Abstract
Amorphous alloys were prepared in a wide composition range by the addition of tantalum to manganese by the sputter deposition method. The effect of alloying tantalum on the corrosion behavior and the stability of sputter-deposited amorphous Mn-Ta alloys was studied. Different techniques such as weight-loss measurements, electrochemical techniques, and X-ray photoelectron spectroscopy (XPS) analysis were used. The addition of Ta remarkably reduces the rate of dissolution of the alloy and Ta passivates spontaneously. No signs of pitting corrosion or transpassive dissolution of Mn were observed. The anodic current density decreases significantly with increasing the tantalum content. XPS studies show a preferential oxidation of Ta in the air-formed (native) oxide. The preferential oxidation was remarkable after immersion in 1 M  solution for 30 min. The thickness of the passive film formed on the sputter-deposited Mn-30Ta alloy increases linearly with the applied potential as that observed in valve metals. The formed passive film consists mainly of
solution for 30 min. The thickness of the passive film formed on the sputter-deposited Mn-30Ta alloy increases linearly with the applied potential as that observed in valve metals. The formed passive film consists mainly of  At potentials higher than +0.7 V (standard calomel electrode) high oxidation states of Mn were found to be incorporated in the passive film. © 2001 The Electrochemical Society. All rights reserved.
At potentials higher than +0.7 V (standard calomel electrode) high oxidation states of Mn were found to be incorporated in the passive film. © 2001 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
In recent years, the sputter deposition technique is being realized as important in alloy preparation and thin film technology as well as in different scientific studies.1 2 3 4 5 6 Some advantages of the sputtering technique are the preparation of sputtered film thickness with controlled thickness and well-defined stoichiometry, and the exclusion of oxide/metal interactions by depositing the oxide films on inert substrates. In addition, sputtering is particularly suitable to form single phase solid solution even when the boiling point of one component is higher than the melting points of the rest of components and/or when one component is immiscible with another in the liquid state.7 By utilizing these advantages, the sputter deposition method was widely used to produce highly corrosion-resistant materials.6 7 8 9 10 11 In this regard, Frankel et al. used sputter-deposited thin films to investigate the effect of metal film thickness on the behavior of pits in Ni-20% Fe and the effect of alloying addition on the repassivation behavior of pit in Al and Al-alloy.1 2 3 Pitting of sputter-deposited films is interesting because growing pits quickly penetrate the thin film and propagate radially, essentially as two-dimensional pits with the walls perpendicular to the substrate. In such cases, the pit depth can be carefully controlled and hence, assumptions and approximations can be minimized.
It is well known that manganese is one of the most reactive elements in the galvanic series, and that it is readily attacked by water to form manganese hydroxide with the evolution of hydrogen. However, manganese is used to form many important alloys. With other elements (such as Al, Sb, and Cu), the metal forms highly ferromagnetic alloys. After special treatment, manganese metal is ferromagnetic. Therefore, forming highly corrosion-resistant materials of manganese may create a wider application of this important metal. A series of homogenous single phase binary manganese valve metal alloys have, recently, been prepared by sputter deposition in order to obtain highly corrosion-resistant materials.9 10 11
In the present study, the stability of the sputter-deposited amorphous Mn-Ta alloys, as corrosion resistant materials, has been investigated in chloride-free and chloride-containing  solutions. In this respect, different techniques were employed such as weight-loss measurements, electrochemical techniques, and X-ray photoelectron spectroscopy (XPS) analysis. Special attention was directed to the effect of film composition, structure, and thickness of the surface films on the corrosion behavior of the alloys.
solutions. In this respect, different techniques were employed such as weight-loss measurements, electrochemical techniques, and X-ray photoelectron spectroscopy (XPS) analysis. Special attention was directed to the effect of film composition, structure, and thickness of the surface films on the corrosion behavior of the alloys.
Experimental
The Mn-Ta alloys were prepared by direct current (dc) magnetron sputtering on glass substrates. The target was composed of 99.95% pure tantalum disk of 100 mm diam and 6 mm thickness, on the sputter erosion region of which 99.9% pure electrodeposited manganese (ED-Mn) plates of irregular shapes were placed. The substrates were made from glass plates which were rinsed by immersion in water, containing a commercial detergent, at 75°C. Three water-cooled substrates were installed downward in the sputtering chamber facing the target. For the sake of homogeneity of the sputter deposits, the substrates were revolved around the central axis of the chamber, so that the substrates passed directly above the target. Also, the substrates revolved around their own axes. After the installation of the target and substrates, the sputtering chamber was evacuated to about  After presputtering of the target for 30 min, the sputtering of the substrate was conducted at
After presputtering of the target for 30 min, the sputtering of the substrate was conducted at  in argon gas. The composition of sputter-deposits was controlled by changing the number of manganese plates on the tantalum disk, and was determined by electron probe microanalysis (Shimadzu EPMA-C I). The structure of sputter-deposited alloys was identified by X-ray diffraction (XRD) with Cu Kα radiation at θ-2θ mode.
in argon gas. The composition of sputter-deposits was controlled by changing the number of manganese plates on the tantalum disk, and was determined by electron probe microanalysis (Shimadzu EPMA-C I). The structure of sputter-deposited alloys was identified by X-ray diffraction (XRD) with Cu Kα radiation at θ-2θ mode.
