KF with and without Li2O at 873 K to examine the effects of O2− ions on the Si electrodeposition process. Then, the electrodeposited Si layers on the Ag substrates were characterized using scanning electron microscopy (SEM), energy dispersive X-ray spectrometry (EDS), and X-ray diffraction (XRD).
Experimental
LiF (98.0%, Wako Pure Chemical Co. Ltd.), NaF (99.0%, Wako Pure Chemical Co. Ltd.), KF (99.0%, Wako Pure Chemical Co. Ltd.), SiO2 (99.0%, Wako Pure Chemical Co. Ltd.), and Li2O (99.0%, Wako Pure Chemical Co. Ltd.) were used as the starting materials. Depending on the experimental conditions, these reagents were then mixed in various compositions. The mixture was kept under vacuum for more than 24 h at 473 K to eliminate any water.
For the calorimetry studies, KF and SiO2 powder were mixed by mechanical grinding using mortar and pestle for over 5 minutes to obtain homogeneous mixture. The melting points of the KF-SiO2 mixture were measured using TG-DTA (DTG-60H, Shimadzu Corporation Co. Ltd.). The phase diagram of the binary KF-SiO2 salt mixtures were constructed by plotting the temperatures of endothermic peaks identified on the DTA curves obtained in the heating process to avoid uncertainty because of supercooling.
For the Raman spectroscopic analysis, LiF, KF, SiO2, and Li2O powders, mixed in various compositions and subject to a drying process, were introduced into a platinum crucible. The crucible was sealed in a hot stage device (10016, Japan HighTech Co. Ltd.) and continuously heated at 100 K min−1 under an Ar atmosphere. Raman spectra were obtained using a micro-Raman spectrometer (LabRAM Spectrometer, Horiba Jobin-Yvon GmbH) with a YAG laser (532 nm).
For the electrochemical experiments, all measurements were performed in a dry Ar atmosphere. We heated the cell using a programmable furnace, and the temperature of the cell was measured using a Chromel-Alumel thermocouple with an accuracy of ±1 K. Ag wires (1 mm diameter; 99.99%, Nilaco Co. Ltd.) and Ag plates (5 mm × 8 mm × 0.5 mm; 99.5%, Nilaco Co. Ltd.) were used as the working electrodes. The potentials of the electrodes were measured using a nickel wire immersed in a molten electrolyte, which acted as a quasi-reference