Abstract
Superhydrophobic cobalt (Co) coating with micro-nanofiber structure was rapidly fabricated by a one-step electrodeposition process. The wettability, microstructure, and corrosion resistance of the as-prepared Co coating were evaluated under different test conditions. The Co coating electrodeposited at 20 V for 5 min displays superhydrophobicity with a contact angle of 160° and a sliding angle of 6.2°. The superhydrophobicity of the coating is derived from the form of the cobalt myristate on its surface and the special micro-nanofiber structure. Excessive electrodeposition time or voltage change the micro-nanofiber structure of the coating and then decrease its superhydrophobicity. The coating surface can be switched from superhydrophobic to hydrophilic by heating in an oven and then dipping in the ethanol solution of 0.1 M myristic acid. In addition, the prepared Co coating displays better corrosion resistance than the bare Cu substrate. The corrosive medium with strong alkalinity or highly acidity decreases the corrosion resistance of the Co coating. The corrosion resistance of the coating is consistent with its hydrophobicity. The superhydrophobicity favored the improvement of the corrosion resistance for the coatings.
Export citation and abstract BibTeX RIS
Superhydrophobic surfaces have attracted much attention due to its great importance not only in fundamental research but also in industrial application, such as in the prevention of the adhesion of snow to antennas and windows, self-cleaning traffic indicators, the reduction of frictional drag on ship hulls, metal refining and strain resistant textiles.1,2 The superhydrophobic property of a surface is believed to be governed by a surface chemistry and a rough surface with special micro- and nano-structures. With this respective in mind, numerous researchers have designed superhydrophobic surface via creating rough surface on low surface energy substrate or chemically modifying rough substrate with low surface materials.3–17
Various methods including chemical vapor deposition,3,4 chemical etching,5–8 sol-gel,9,10 eletrospinning,11–13 laser treatment of surface,14,15 and immersion method,16,17 had been reported for constructing superhydrophobic surfaces. However, most of these methods involve severe conditions, such as tedious chemical treatments, expensive materials and complex multi-step processing, limiting their practical applications. Electrodeposition is thought to be one effective technique to create artificial superhydrophobic surfaces due to its simplicity, low-cost and low-temperature method.18–28 Furthermore, electrodeposition technique is suitable for mass production and the micro-nanostructure array can be easily controlled by adjusting the electrodeposition parameters. Xi et al.,18 fabricated the superhydrophobic surface on a series of substrates such as copper, titanium, iron, zinc, etc. They found that the superhydrophobicity of the resulting surfaces resulted from the hierarchical micro- and nano-structures. Chen et al.,19 developed a rapid one-step electrodepositing process to fabricate superhydrophobic nickel deposit on the cathodic surface by copper plate in an electrolytic solution containing nickel chloride, myristic acid and ethanol. However, the evolution of the microstructure and the wettability of the as-prepared coatings with increasing depositing time and voltage were not paid much attention in their reports.
Cobalt (Co) is usually used in the preparation of magnetic, wear-resistant and high-strength alloys.29–31 However, the surface hydrophilicity of the Co coating resulted in its poor corrosion resistance and self-cleaning properties. Chen et al.,32 fabricated Co-based superhydrophobic powder coating on the stainless steel surface. They though the superhydrophobicity was derived from the low surface energy of the coating surface and its special hierarchical structures that consists of mutually stacked spherical particles with a diameter of 2–5 μm. However, Qiu et al.,33 thought the superhydrophobicity of their deposited Co coating resulted from the asthetic flower-like morphology. It is clear that the superhydrophobic mechanism for the Co coating remain controversial in these reports. Moreover, the reports concerning the relation between the electrodeposition parameters, the crystal growth mechanism, and the microstructure of the different superhydrophobic surface are still limited.
The corrosion resistance is an important property for the application of the superhydrophobic surface. Ishizaki et al.,3 revealed that the modified magnesium alloy with a superhydrophobic surface thereon had a higher corrosive resistance than the untreated one. However, the corrosion resistance was not paid much attention referring to the superhydrophobic Co coating.32,33 In this work, the corrosion resistant and superhydrophobic Co coatings with micro-nanofiber structure were prepared by a rapid one-step electrodeposition. A short electrodeposition time of 0.5 min is sufficient to render the superhydrophobic surface with a contact angle over 150°. In addition, the relationships between the electrodeposition parameters, the microstructure, and the wettability of the deposited Co coating were discussed in detail. Finally, the electrochemical corrosion properties of the coatings were investigated under different conditions.
Experimental
Materials and sample preparation
Anhydrous ethanol (Guangzhou Donghong Chemical Plant, China) and myristic acid (CH3 (CH2)12COOH, National Medicine Group Chemical Reagent Co., LTD, China) are analytical reagents and used without purification. Six hydrated cobalt dichloride (CoCl2· 6H2O), analytical reagent, was supplied from Tianjin Fuchen Chemical Factory, China, and used as received.
Two copper plates with a size of 40 × 20 × 1 mm were ultrasonically cleaned in a soap solution for 30 min for degreasing, followed by oxide removal by immersing in 5 vol% H2SO4 and rinsing in water and acetone. The two copper plates were taken as the cathode and anode in an electrolyte cell and a direct current (DC) voltage was applied to two electrodes with a distance of 1.0 cm. The electrolyte is a uniform 100 mL ethanol solution of CoCl2· 6H2O (0.05 M) and myristic acid (0.10 M). The electrodeposition was conducted under room temperature with varying DC voltage and depositing time. After deposition, the Co deposit on the substrate were rinsed with distilled water and dried at ambient temperature for the subsequent characterizations.
Characterizations
Surface morphology of the obtained coating was characterized by a scanning electron microscopy (SEM, S-3700N). The corresponding element distributions were measured by an Energy Dispersed X-ray (EDX) microanalyzer attached with the SEM. The surface roughness of the deposit surface was characterized with a BMT Expert 3D surface profile measurement. The infrared transmission spectra of the scraped powders from the Co deposit were recorded at room temperature on Fourier-transform infrared spectrophotometer with KBr pellet technique (FTIR, Bruker Vector 33). The crystal structures of the scraped powders were studied using an X-ray diffractometer (D/max-IIIA) fitted with a Cu Ka radiation (1.5405 Å). The scanning angle (2θ) ranged from 1.0° to 60.0°, with a step size of 0.02° and counting time of 2 s/step. The water contact angle (CA) and slide angle (SA) were measured with approximately 5 μL water droplets using a measuring apparatus (JC2000A) at ambient temperature. The values reported are averages of five measurements made on different positions of the sample surface. The pH value of the water droplets was adjusted by sulfuric acid and sodium hydroxide.