Prior to the electrochemical experiments, the samples were mechanically polished with diamond spray down to 0.25 μm. All tested samples were cleaned in acetone after mechanical polishing. The open-circuit potential of the prepared alloys was recorded, up to 60 min, from the moment of electrode immersion in the test solution until it reached steady state, using a high impedance digital multimeter. The electrochemical impedance investigations and the polarization measurements were carried out using an IM5d system (Zahner Elektrik GmbH & Co., Kronach, Germany).
The excitation amplitude was 10 mV peak-to-peak in a frequency domain  to
to  Potential and current control in the cell were achieved using the same system. All experiments were performed at constant temperature of
Potential and current control in the cell were achieved using the same system. All experiments were performed at constant temperature of  using a water thermostat with a double-walled all glass electrochemical cell. The potentials were measured against and referred to the saturated calomel as a reference electrode. Potentiodynamic polarization was carried out after immersion in the test solution and the steady open-circuit potential was reached. A potential sweep rate of 0.5 mV s−1 was used and potentiostatic polarization measurements were performed for 30 min at each potential. The counter electrode was a large area platinum wire. Details of the experimental procedures are described elsewhere.12
13
using a water thermostat with a double-walled all glass electrochemical cell. The potentials were measured against and referred to the saturated calomel as a reference electrode. Potentiodynamic polarization was carried out after immersion in the test solution and the steady open-circuit potential was reached. A potential sweep rate of 0.5 mV s−1 was used and potentiostatic polarization measurements were performed for 30 min at each potential. The counter electrode was a large area platinum wire. Details of the experimental procedures are described elsewhere.12
13
X-ray photoelectron spectra were measured by a Shimadzu-ESCA 850 photoelectron spectrometer with Mg Kα excitation for surface analysis. Binding energies were calibrated as reported previously.14 The binding energies of Au  and
and  electrons of gold metal and Cu
electrons of gold metal and Cu  and
and  electrons of copper metal were taken as 84.07, 87.74, 952.53, and 952.35 eV, respectively, and the kinetic energy of the Cu
electrons of copper metal were taken as 84.07, 87.74, 952.53, and 952.35 eV, respectively, and the kinetic energy of the Cu 

 Auger electrons of copper metal was taken as 918.65 eV.14 The binding energies at the peaks observed in photoelectron spectra were further corrected by taking the binding energy of C 1s as 285.0 eV when the specimen showed charging effect. The composition and thickness of the surface film were quantitatively determined by a previously proposed method using integrated intensities of X-ray photoelectron spectra.15
16 The photoionization cross section of Mn
Auger electrons of copper metal was taken as 918.65 eV.14 The binding energies at the peaks observed in photoelectron spectra were further corrected by taking the binding energy of C 1s as 285.0 eV when the specimen showed charging effect. The composition and thickness of the surface film were quantitatively determined by a previously proposed method using integrated intensities of X-ray photoelectron spectra.15
16 The photoionization cross section of Mn  and Ta 4f electrons relative to that of O 1s electrons used were 2. 4729 and 2.617,11 respectively. For angle-resolved XPS, the angle between the specimen surface and the direction of photoelectron to the detector (take-off angle) was changed by using tilted specimen stages. Other XPS measurements were carried out at fixed take-off angle of 90°.
and Ta 4f electrons relative to that of O 1s electrons used were 2. 4729 and 2.617,11 respectively. For angle-resolved XPS, the angle between the specimen surface and the direction of photoelectron to the detector (take-off angle) was changed by using tilted specimen stages. Other XPS measurements were carried out at fixed take-off angle of 90°.
Results and Discussion
Hereafter, all alloy compositions are denoted in atomic percentage. XRD patterns of sputter-deposited Mn-Ta alloys revealed that the alloys containing less than 25 atom % Ta consist of a mixture of amorphous phase and unidentified metastable phase or α-Mn single phase, whereas the alloys containing 25-92 atom % Ta show the halo pattern typical of an amorphous structure.9 Consequently, the sputter-deposited Mn-Ta alloys are interesting materials for the corrosion resistance investigation, especially in aggressive solutions.
Corrosion rates and open-circuit potential measurements
The corrosion rates of the alloys containing less than 25 atom % Ta is significantly high, and due to the limited thickness of the sputtered film (∼2 μm), it dissolves in a few seconds. The corrosion rates were calculated for different compositions of sputter-deposited amorphous Mn-Ta alloys using the weight-loss method. Table I presents the measured corrosion rates of these alloys after immersion for 1 week in 1 M  solution at 30°C. The corrosion rate of ED-Mn after 1 h immersion was also presented for comparison. ED-Mn was found to dissolve abruptly with evolution of hydrogen gas after immersion in the 1 M
solution at 30°C. The corrosion rate of ED-Mn after 1 h immersion was also presented for comparison. ED-Mn was found to dissolve abruptly with evolution of hydrogen gas after immersion in the 1 M  solution. The corrosion rate of ED-Mn was estimated to be
solution. The corrosion rate of ED-Mn was estimated to be  The data of Table I shows that the corrosion rate remarkably decreases with increasing Ta content in the alloy.