electrode (QRE). The potential of Ni QRE was calibrated with respect to a K+/K electrode, which was prepared by electrodepositing K metal on a nickel electrode. The counter electrode was a glassy carbon rod (3 mm diameter; Tokai Carbon Co. Ltd.). A potentiostat/galvanostat (VSP, BioLogic Co. Ltd.) was used for electrochemical measurements.
All Si samples were prepared by potentiostatic electrolysis and rinsed with hot water heated to 363 K for more than 6 h. The obtained samples were analyzed by XRD (Multi Flex, Rigaku Industrial Co. Ltd.) with a Cu Kα line and SEM (JSM-7001, JEOL). Finally, we prepared the cross-sectional samples using a cross-sectional polisher.
Results and Discussion
The coordination structure of Si ions in a KF-SiO2 system
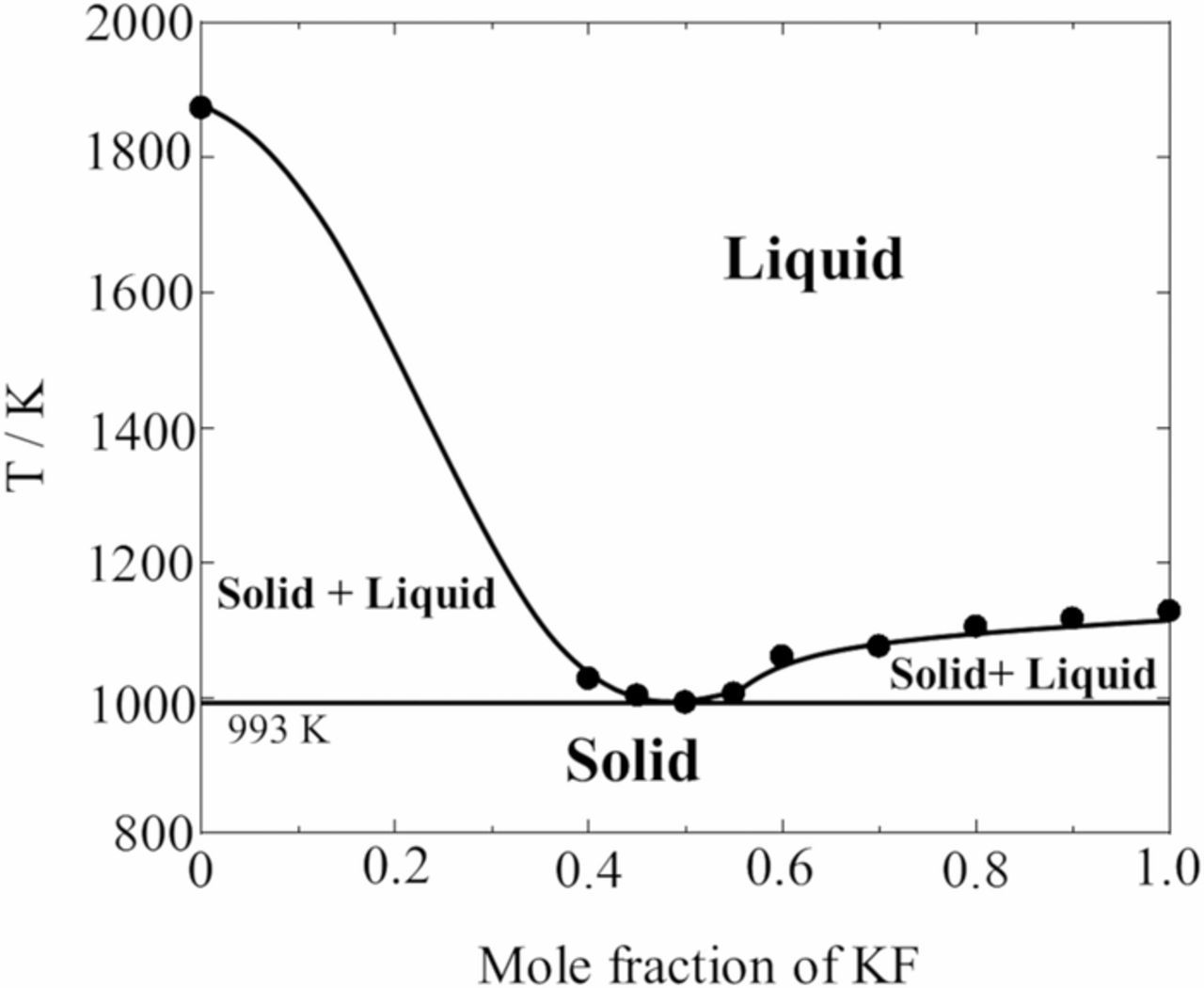
In order to investigate the dissolution behavior of SiO2 powder in molten fluoride systems, we selected KF single melt as the simple fluoride melt. To examine the thermal properties of the KF-SiO2 system, the melting points at various mole fractions of the KF-SiO2 were measured using TG-DTA. Fig. 1 shows the phase diagram of the KF-SiO2 binary system. These data were collected by continuous heating at 10 K min−1 for the TG-DTA measurement so that the scan rate was sufficient to detect the transition temperature. The phase diagram was a simple eutectic type, and the eutectic composition of the KF-SiO2 system was 50 mol% KF at a temperature of 993 K. This temperature was 990 K lower than the melting point of single SiO2, which indicates that SiO2 can be dissolved into a new coordination structure of Si ions by interactions with KF molecules. The eutectic composition determined in this study agreed with that reported in a previous study,19 although the temperature was 73 K lower than the reported value, which might be caused by the difference in sample preparation for DTA measurement between the previous study19 and our measurement.
Figure 1. Phase diagram of the KF-SiO2 system.
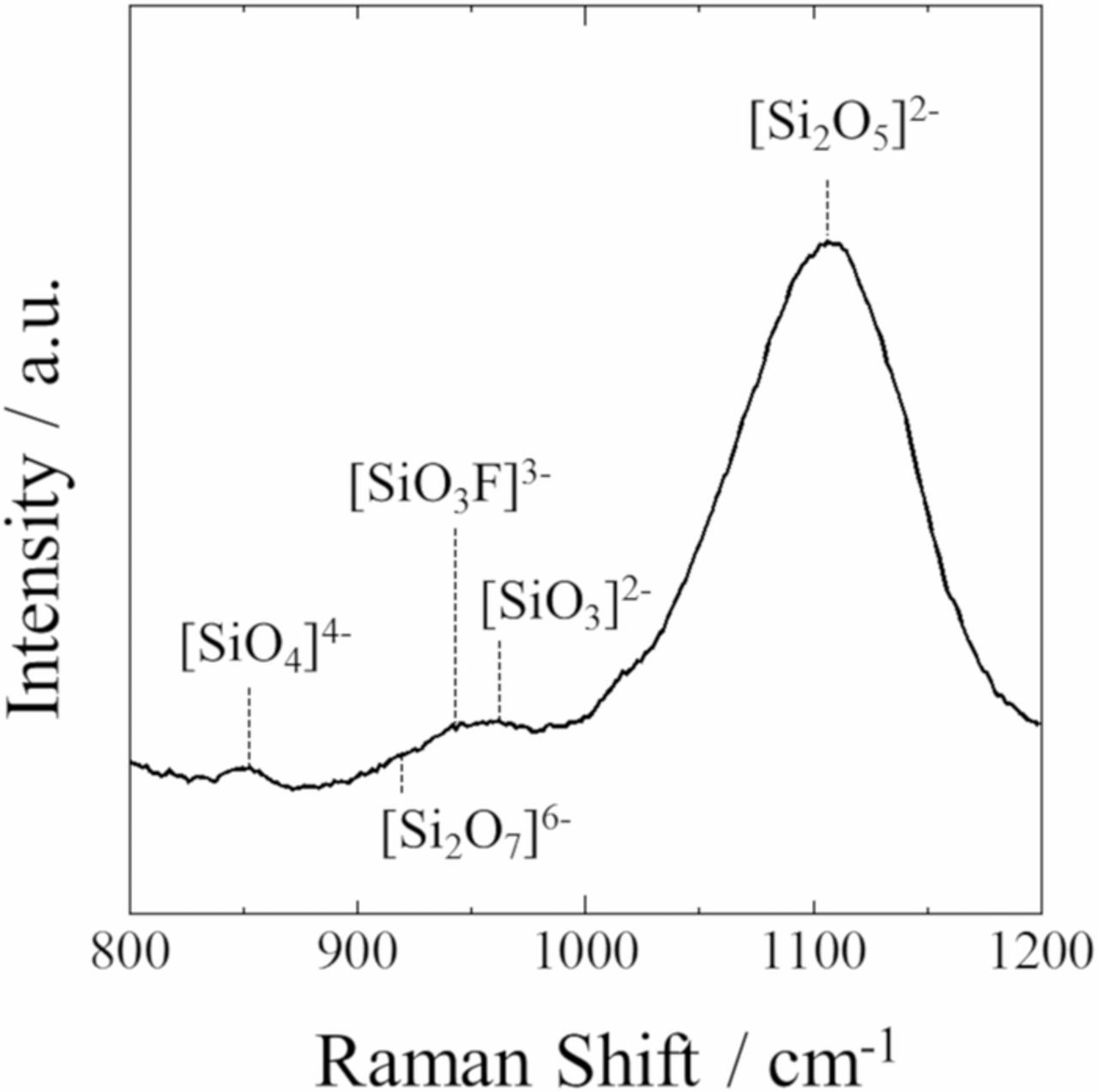