Electrochemical corrosion test was carried out in a three electrode cell. A platinum plate and saturated calomel electrode (SCE) were used as the counter and reference electrode, respectively. The as-prepared Co deposit was used as a working electrode. Measurements were performed by electrochemical workstation (CorrTest CS310, Wuhan Corr Test Instrument Co. Ltd., China) at room temperature in the different corrosive medium. The pH value of the corrosive medium was adjusted by sulfuric acid and sodium hydroxide. Before the electrochemical tests, coatings were mounted using paraffin wax with a surface area of 1 cm2 exposed to the corrosive medium. The potentiodynamic anodic polarization curves of the electrolyte were recorded at a sweep rate of 0.5 mV·s−1 from −400 to 600 mV of the open circuit potential. The corrosion potential (Ecorr) and corrosion current density (icorr) were determined using the Stern-Geary equation from polarization curves. The linear polarization resistance (Rp) was determined by performing linear sweep voltammetry (LSV) at a scanning rate of 0.1 mV· s−1 in the range from −10 mV to +10 mV of the open-circuit potential. The average value from four replicates for each kind of specimen was reported.
Results and Discussion
Wettablility and microstructure
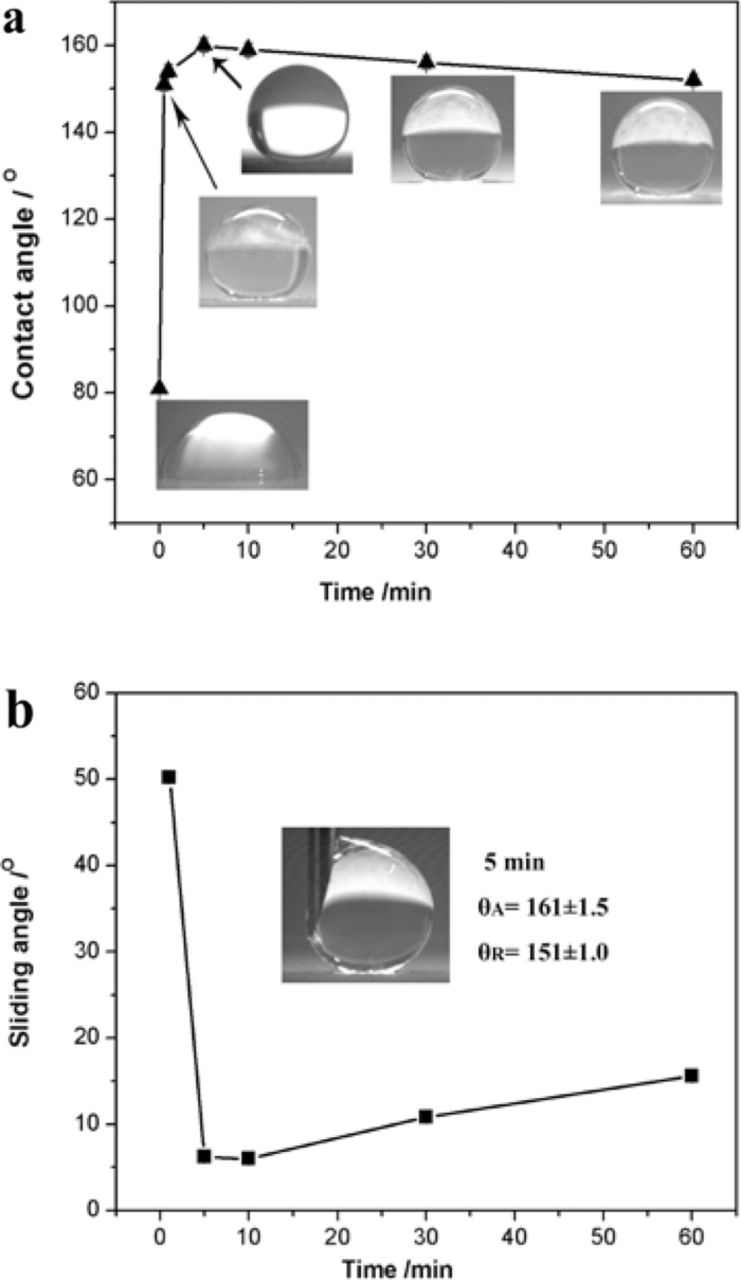
Influence of the electrodeposition time on the contact angle and the sliding angle of a water droplet on the Co deposit produced at 20 V are shown in Fig. 1. The surface of the pure Cu surface was hydrophilic as it had the contact angle of 82°, as shown in Fig. 1a. When electrodeposition time is 0.5 min, the contact angle on the deposited Co surface reached 151°, however the water droplet still could not slide on this surface. With the increase of the electrodeposition time, the contact angle increased and the slide angle decreased. The biggest contact angle of 160° was obtained when the Co coating was electrodeposited for 5 min at 20 V (Fig. 1a). At the same time, the smallest slide angle of 6.2° was acquired (Fig. 1b). The results suggest that a short electrodeposition time within 5 min can fabricate the superhydrophobic Co deposit. This technique has fast and facile advantages. But, as the deposition time went by, the contact angle began to decrease and the slide angle began to increase. When the electrodeposition time was extended to 60 min, the contact angle was only 152° and the slide angle reached 15.6°. The decreased superhydrophobicity with increasing electrodeposition time might be related to the variation of the microstructure of the deposited Co surface.
Figure 1. (a) Contact angle of a water droplet on the Co coating deposited at 20 V with increasing electrodeposition time (Inserted figures are water droplets on the surface); (b) sliding angle on the coating (Inserted figure is the typical advancing/receding contact angle of water droplets on the surface).