The data of Table I shows that the corrosion rate remarkably decreases with increasing Ta content in the alloy.
Table I.
Corrosion rates for ED-Mn and amorphous Mn-Ta alloys measured in 1 M 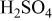 solution at 30°C. solution at 30°C. | ||||||
|---|---|---|---|---|---|---|
| Ta content (atom %) | 0 | 24 | 30 | 36 | 40 | 48 |
| Corrosion rate (mm year−1) |
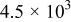 | 9 |
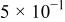 |
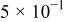 |
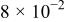 |
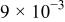
|
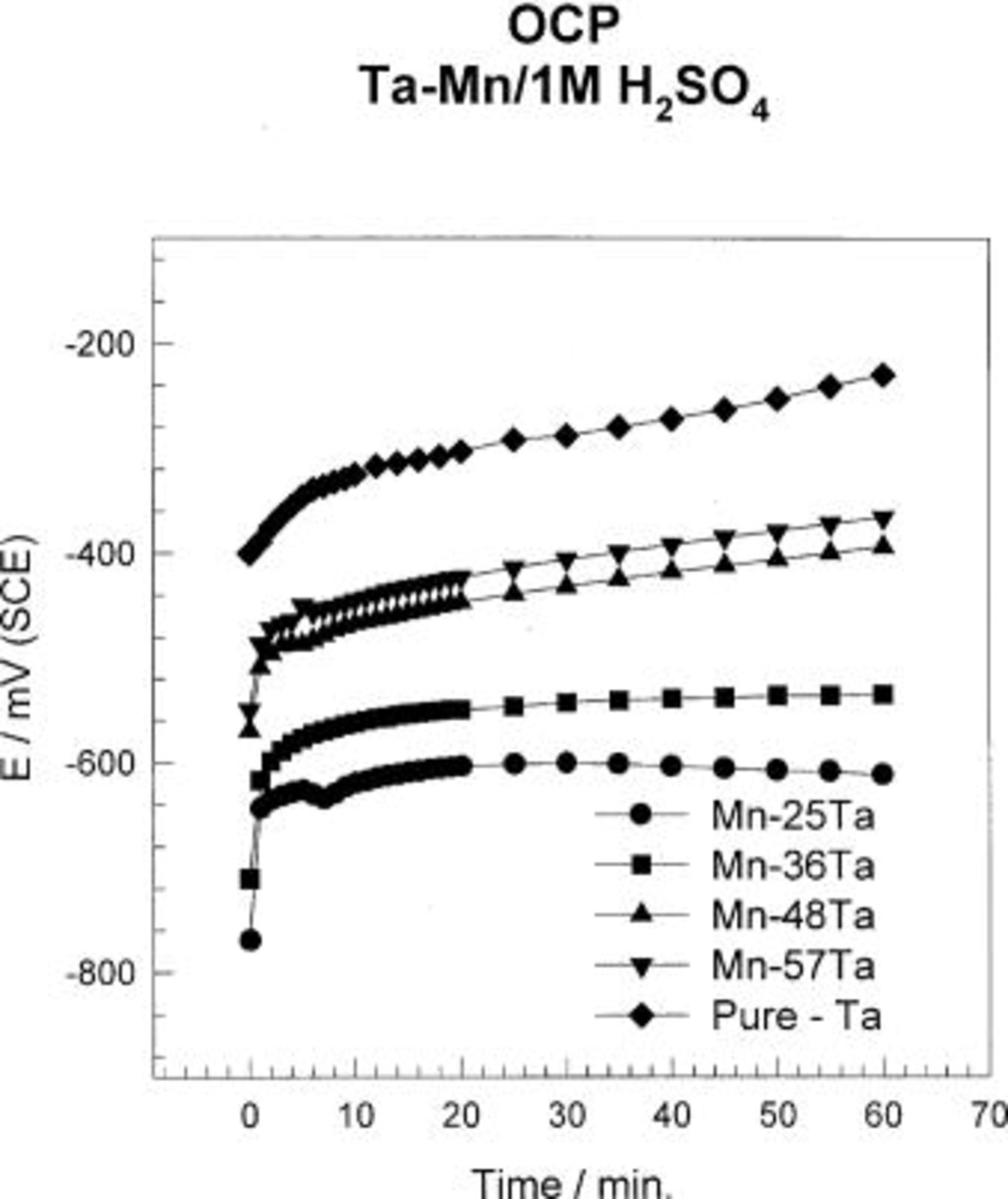
The open-circuit potential of different amorphous Mn-Ta alloy compositions, Mn-(25-57) Ta, was recorded with time over a period of 60 min in 1 M  solution (cf. Fig. 1). For comparison, the open-circuit potential of sputter-deposited Ta was also recorded. Alloys containing ⩾25 atom % Ta show high stability in the acid solution. In general, the open-circuit potential of samples containing ⩾25 atom % Ta shifts toward more positive potential with time. The steady-state potential for samples containing
solution (cf. Fig. 1). For comparison, the open-circuit potential of sputter-deposited Ta was also recorded. Alloys containing ⩾25 atom % Ta show high stability in the acid solution. In general, the open-circuit potential of samples containing ⩾25 atom % Ta shifts toward more positive potential with time. The steady-state potential for samples containing  was reached within 20 min from sample immersion in the test solution. Alloys containing ⩾40 atom % Ta and sputter-deposited Ta show continuous shift of the open-circuit potential toward a more positive direction, indicating continuous passivation over the period of measurements.
was reached within 20 min from sample immersion in the test solution. Alloys containing ⩾40 atom % Ta and sputter-deposited Ta show continuous shift of the open-circuit potential toward a more positive direction, indicating continuous passivation over the period of measurements.