In order to examine the coordination structure of Si ions in the eutectic composition of the KF-SiO2 (50:50 mol%) system, high-temperature Raman spectroscopy was carried out. Fig. 2 shows the Raman spectrum of the melt from 800 to 1200 cm−1 at 1073 K, which contained several wide vibrational bands that could be assigned based on previously reported studies as follows: the band with a maximum at 1100 cm−1 corresponded to [Si2O5]2− with one non-bridging oxygen atom. Smaller bands near 850, 920 and 960 cm−1 could be attributed to [SiO4]4−, [Si2O7]6−, and [SiO3]2− with four, three and two non-bridging oxygen atoms, respectively.20,21 Also, the band with a maximum at 950 cm−1 was related to [SiO3F]3−.22 From the Raman spectrum, it was revealed that SiO2 dissolved as the states of various silicate ions in the KF-SiO2 system. Based on the Raman spectrum, although it is difficult to quantitatively evaluate the amount of each silicate ion, the [Si2O5]2− ion was considered to be the dominant species because it was obvious that the intensity of the Raman band at 1100 cm−1 was considerably larger. These results indicate that fluoride anions could have caused Si-O-Si bond breakage of SiO2, after which the oxyfluoride or silicate ions would have formed.
Figure 2. A Raman spectrum of the KF-SiO2 melt at 1073 K.
Coordination structure of Si ions in molten LiF-KF-SiO2 and molten LiF-NaF-KF-SiO2 systems with and without Li2O
The coordination structure of Si ions was investigated in a molten LiF-KF (51.0:49.0 mol%) system containing 5.0 mol% SiO2 and a molten LiF-NaF-KF (46.5:11.5:42.0 mol%) system containing 5.0 mol% SiO2 with and without Li2O. Compared to KF-SiO2, LiF-KF and LiF-NaF-KF were more suitable electrolytes for Si electrodeposition because of their low melting points (LiF-KF: 765 K and LiF-NaF-KF: 730 K) and low viscosity.
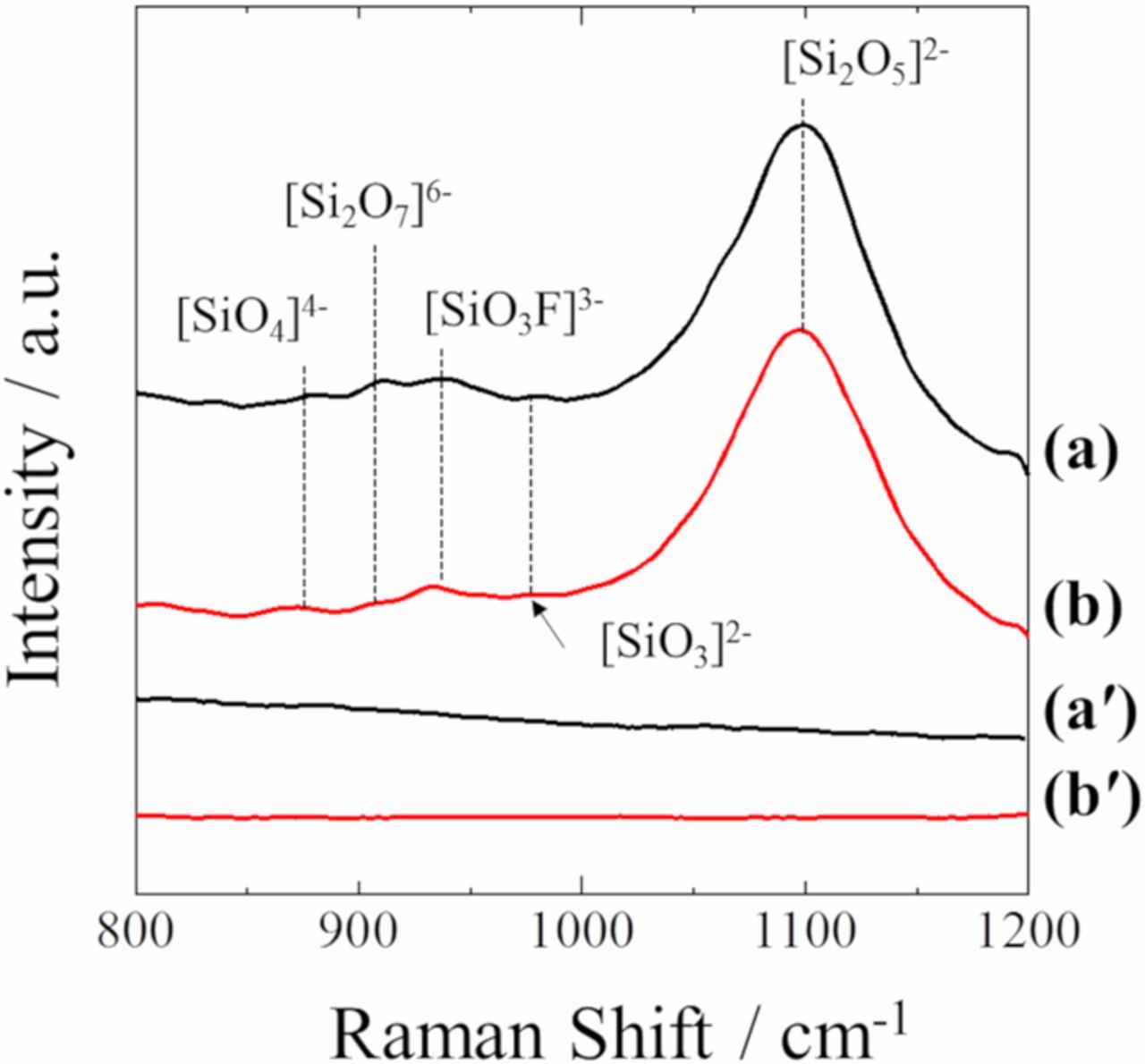
Fig. 3 shows the Raman spectra for molten (a) LiF-KF-SiO2, (b) LiF-NaF-KF-SiO2, (aʹ) LiF-KF, and (bʹ) LiF-NaF-KF at 873 K. Both LiF-KF-SiO2 and LiF-NaF-KF-SiO2 showed several wide vibrational bands, while no bands or peaks were confirmed in the blank LiF-KF and LiF-NaF-KF. Moreover, the Raman spectra of both LiF-KF-SiO2 and LiF-NaF-KF-SiO2 systems were very similar to those of the KF-SiO2 system. This indicated that the dissolution behavior of SiO2 was almost the same in molten fluoride because of the presence of fluoride anions.