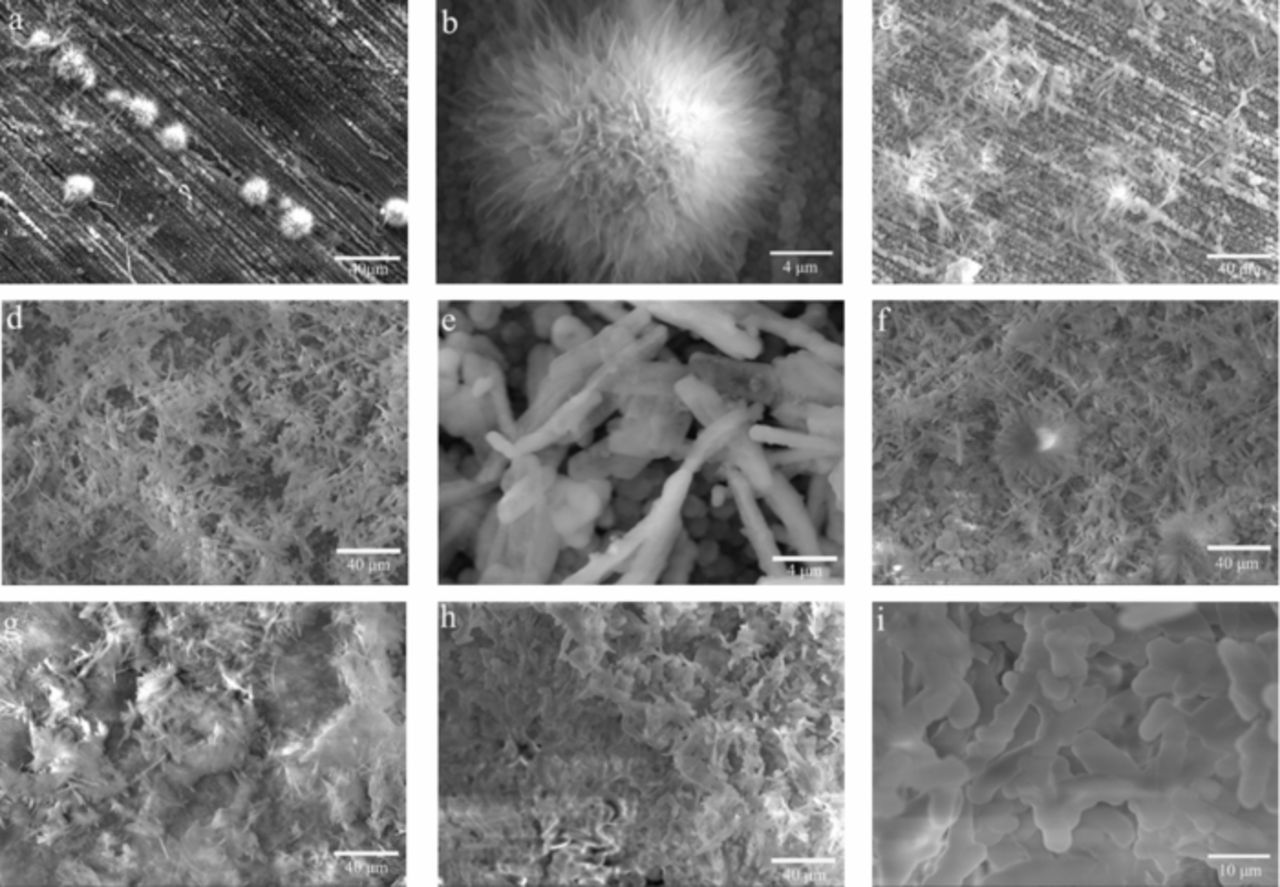
Fig. 2 shows the SEM images of the Co coating produced at 20 V with increasing depositing time. As shown in Fig. 2a, a few globular crystallites with a diameter around 10 μm formed on the substrate surface as the electrodeposition was conducting for 0.5 min. Fig. 2b, the high magnification of Fig. 2a, confirmed that the crystallite particles was like a flower and featured with the nano-sheet structure. When the electrodeposition time was increased to 1 min, the globular crystallites grew to the flocculent crystallites consisting of many micro-nanofiber particles (Fig. 2c). As the electrodeposition time prolonged to 5 min, the number and the size of the micro-nanofibers increased and the uniformity was improved, as shown in Fig. 2d. The high magnification (Fig. 2e) displayed that the micro-nanofiber was featured with a diameter around 1 μm and a length about 5–10 μm. With the further increase of the electrodeposition time to 10 min, the homogeneous crystallites were dense and the surface was almost coated with these micro-nanofibers (Fig. 2f). When the electrodeposition time was extended to 30 min, the coating surface was completely coated with these crystallites, as shown in Fig. 2g. Moreover, it seemed that these micro-nanofibers merged together to form a few bulky sheet crystallites (Fig. 2g). When the growth process lasted to 60 min, most of the micro-nanofibers merged together and a lot of bulky sheet crystallites formed, as shown in Fig. 2h. The magnified image of Fig. 2h showed the bulky sheet crystallites consisting of many micro-rods with a length of 10–20 μm and a diameter of 2–4 μm (Fig. 2i). These micro-rods might derive from the growth of the micro-nanofiber crystallites with increasing electrodeposition time. Generally, the crystallite structure of the Co deposit transforms from the flower to micro-nanofiber, and then to micro-rod with the increase of deposition time.
Figure 2. SEM images of the Co coating deposited at 20 V for different electrodeposition time. ((a) and (b) 0.5 min; (c) 1 min; (d) and (e) 5 min; (f) 10 min; (g) 30 min; (h) and (i) 60 min).
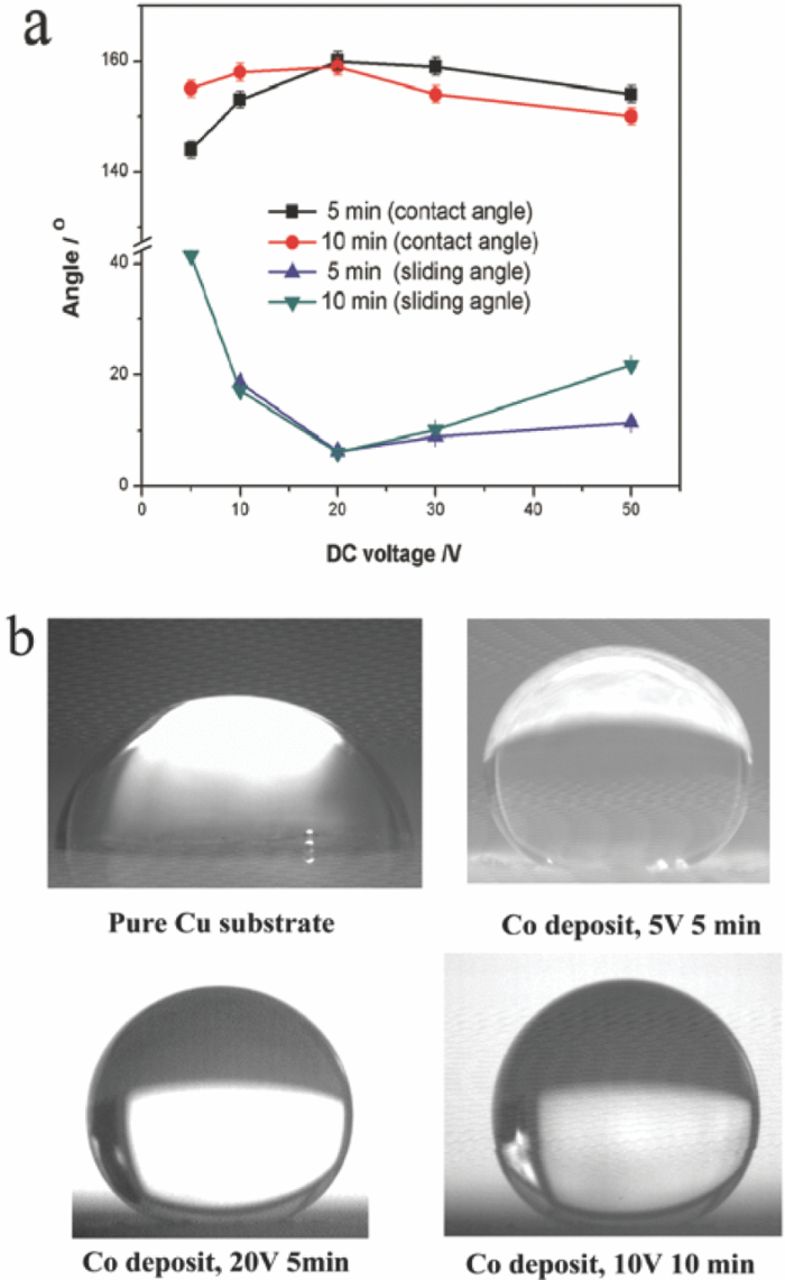
Variations of the contact angle and the sliding angle of a water droplet on the deposited Co surface with increasing DC voltage are shown in Fig. 3a. The typical images of the water droplets on the deposits are shown in Fig. 3b. The contact angle increased with increasing DC voltage up to 20 V and then decreased with the further increase of the voltage. The contact angle of 82° for the pure Cu substrate increased to 144° for