Figure 1. Variations of the potentials of sputter-deposited Mn-Ta alloys with time in naturally aerated unstirred  solution at 30°C.
solution at 30°C.
Potentiodynamic and electrical impedance spectroscopy (EIS) measurements
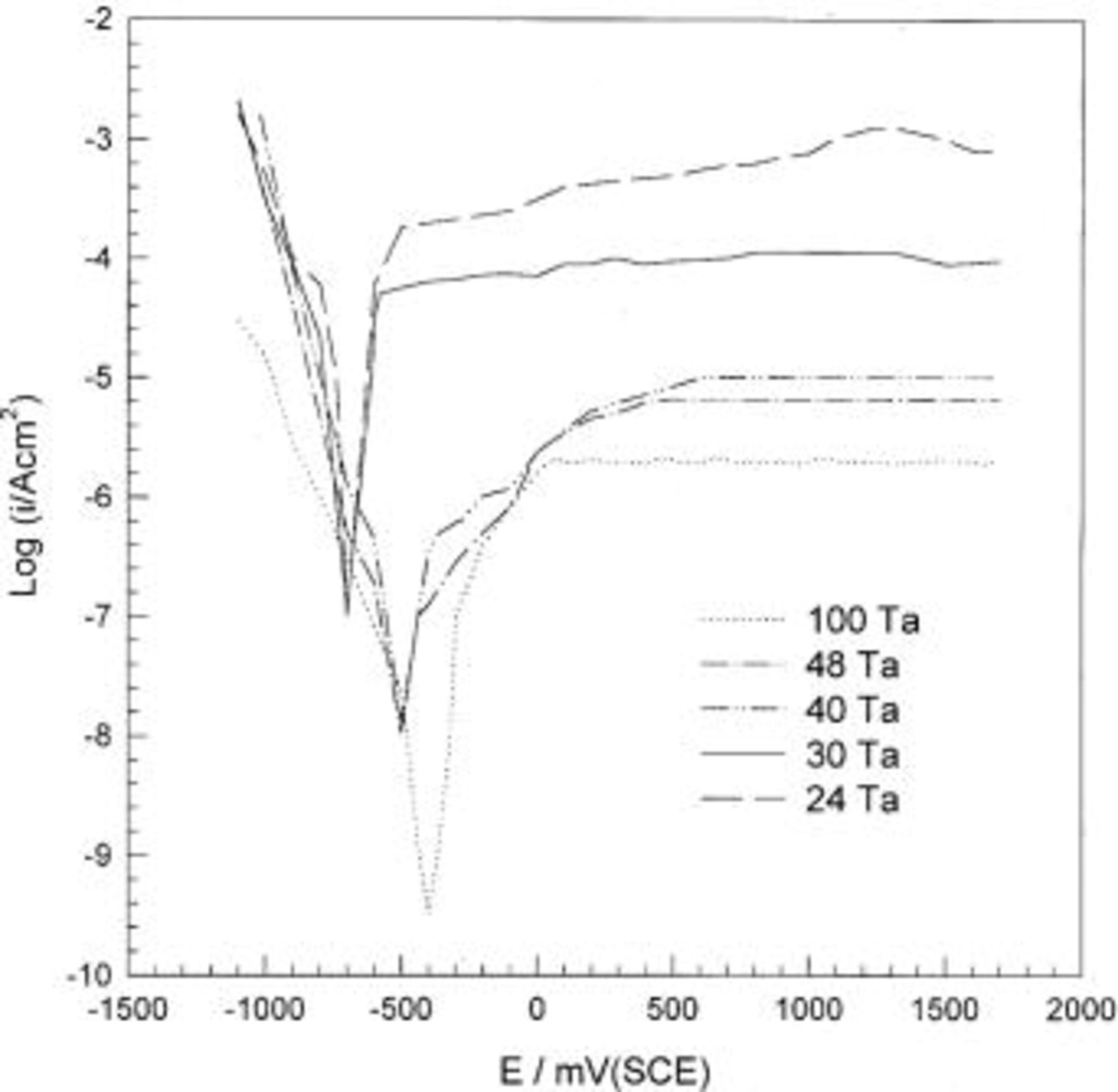
In order to explore and to understand the role of the alloying element on the stability of the prepared binary amorphous alloys, electrochemical measurements were performed. Potentiodynamic curves of the sputter-deposited amorphous Mn-Ta alloys in 1 M  solution were traced (cf. Fig. 2). In spite of the fact that ED-Mn dissolves instantaneously in 1 M
solution were traced (cf. Fig. 2). In spite of the fact that ED-Mn dissolves instantaneously in 1 M  the addition of Ta remarkably reduces the rate of dissolution of Mn, and the alloys passivate spontaneously. Neither pitting nor transpassivation was observed by anodic polarization. The anodic current density decreases significantly with increasing Ta content. Nevertheless, the alloys still show high current density compared to that of sputter-deposited tantalum. This means that the passive films formed on these alloys contains defects from which the active dissolution current flows.