Figure 3. Raman spectra of molten (a) LiF-KF-SiO2, (b) LiF-NaF-KF-SiO2, (aʹ) LiF-KF, and (bʹ) LiF-NaF-KF at 873 K.
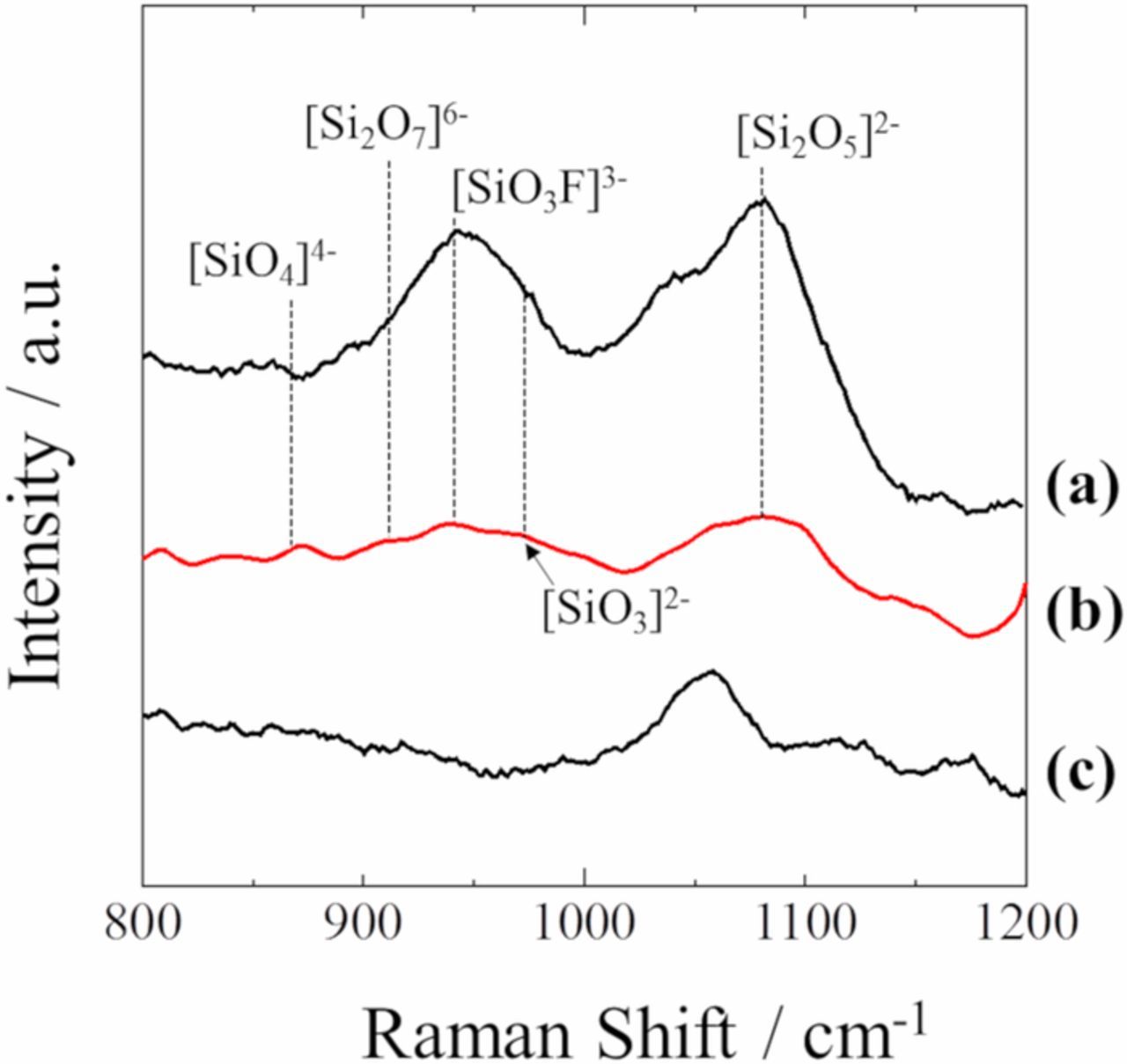
Fig. 4 shows the Raman spectra for molten (a) LiF-KF-SiO2 with 3.0 mol% Li2O, (b) LiF-NaF-KF-SiO2 with 3.0 mol% Li2O, and (c) LiF-KF with 3.0 mol% Li2O at 873 K. The Raman band near 1050 cm−1 was attributed to Li2O. The Raman spectra of both types of SiO2 (Figs. 4a and 4b) were almost similar. Comparing the spectra with Li2O system (Figs. 4a and 4b) to without Li2O system (Figs. 3a and 3b), the intensity of the Raman band at 935 cm−1 due to the [SiO3F]3− ion increased after the addition of Li2O. This indicates that the O2− ions could cleave the Si-O-Si bonds of SiO2 or silicate ions such as [Si2O5]2−; consequently, the formation of tetrahedron [SiO3F]3− species was promoted.