the Co surface deposited at 5 V for 5 min, 160° for the surface deposited at 20 V for 5 min, and 158° for the surface deposited at 10 V for 10 min, as shown in Fig. 3a and 3b. The sliding angle rapidly decreased with the increase of the deposition voltage up to 20 V, and then increased with the further increase of the voltage. The Co coating deposited at 20 V for 5 min or 10 min displayed the contact angle around 160° and the sliding angle around 6.0°, indicating that DC voltage of 20 V was sufficient to deposit the superhydrophobic Co surface on the copper substrate. The excessive voltage resulted in the decreased superhydrophobicity for the produced deposit.
Figure 3. (a) Contact angle and sliding angle of the Co coating deposited at different DC voltage for 5 min and 10 min. (b) Typical images of water droplets on the Cu substrate and the Co coating deposited at different conditions.
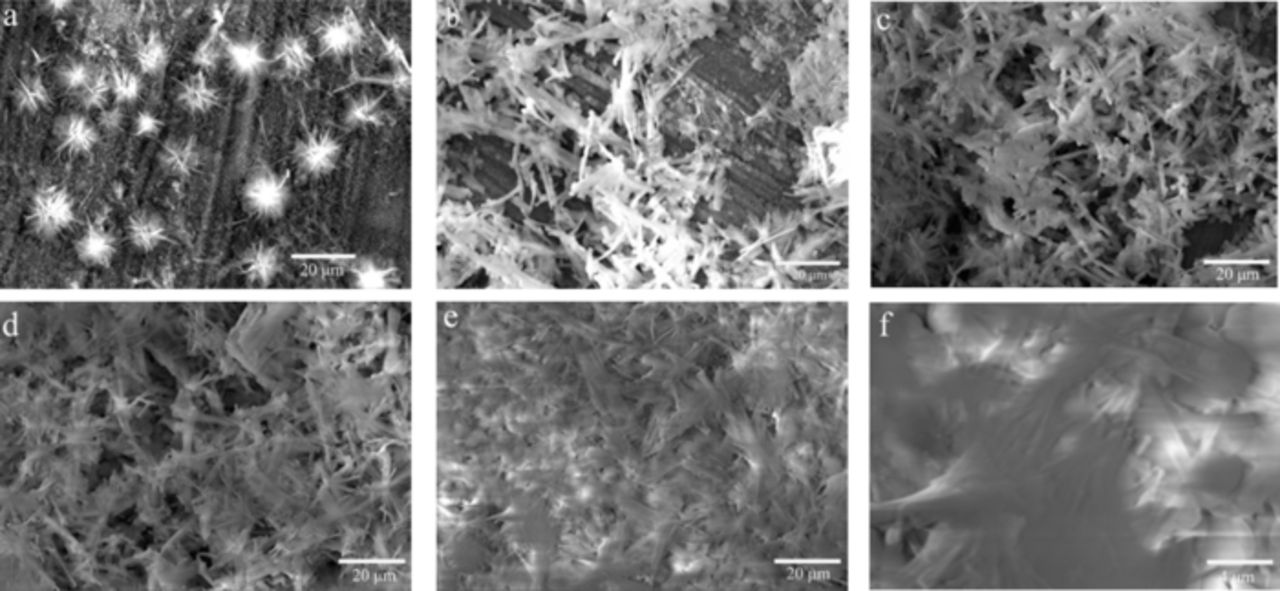
The variations of the wettability for these deposited Co surfaces with increasing DC voltage have relation with their microstructures. SEM images of the coatings produced at different DC voltage for 5 min are shown in Fig. 4. As shown in Fig. 4a, the surface of the substrate was sparsely covered with a few crystallites like flowers, as the electrodeposition was carried out at 5.0 V. With the increase of the DC voltage to 10 V, the crystallites showed the micro-nanofiber structure (Fig. 4b). When the electrodeposition was conducted at 20 and 30 V, the micro-nanofibers became continuous and completely covered the substrate surface, as shown in Fig. 4c and 4d. It seemed that the micro-nanofiber crystallites merged together to form the bulky sheet crystallites, as the deposit was produced at 50 V for 5 min (Fig. 4e and 4f). Generally, the above results from Fig. 1-4 showed that the micro-nanofiber structure had a positive effect on the superhydrophobicity of the produced Co deposit. Besides the morphology, the surface roughness might be another crucial factor to determine the wettability of the deposit surface. However, the surface profile measurements showed that the surface of these coatings were very rough with the average surface roughness more than 4.0 μm and no great differences were found between these coatings produced by different deposition conditions. It can be concluded that the surface roughness was not an important factor to affect the superhydrophobicity of the as-prepared Co deposits in this work.
Figure 4. SEM images of the Co coating deposited at different DC voltage for 5 min. ((a) 5 V; (b) 10 V; (c) 20 V; (d) 30 V; (e) and (f) 50 V).