the addition of Ta remarkably reduces the rate of dissolution of Mn, and the alloys passivate spontaneously. Neither pitting nor transpassivation was observed by anodic polarization. The anodic current density decreases significantly with increasing Ta content. Nevertheless, the alloys still show high current density compared to that of sputter-deposited tantalum. This means that the passive films formed on these alloys contains defects from which the active dissolution current flows.
Figure 2. Potentiodynamic polarization curves of sputter-deposited Mn-Ta alloys in naturally aerated unstirred  solution at 30°C.
solution at 30°C.
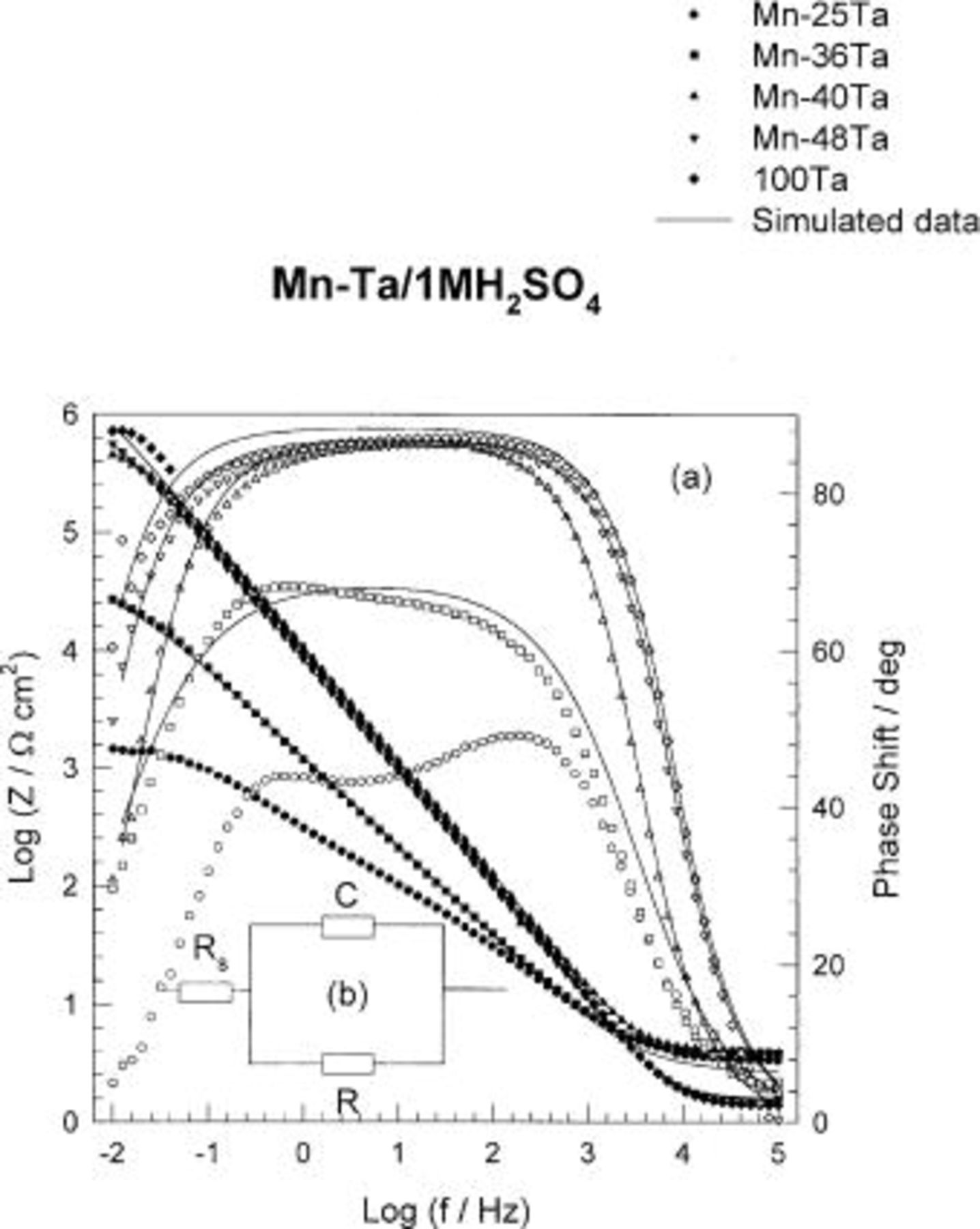
The impedance behavior of the sputter-deposited films of pure Ta and Mn-Ta alloys containing 25-57 Ta was investigated. The impedance data recorded after 60 min of sample immersion in 1 M  solution at 30°C were presented as Bode plots in Fig. 3a. For sputter-deposited films of Ta and Mn-Ta alloys containing ⩾40 atom % Ta, Bode plots show high impedance values and broad single phase maxima. This behavior is characteristic for valve metals, especially tantalum, which is characterized by the formation of stable passive film on its surface.12
17 For a sample containing 25-36 Ta, lower impedance values and two phase maxima were recorded. This indicates the formation of less protective film and also the dissolution of the sample which could be observed by the naked eye.