Figure 4. Raman spectra of molten (a) LiF-KF-SiO2-Li2O, (b) LiF-NaF-KF-SiO2-Li2O, and (c) LiF-KF-Li2O at 873 K.
Electrodeposition of Si in a molten LiF-NaF-KF-SiO2-Li2O system
According to high-temperature Raman spectroscopy, the addition of O2− ions into the molten fluorides changed the bonding states of the Si ions. In order to understand the effect of O2− ions on the electrodeposition mechanism in the fluoride melt, Si was electrodeposited in molten LiF-NaF-KF with the Li2O system. In our previous study, we showed that 1–2 μm polycrystalline and dense Si films were electrodeposited on Ag substrates by potentiostatic electrolysis in molten LiF-NaF-KF without Li2O at 873 K.17
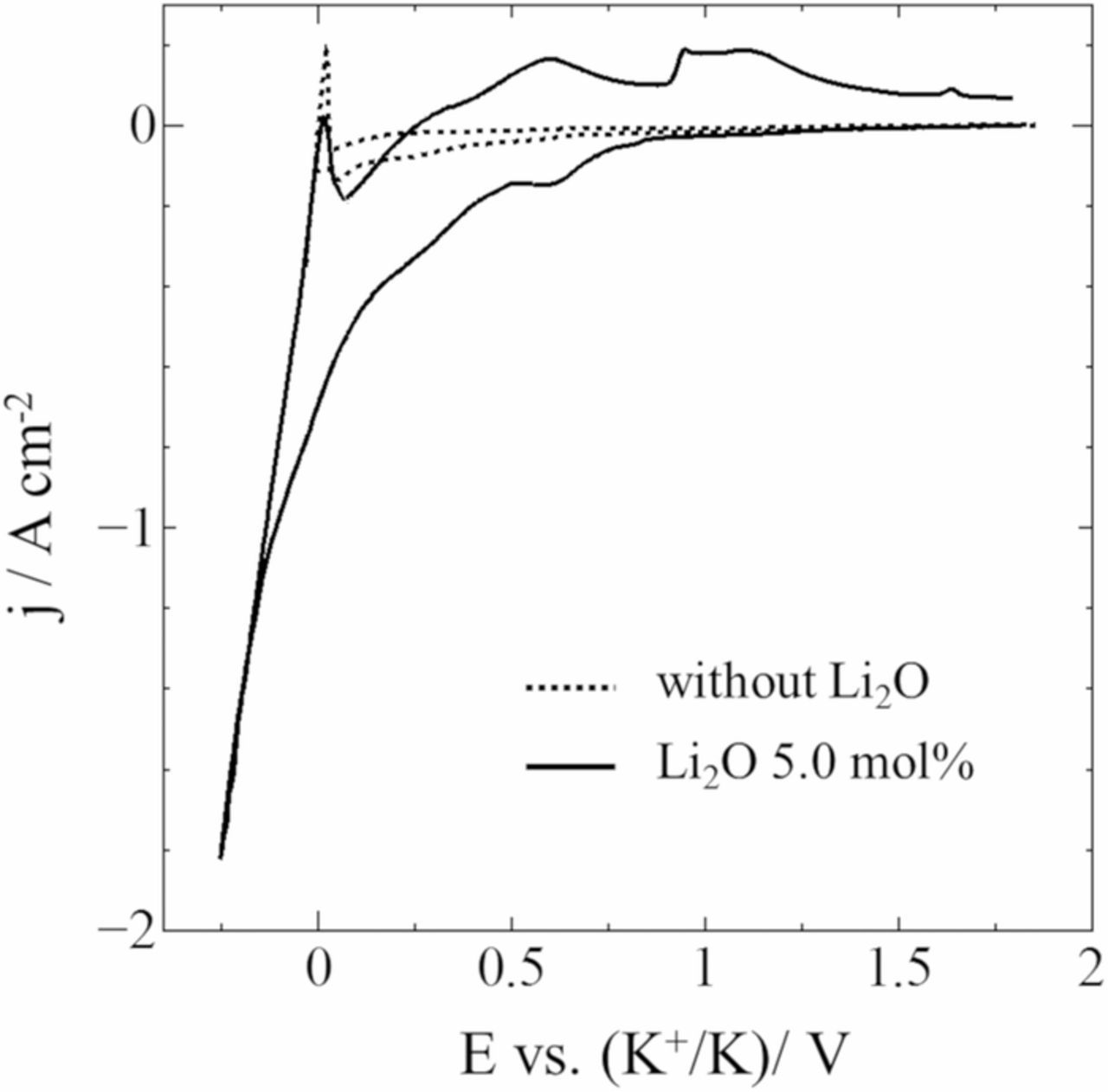
Fig. 5 shows the cyclic voltammograms for Ag substrates before and after adding 5.0 mol% Li2O into the LiF-NaF-KF at 873 K. Before adding Li2O, a broad and slight cathodic current was observed from ca. 1.0 V during the cathodic sweep, which was attributed to the reduction of Si ions dissolved in the melt.17 The broad cathodic curve represented the multiple coordination structures of the Si ions such as [SiO3]2−, [Si2O5]2−, [Si2O7]6−, [SiO4]4−, and [SiO3F]3−, which were confirmed by Raman spectroscopy. After adding Li2O, the reduction current significantly increased. This suggested that the solubility of SiO2 could be enhanced by the change in the coordination structure of Si ions associated with the addition of O2− ions.