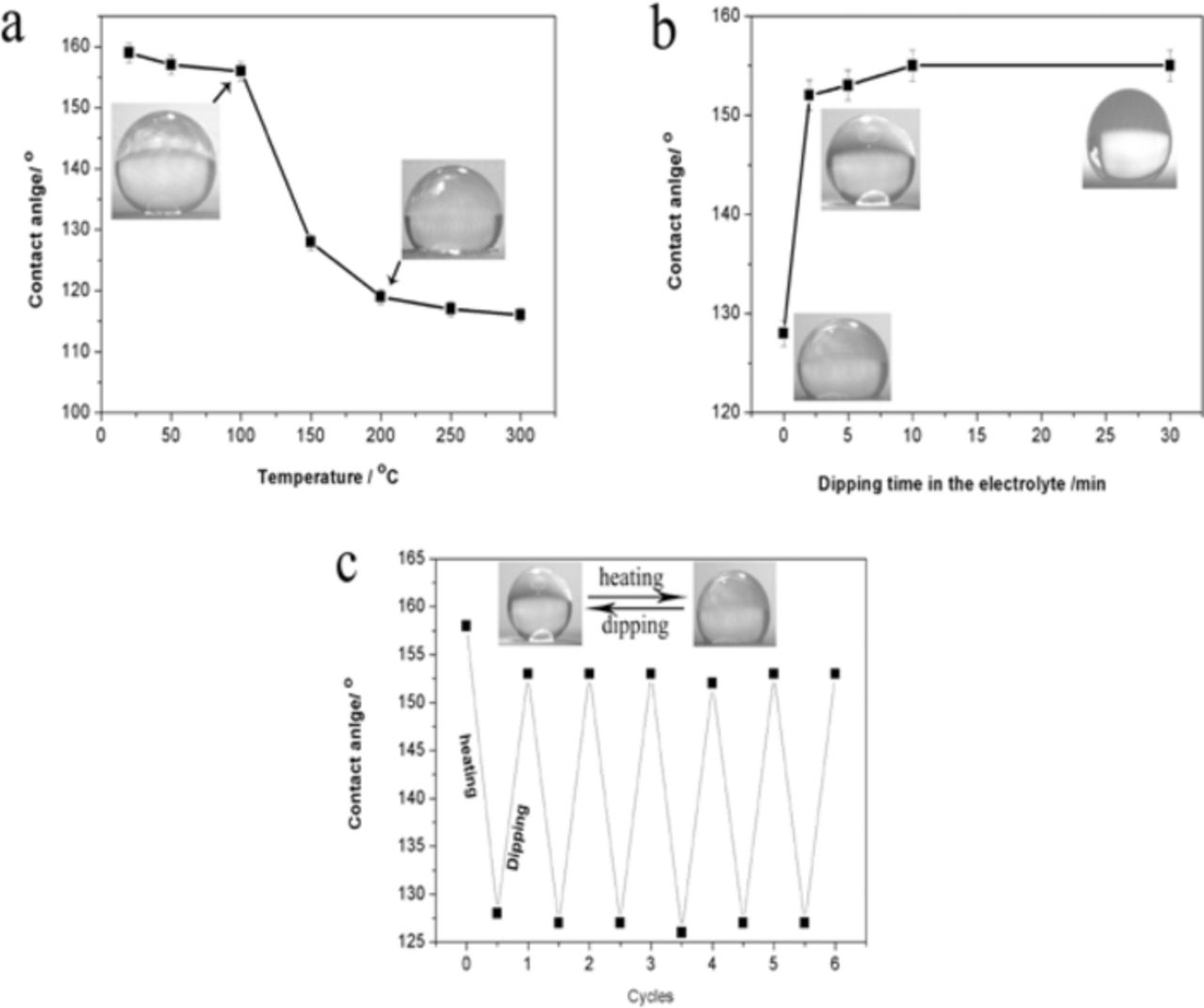
To investigate the environmental temperature on the coating wettability, the coating was kept in an oven for 10 min under different temperatures. Fig. 5a shows the effect of the environmental temperature on the contact angle of the Co coating deposited at 20 V for 5 min. The contact angle slightly decreased with increasing temperature up to 100°C and then rapidly decreased with the further increase of the temperature. As the coating was kept in the oven with 200°C for 10 min, its surface changed to be hydrophilicity with a contact angle lower than 120° from the superhydrophobicity with a contact angle around 160°. Interestingly, the superhydrophobicity of the coating was recovered by dipping in the ethanol solution of 0.1 M myristic acid, as shown in Fig. 5b. The dipping time of 2 min was enough for regaining the superhydrophobic surface with a contact angle over 150°. Fig. 5c shows that the deposit surface can be switched from superhydrophobic to hydrophilic by heating in an oven and then dipping in the ethanol solution of 0.1 M myristic acid.
Figure 5. (a) Variation of the contact angle with the increasing temperature for the Co coating deposited at 20 V for 5 min; (b) Variation of the contact angle with increasing dipping time in ethanol solution of 0.1 M myristic acid; (c) Reversible superhydrophobicity and hydrophilicity transitions of the coating (heating at 150°C for 10 min and dipping for 2 min).