solution at 30°C were presented as Bode plots in Fig. 3a. For sputter-deposited films of Ta and Mn-Ta alloys containing ⩾40 atom % Ta, Bode plots show high impedance values and broad single phase maxima. This behavior is characteristic for valve metals, especially tantalum, which is characterized by the formation of stable passive film on its surface.12
17 For a sample containing 25-36 Ta, lower impedance values and two phase maxima were recorded. This indicates the formation of less protective film and also the dissolution of the sample which could be observed by the naked eye.
Figure 3. (a) Bode plots of sputter-deposited Mn-Ta alloys after 60 min of immersion in naturally aerated unstirred  solution at 30°C. (b) Equivalent circuit used in the fitting of the impedance data of Mn-Ta alloys at different conditions;
solution at 30°C. (b) Equivalent circuit used in the fitting of the impedance data of Mn-Ta alloys at different conditions; 
 film resistance, and
film resistance, and  film capacitance.
film capacitance.
The impedance data for Mn-Ta sputter-deposited films in 1 M  solution under open-circuit potential conditions were analyzed. The equivalent circuit model consists of a capacitor, C, in parallel with a resistor, R, representing the passive film capacitance and resistance, respectively, and the solution resistance is represented by a resistor,
solution under open-circuit potential conditions were analyzed. The equivalent circuit model consists of a capacitor, C, in parallel with a resistor, R, representing the passive film capacitance and resistance, respectively, and the solution resistance is represented by a resistor,  in series with the above parallel combination (cf. Fig. 3b). The electrode impedance modulus, Z, in this study was found to be represented by the mathematical formulation
in series with the above parallel combination (cf. Fig. 3b). The electrode impedance modulus, Z, in this study was found to be represented by the mathematical formulation
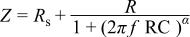
where α denotes an empirical parameter  and f is the frequency in hertz. The above relation is known as the dispersion formula and it takes into account the deviation from the ideal RCbehavior in terms of a distribution of time constants due to surface inhomogeneties, roughness effects, and variations in properties or compositions of surface layers,18
19
20
and f is the frequency in hertz. The above relation is known as the dispersion formula and it takes into account the deviation from the ideal RCbehavior in terms of a distribution of time constants due to surface inhomogeneties, roughness effects, and variations in properties or compositions of surface layers,18
19
20