Figure 5. Cyclic voltammograms for an Ag electrode before and after adding Li2O into molten LiF-NaF-KF-SiO2 at 873 K. Scan rate: 0.1 V s−1.
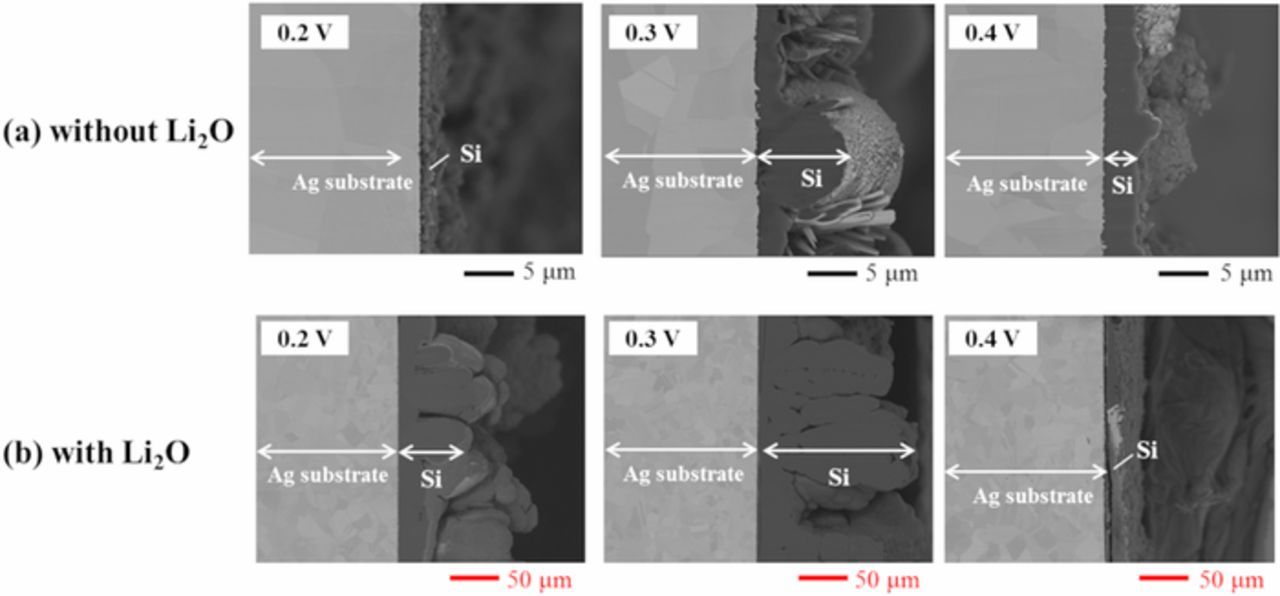
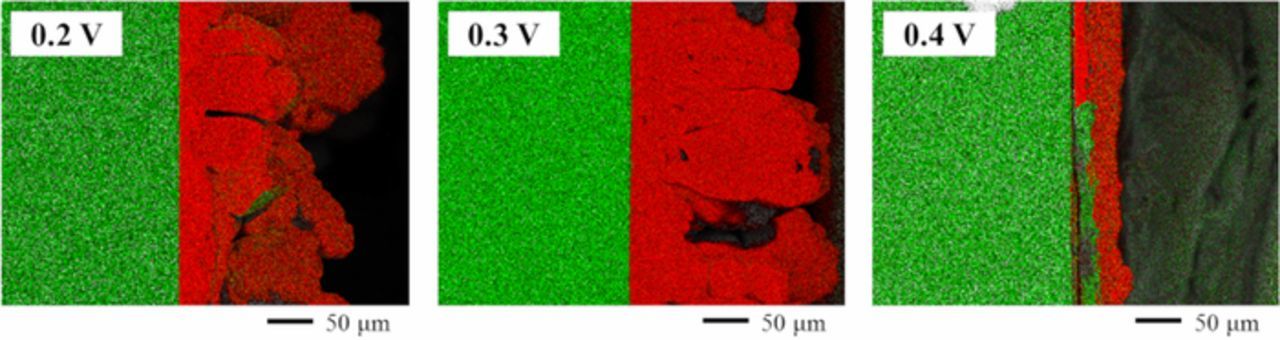
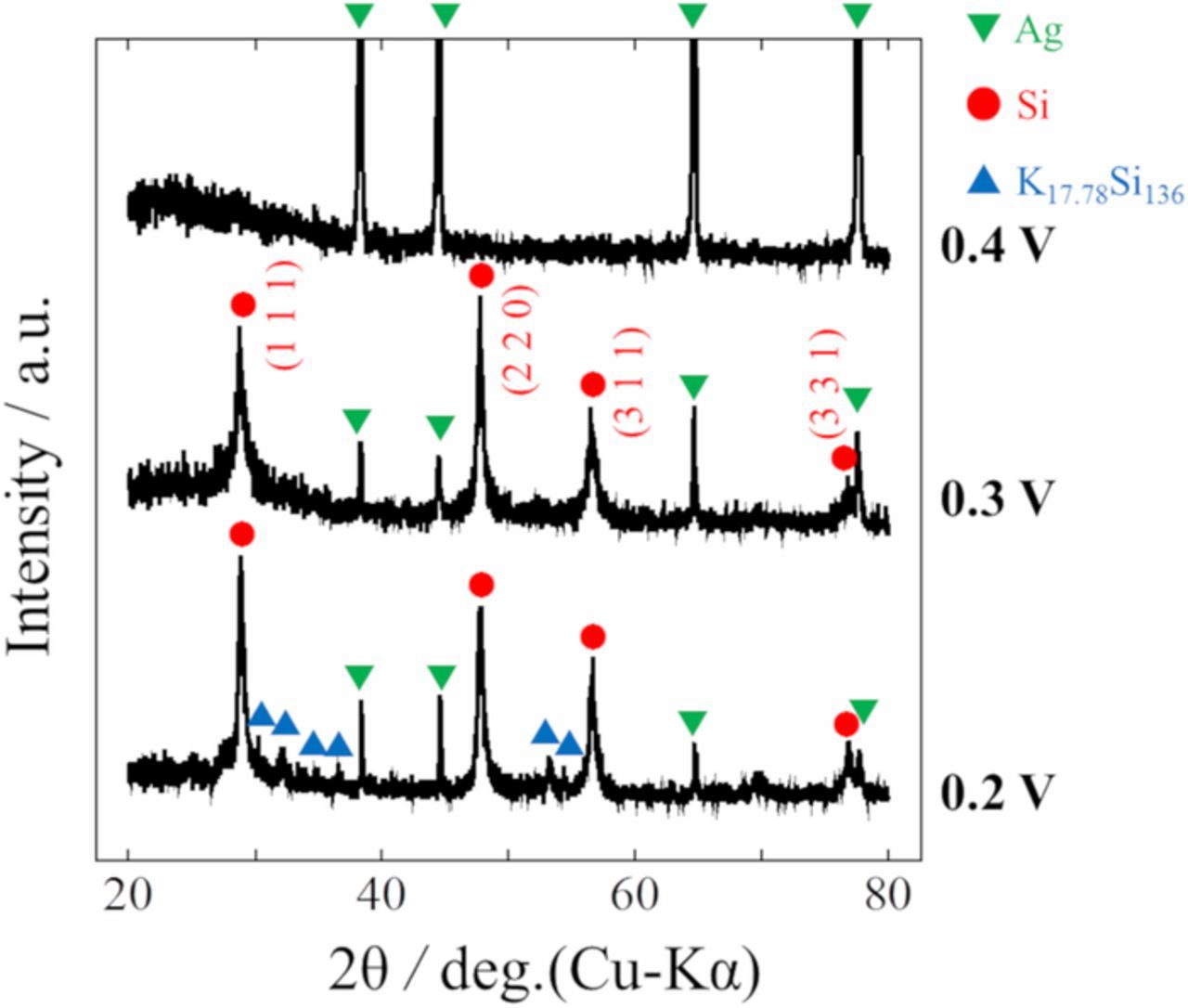
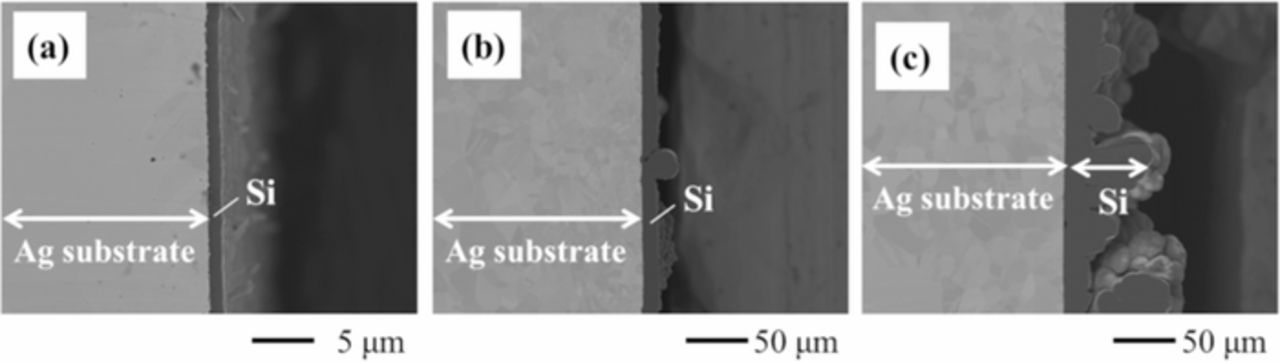
Based on the results of cyclic voltammetry, potentiostatic electrolysis was conducted at 0.2, 0.3, and 0.4 V, where the potentials were considered to be negative enough to form metallic silicon, for 3600 s using Ag plate cathodes in molten LiF-NaF-KF containing 0.5 mol% SiO2 with or without 5.0 mol% Li2O. Fig. 6 shows the cross-sectional SEM images of the samples in both electrolytes. Si layers of few micrometers were obtained in the cases without Li2O, whereas the thicknesses of Si layers increased from tens to hundreds of micrometers in the case with Li2O. As shown in Fig. 7, the electrodeposits of Si were identified using EDS analysis. In Fig. 7, the green and red area correspond to Ag and Si. Fig. 8 shows the results of the XRD patterns for the samples. The sample obtained at 0.4 V was partially deposited on the substrate and the majority of the deposits obtained at 0.4 V were exfoliated while rinsing with hot water, which indicated low adhesion between the electrodeposited Si and Ag substrate at that potential. The peaks attributed to polycrystalline Si were recognized in the samples with electrolytic potentials at 0.2 and 0.3 V, whereas only peaks that could be attributed to the Ag substrate were observed at 0.4 V. The K-Si alloy was also detected in the sample at 0.2 V.