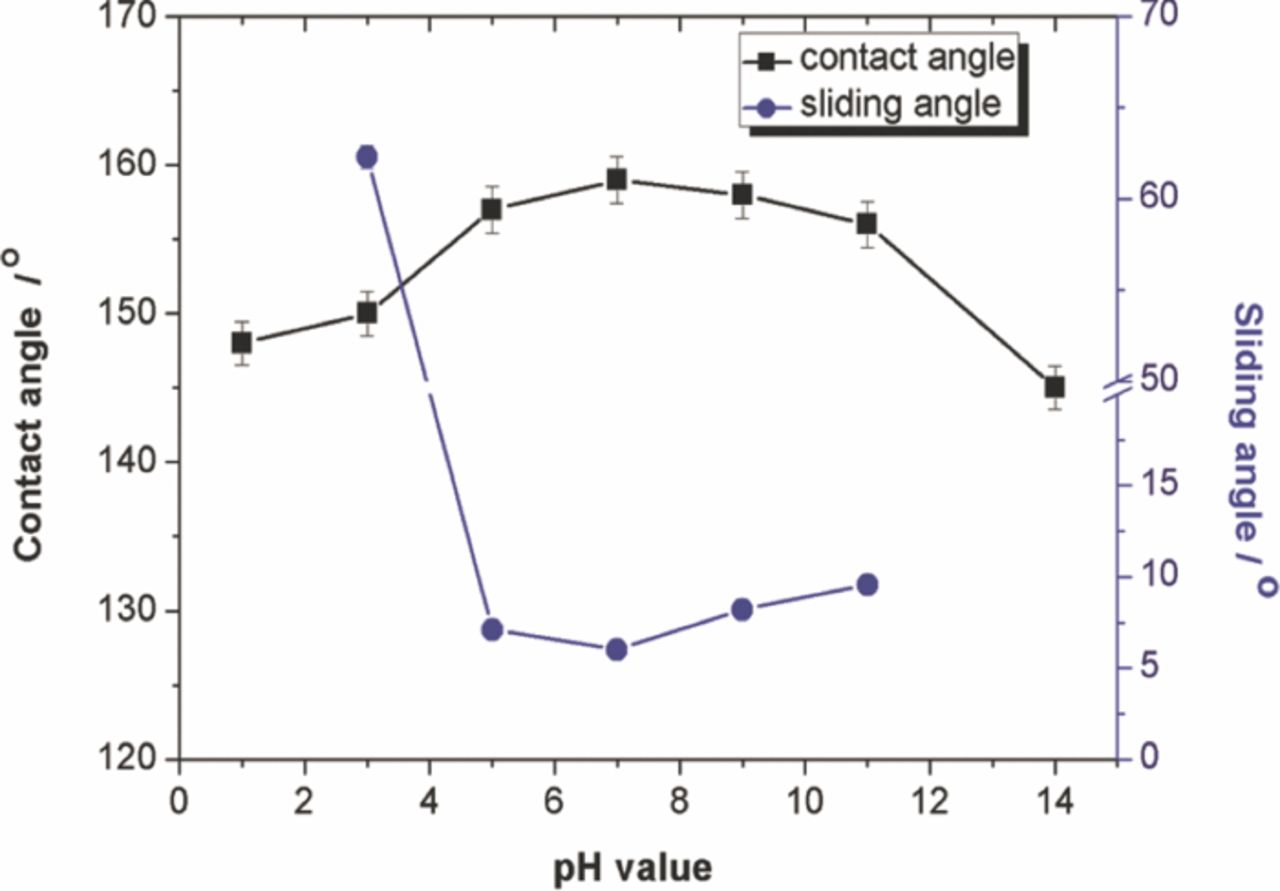
Influences of the pH value of the water droplet on the contact angle and the sliding angle of the Co coating produced at 20 V for 5 min are shown in Fig. 6. The pH value of the water droplet was adjusted by sulfuric acid and sodium hydroxide. The contact angle on the coating surface was larger than 150° in pH values ranging from 3.0 to 11.0. But it was lower than 150° as the pH value was 1.0 or 14.0. At the same time, it was seen that the sliding angle was lower 10° in pH values ranging from 5.0 to 11.0. These results showed that the as-prepared Co deposit had good hydrophobicity in the aqueous solution with pH value from 3.0 to 11.0. The solution with extreme acidity or alkalinity increased the hydrophilicity for the produced Co deposit.
Figure 6. Influence of pH value of water droplet on the contact angle and sliding angle of the Co coating produced at 20 V for 5 min.
Formation mechanism
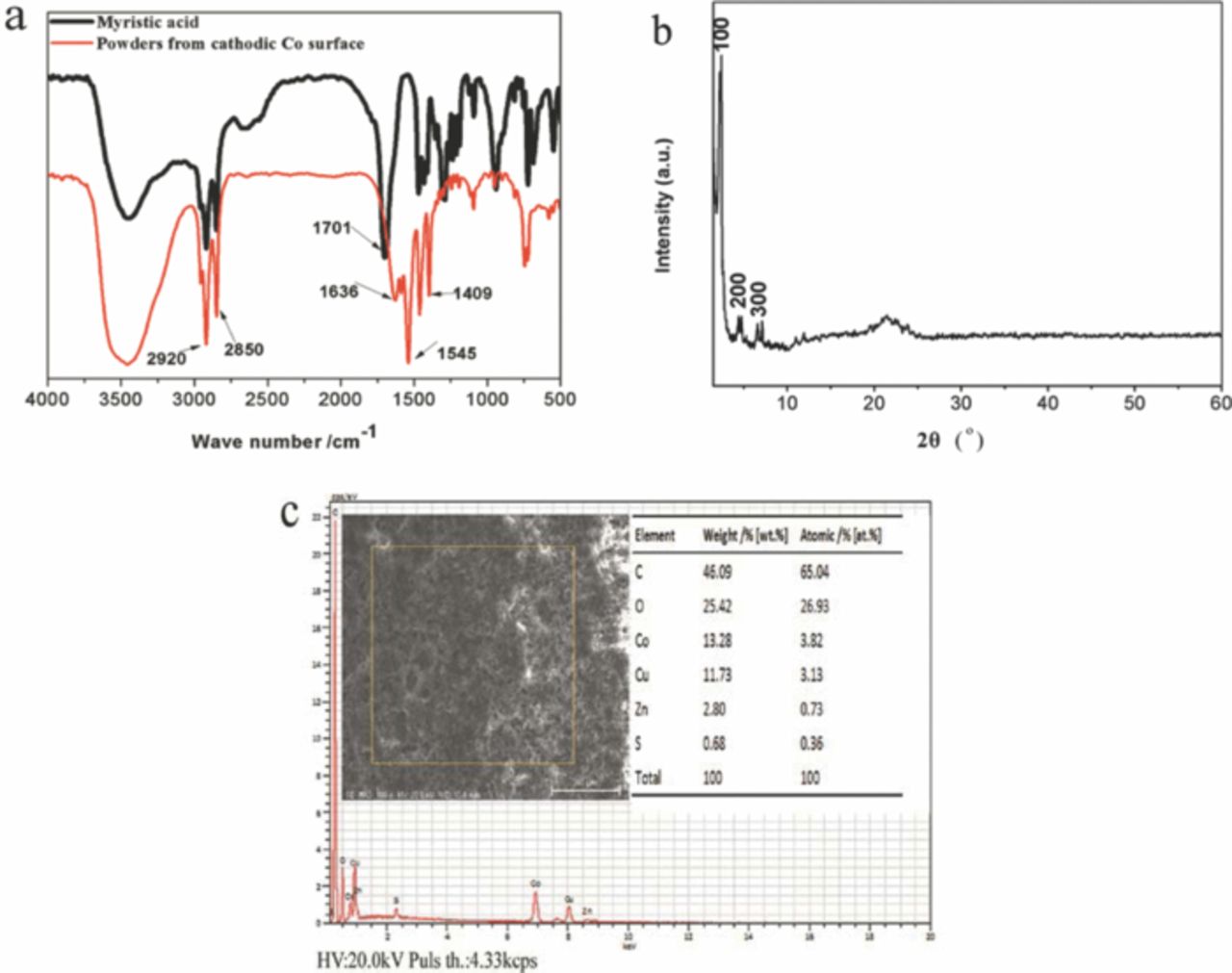
FTIR spectrum of the myristic acid and the powders scraped from the Co deposit produced at 20 V for 5 min is shown in Fig. 7a. The absorbing peaks at 2850 cm−1 and 2920 cm−1, which are attributed to the methylene and methyl symmetric stretch, appeared in the spectrum of the powders from the Co deposit. The peak at 1701 cm−1, the carboxyl group (-COO) in myristic acid, disappeared in the powder from the Co deposit. However, a few new absorbing peaks at 1636, 1545 and 1409 cm−1 are shown in the spectra of the powders, which stem from the asymmetric and symmetric stretches of –COO group.18 Fig. 7b plots the XRD spectrum of the powders scraped from the Co deposit produced at 20 V for 5 min. There are a set of well defined diffraction peaks in the small angle region from 1.5° to 10°, suggesting that the resulting superhydrophobic Co surface was crystallized and had a layer structure.17,21 According to the Bragg's law,34 the d-spacing of the (100), (200) and (300) in this report are 3.47, 1.95 and 1.29 nm, respectively. EDX spectrum, in Fig. 7c, shows that the deposited Co surface are mainly composed from the elements of C, O and Co beside the substrate Cu, which agreed well with the analysis results from FTIR spectrum.