The calculated parameters showed that the passive film resistance is quite low, 1-12 kΩ cm2, for less than 36 Ta content (Table II). For alloys of higher Ta contents remarkable increase in the passive film resistance with increase of the Ta content (cf. Table II) was recorded. Also, the values of the empirical parameter, α, are very close to 1 for sputter Ta and high Ta content alloys reflecting smooth surfaces.
Table II.
Equivalent circuit parameters for Mn-Ta sputter-deposited film after 60 min of sample immersion in 1 M 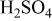 solution at 30°C. solution at 30°C. | ||||
|---|---|---|---|---|
| % Ta |
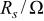 |
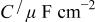 | α |
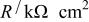 |
| 25 | 19.5 | 28.64 | 0.63 | 1 |
| 31 | 20.4 | 12.95 | 0.84 | 12 |
| 36 | 24.0 | 26.5 | 0.78 | 35 |
| 40 | 11.5 | 11.9 | 0.97 | 536 |
| 48 | 5.2 | 14.9 | 0.97 | 933 |
| 57 | 4.7 | 14.1 | 0.97 | 1212 |
| 100 | 3.5 | 14.3 | 0.99 | 1896 |
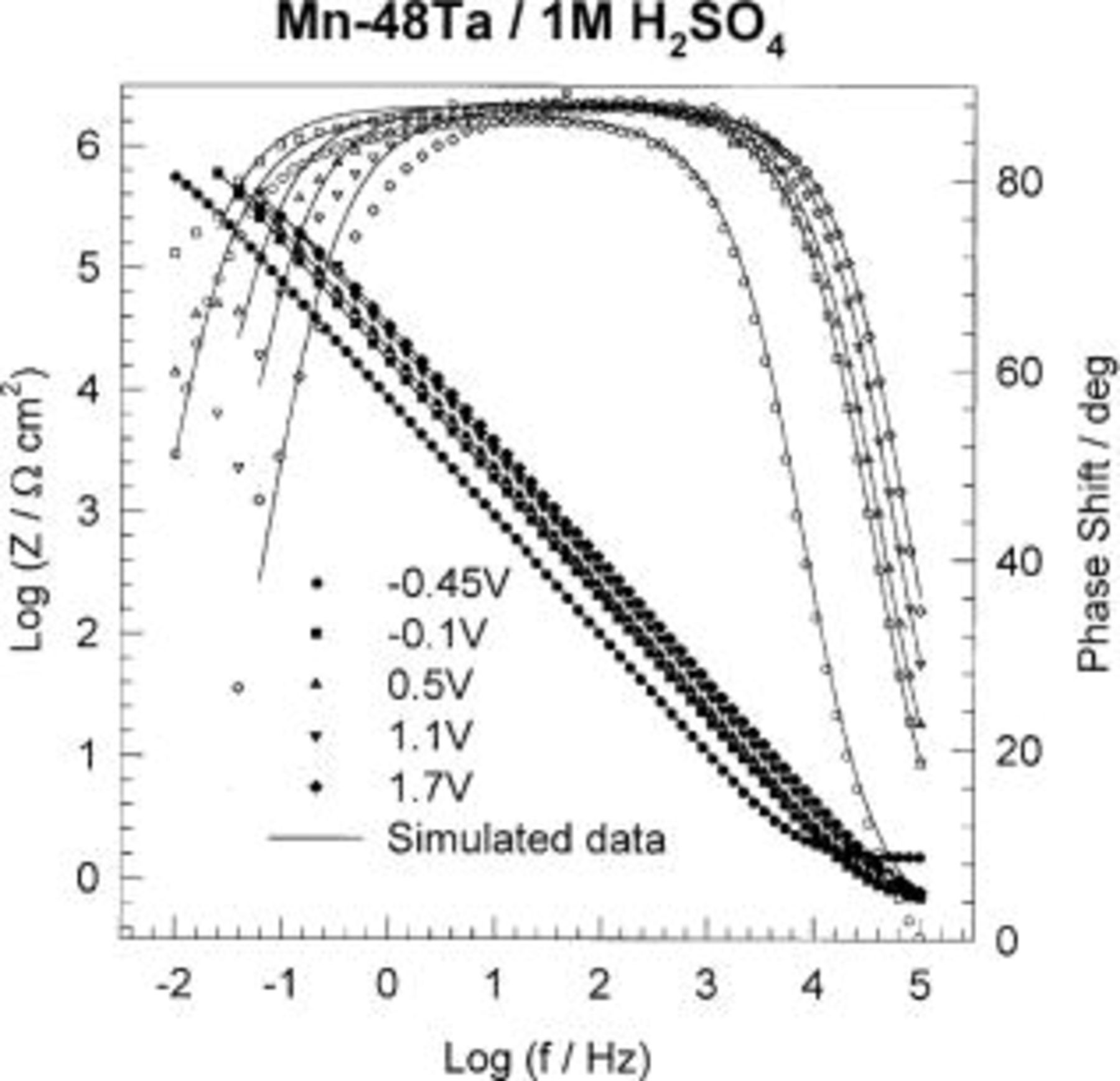
To study the passivation process occurring at the Mn-Ta surface, it was important to investigate the impedance characteristics of the sputter-deposited film under anodic polarization. Therefore, a constant potential was applied to the Mn-48 Ta sputter-deposited film in 1 M  solution until a steady state was reached and then the impedance spectra were recorded. The results of these measurements at different potentials are presented as Bode plots in Fig. 4. Although the electrochemical impedance was measured down to very low frequencies (10 mHz), the Bode plots do not show a resistive region (horizontal line and a phase angle ≈0°) at these frequencies. The impedance measurements at different potentials were analyzed using the same equivalent circuit model. The data analysis at different potentials is presented in Table III. The reciprocal capacitance of the passive film, 1/C, is directly proportional to the thickness of the passive film. The value of reciprocal capacitance can be obtained either from the Bode plot at a frequency of 0.158 Hz where the impedance data give a straight line with a slope of
solution until a steady state was reached and then the impedance spectra were recorded. The results of these measurements at different potentials are presented as Bode plots in Fig. 4. Although the electrochemical impedance was measured down to very low frequencies (10 mHz), the Bode plots do not show a resistive region (horizontal line and a phase angle ≈0°) at these frequencies. The impedance measurements at different potentials were analyzed using the same equivalent circuit model. The data analysis at different potentials is presented in Table III. The reciprocal capacitance of the passive film, 1/C, is directly proportional to the thickness of the passive film. The value of reciprocal capacitance can be obtained either from the Bode plot at a frequency of 0.158 Hz where the impedance data give a straight line with a slope of  or from the imaginary part of impedance,