Figure 6. SEM images of the samples after potentiostatic electrolysis at 0.2, 0.3, and 0.4 V for 3600 s in molten LiF-NaF-KF containing 0.5 mol% SiO2 (a) without and (b) with 5.0 mol% Li2O at 873 K.
Figure 7. EDS element mapping images of the samples after potentiostatic electrolysis at 0.2, 0.3, and 0.4 V for 3600 s in molten LiF-NaF-KF containing SiO2 with 5.0 mol% Li2O at 873 K. The green and red area in the images correspond to Ag and Si, respectively.
Figure 8. XRD patterns of the samples after potentiostatic electrolysis at 0.2, 0.3, and 0.4 V for 3600 s in molten LiF-NaF-KF-SiO2 with 5.0 mol% Li2O at 873 K.
In order to investigate the time transient of the morphological variations of the Si layer in LiF-NaF-KF with Li2O at 873 K, the Si samples were prepared by potentiostatic electrolysis at 0.3 V for 60, 600, and 1800 s, where the electrolytic potential forms good adhesion of electrodeposited Si without the formation of any K-Si alloys. Fig. 9 shows the cross-sectional SEM images. Although the flat and dense Si layer was formed at the initial stage of the electrolysis (Fig. 9a), the Si layer's surface morphology partially changed to an inhomogeneous shape when the thickness reached ca. 10 μm (Fig. 9b). With increase in electrolysis time, the surface morphology became rougher (Fig. 9c). This morphological change may have been caused by a comproportionation reaction between electrodeposited Si and Si ions in the melt:3
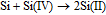
Once the Si deposit became partially rough due to the above reaction, heterogeneous growth could be enhanced from its roughness as starting point. Also, the reduction reaction of Si(II) ions might cause the morphological change because of the difference in reduction processes between Si(IV) and Si(II). Table I lists the thickness of Si layers and current efficiencies of the samples prepared by potentiostatic electrolysis at various potentials for 1 h in molten LiF-NaF-KF, with or without 5.0 mol% Li2O, at 873 K. The thickness and current efficiencies were significantly enhanced by the addition of Li2O, which indicated that the coordination structures of Si ions affected characteristics of the electrodeposited Si such as denseness, adhesion, and morphology. This indicated that the comproportional reaction could be suppressed by adding Li2O because of the structural change in the Si ions. These results suggested that the design of the molten salts bath is a key technology for fabricating high-quality Si layers with high current efficiency.
Figure 9. SEM images of the samples after potentiostatic electrolysis at 0.3 V for (a) 60 s, (b) 600 s, and (c) 1800 s in molten LiF-NaF-KF containing 0.5 mol% SiO2 with 5.0 mol% Li2O at 873 K.
Table I. Thickness of Si layar and current efficiencies of the samples prepared by the potentiostatic electrolysis at various potentials in molten LiF-NaF-KF with or without 5.0 mol% Li2O at 873 K.
| Thickness of Si layer/μm | Current efficiency/% | |||
|---|---|---|---|---|
| Potential/V | without Li2O | with Li2O | without Li2O | with Li2O |
| 0.7 | 1 | - | 23.2 | - |
| 0.4 | 1–3 | (island-like deposit) | 19.0 | 6.5 |
| 0.3 | 2–6 | 40–160 | 13.3 | 50.8 |
| 0.2 | 0.2–1 | 30–100 | 13.2 | 49.9 |
The growth rate of the thickness of the Si layers could be enhanced by adding O2− ions into the molten fluoride; however, the surface morphology of the Si layers must be more carefully controlled by electrochemical parameters. The molten fluoride system, which can fabricate Si films from SiO2 at low temperatures, has the potential to develop environmentally friendly and sustainable electrolytic systems by combining non-consumable oxygen-evolving anodes such as boron-doped diamond electrodes.23
Conclusions
We investigated the effects of O2− ions on the coordination structure of Si ions and electrodeposition process of Si films in molten LiF-NaF-KF mixed with SiO2 powder, both with and without an Li2O system, at 873 K and obtained the following results:
- (1)The phase diagram of the KF-SiO2 binary system was constructed via TG-DTA measurements. The eutectic composition was 50 mol% and the eutectic temperature was 993 K.
- (2)High-temperature Raman spectroscopic data revealed that SiO2 was dissolved by forming Si-F and Si-O bands in molten LiF-NaF-KF and LiF-KF at 873 K and molten KF-SiO2 at 1073 K.
- (3)When Li2O was added into molten LiF-KF and LiF-NaF-KF, the intensity of Raman band due to the [SiO3F]3− structure was increased compared to that without Li2O system. This indicates that the O2− ions can cause breakage of the Si-O-Si bonds of SiO2 or silicate ions such as [Si2O5]2−.
- (4)Electrochemical measurements showed that the cathodic current, attributed to the reduction of Si ions, was significantly increased by adding Li2O into the molten LiF-NaF-KF. The thickness and the current efficiency of the polycrystalline Si layers, prepared by potentiostatic electrolysis at 0.2, 0.3, and 0.4 V for 1 h, were improved by adding Li2O. These results indicated that O2− ions play a key role in the electrodeposition process of Si in molten fluoride and that the design of the molten salts bath is important for obtaining high-quality Si layers with high current efficiency.
Acknowledgment
We sincerely appreciate Professor Y. Fukunaka (Kyoto University) for the valuable discussions. This work was supported by Grant-in-Aid for Scientific Research (No. 15K05652) from JSPS.
ORCID
Takuya Goto 0000-0001-7245-8764