Figure 7. (a) FTIR spectrum of the powders scraped from the Co coating deposited at 20 V for 5 min; (b) XRD pattern of the corresponding powders; (c) EDS spectrum of the Co coating.
These results from Fig. 7a and 7c suggested that the cobalt myristate (Co [CH3 (CH2)12COO] 2) was formed on the deposit surface. When the DC voltage was applied to the two electrodes, the cobalt ions (Co2+) near the cathodic plate reacted with myristic acid and generated the cobalt myristate. The compound of the metal myristate has been confirmed to play an important role in the formation of superhydrohobic surface.18,19,22 In addition, Fig. 1–4 has showed that the superhydrophobicity of the produced Co deposit agreed well with their micro-nanofiber structure. Therefore, it can be concluded that the superhydrophobicity of the deposited Co coating in this work is attributed to the cobalt myristate with low surface energy formed and its special micro-nanofiber structure.
Corrosion resistance
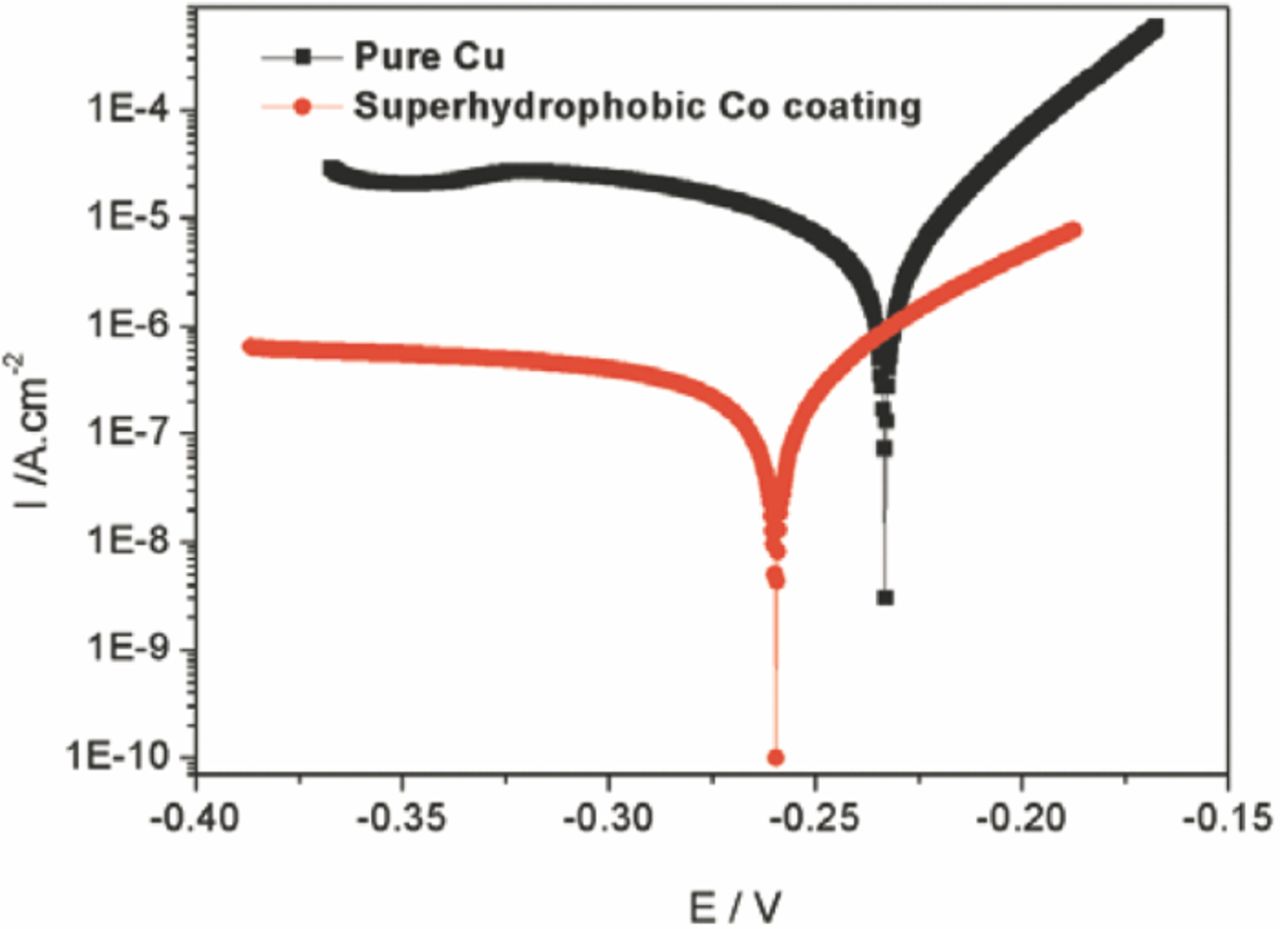
The potentiodynamic polarization curves for the pure Cu and the as-prepared superhydrophobic Co deposit measured in 3.5 wt% NaCl solution are shown in Fig. 8. The corrosion resistances of the samples were derived from the Stern-Geary equation;
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/160/11/D593/revision1/jes_160_11_D593eqn1.jpg)
where icorr is the corrosion current density, Rp is the polarization resistance, βa and βc are the anodic and cathodic Tafel slopes. The calculated corrosion potential (Ecorr), icorr and Rp for the two samples are given in Table I. As shown in Fig. 8 and Table I, the corrosion current density of the as-prepared Co coating was much lower than that of the pure Cu substrate; and the Co coating had much higher polarization resistance when compared to the Cu substrate. These results showed that the as-prepared Co deposit displayed better anticorrosive properties than the bare Cu substrate, which might be attributed to the superhydrophobicity of the deposited Co coating.19
Table I. Corrosion potential (Ecorr), corrosion current (icorr) and polarization resistance (Rp) of the pure Cu and the superhydrophobic Co coating.
| Sample | Ecorr /mV | icorr/A·cm−2 | Rp/ kΩ·cm−2 |
|---|---|---|---|
| Pure Cu | −233.1 | 7.84 × 10−6 | 0.356 |
| Co coating | −259.6 | 4.08 × 10−7 | 24.2 |
Figure 8. Potentiodynamic polarization curves measured in 3.5 wt% NaCl solution for the pure Cu and the superhydrophobic Co coating deposited at 20 V for 5 min.
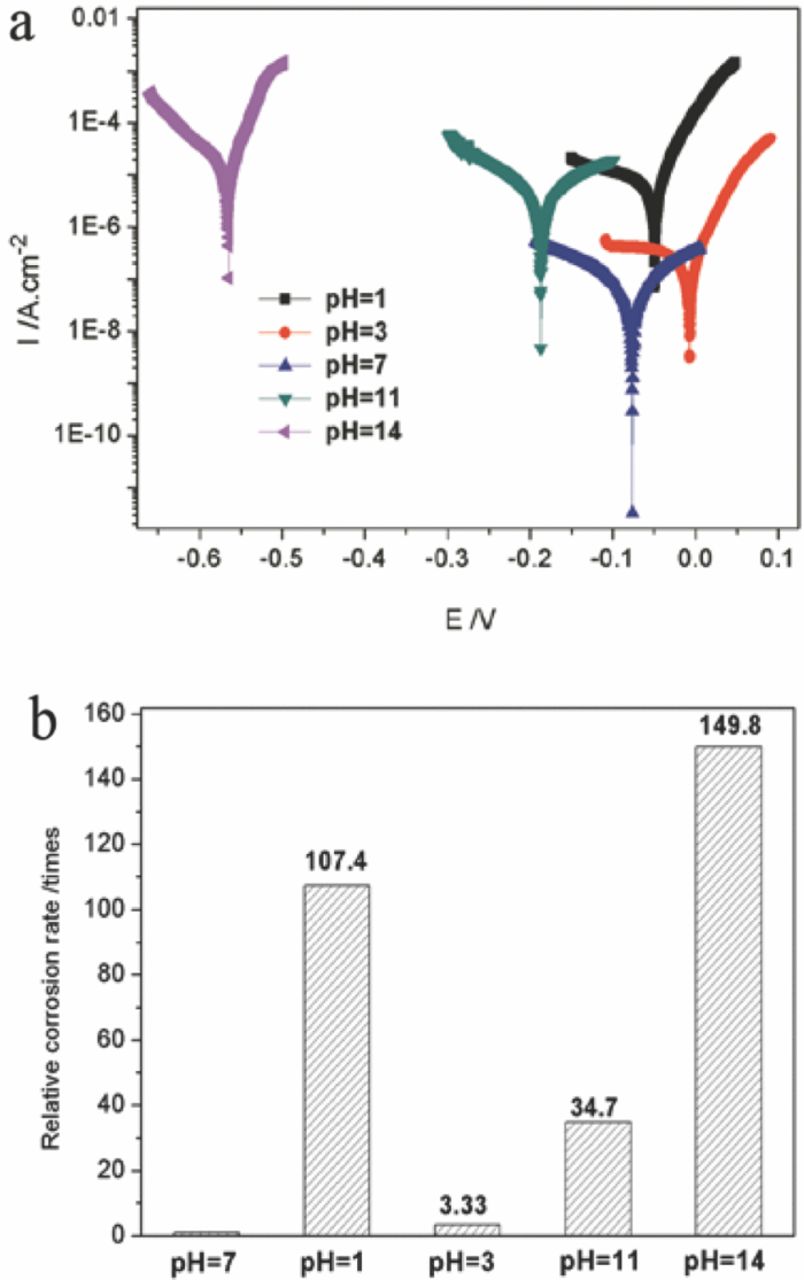
Fig. 9a shows the potentiodynamic polarization curves of the Co coating deposited at 20 V for 5 min measured in the aqueous solution with varying pH value. The coating exhibited the lowest corrosion current density measured in the solution with pH = 7.0. The strong alkali (pH = 14) or acidic solution (pH = 1) leaded to the decreased corrosion resistance due to the rapid increase of corrosion current density. The relative corrosion rates of the Co coating in these corrosive medium are shown in Fig. 9b. The relative corrosion rate was calculated by fitting the potentiodynamic polarization curves using formulas recommended by the equipment manufactures. The corrosion rate of the coating in the solution with pH = 7 was defined as 1. It was seen that the corrosion rates in the solution with pH = 14, 1 and 11 show a 149.8-fold, 107.4-fold and 34.7 fold improvement, respectively, compared to in the solution of pH = 7. These results suggested that the corrosive medium with strong alkality and acidity decreased the corrosion resistance of the as-prepared Co coating, which agreed well with its wettability with increasing pH value (Fig. 6). The results from Fig. 6 and Fig. 9 indicated that the corrosion resistance of the as-prepared Co coating was consistent with its hydrophobicity. The superhydrophobicity of the coating favored the improvement of the corrosion resistance.
Figure 9. Potentiodynamic polarization curves (a) and the relative corrosion rates (b) of the superhydrophobic Co coating deposited at 20 V for 5 min in the solution with varying pH values.
Conclusions
The corrosion resistant and superhydrophobic Co coatings with micro-nanofiber structure were prepared by a rapid one-step electrodeposition. The as-prepared Co deposit displayed superhydrophobicity with a contact angle of 160° and a sliding angle of 6.2° as it was deposited at 20 V for 5 min. The superhydrophobicity of the coating was attributed to the cobalt myristate with low surface energy formed on its surface and the special micro-nanofiber structure. Excessive electrodeposition time or voltage changed the micro-nanofiber structure of the as-prepared Co coating and then decreased its superhydrophobicity. The surface of the Co coating was switched from superhydrophobic to hydrophilic by heating in an oven and then dipping in the ethanol solution of 0.1 M myristic acid. The as-prepared Co deposit displayed better anticorrosive properties than the bare Cu substrate. The corrosive medium with strong alkality and acidity decreased the corrosion resistance of the coating. The corrosion resistance of the coating was consistent with its hydrophobicity.
Acknowledgments
The authors are grateful to the support of the National Natural Science Foundation of China (Grants: 51275176), the Fundamental Research Funds for the Central Universities (Grants: 2011ZZ0055) and Pearl River Science & Technology Star (Grants: 2012J2200068) for financial support.