or from the imaginary part of impedance,  at the same frequency using the relation
at the same frequency using the relation
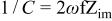
The two methods gave identical values. The oxide film thickness was calculated using the relation
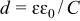
where d is the film thickness and ɛ the dielectric constant of the passive film. It is reasonable to assume that the oxide film is mainly  and its dielectric constant is 27.6,17
21
and its dielectric constant is 27.6,17
21  the permittivity of free space
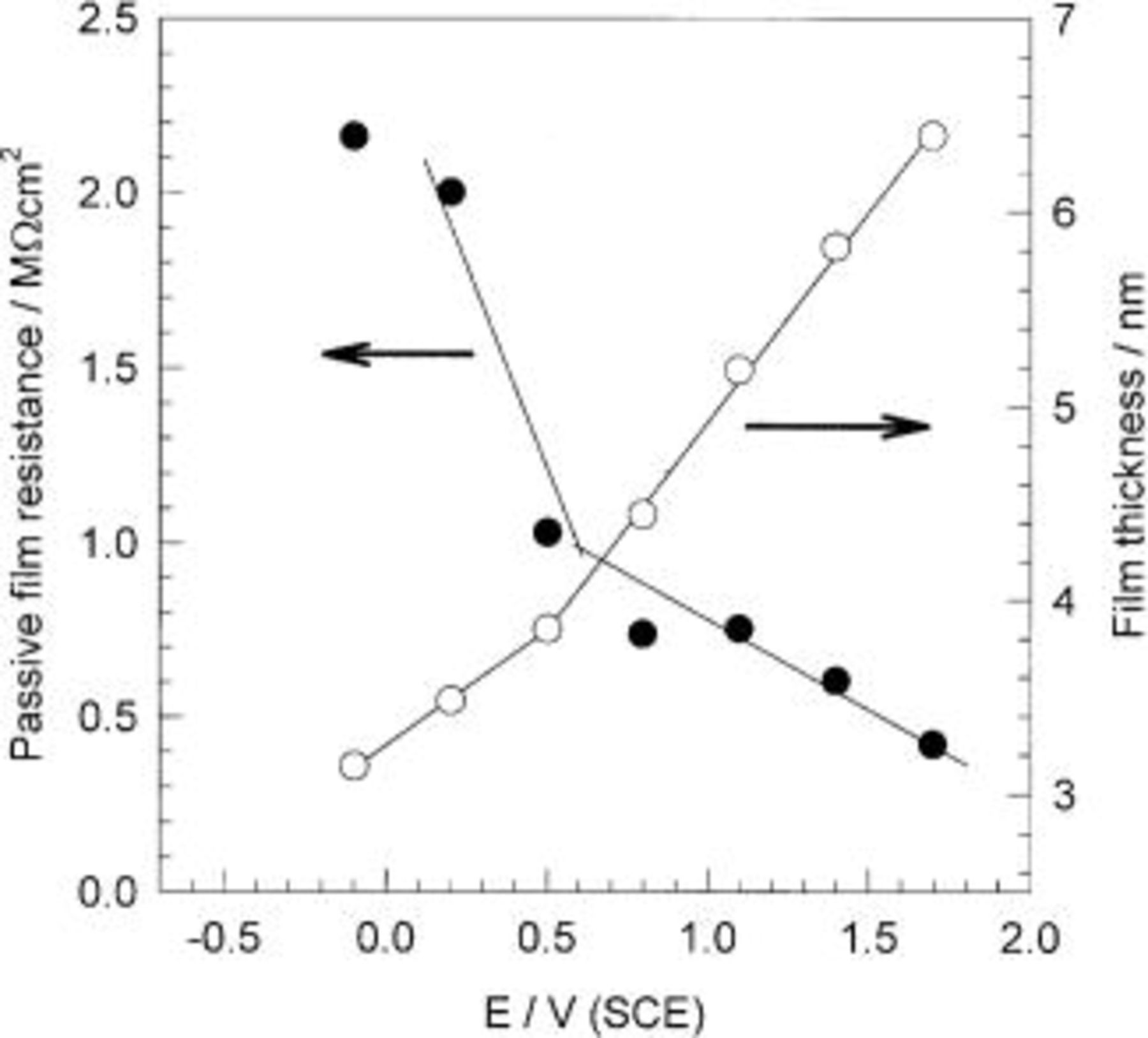
the permittivity of free space  and C is the capacitance of the oxide film. The values of film thickness were calculated at different applied potentials ranging from −0.1 to +1.7 V. Figure 5 shows linear variations of the passive film resistance, R, and the passive film thickness, d, with applied potentials. A break point in the linear variation of the passive film resistance, R, and the passive film thickness, d, was observed at +0.5 V. At low applied potentials (less than +0.5 V), passive film formation with a growth rate of 1.18 nm V−1 was calculated, whereas at higher applied potentials (greater than +0.5 V), a growth rate of 2.15 nm V−1 was achieved. This change in the growth rate of the passive film during anodization indicates a change in the passive film structure and/or composion. The lower growth rate matches that of the growth rate of polycrystalline Ta electrode.17 This means that the passive film formed on the sputter-deposited Ta-Mn electrode at potentials lower than +0.5 V consists mainly of
and C is the capacitance of the oxide film. The values of film thickness were calculated at different applied potentials ranging from −0.1 to +1.7 V. Figure 5 shows linear variations of the passive film resistance, R, and the passive film thickness, d, with applied potentials. A break point in the linear variation of the passive film resistance, R, and the passive film thickness, d, was observed at +0.5 V. At low applied potentials (less than +0.5 V), passive film formation with a growth rate of 1.18 nm V−1 was calculated, whereas at higher applied potentials (greater than +0.5 V), a growth rate of 2.15 nm V−1 was achieved. This change in the growth rate of the passive film during anodization indicates a change in the passive film structure and/or composion. The lower growth rate matches that of the growth rate of polycrystalline Ta electrode.17 This means that the passive film formed on the sputter-deposited Ta-Mn electrode at potentials lower than +0.5 V consists mainly of  At higher applied potentials, the formation of insoluble high oxidation states of Mn species is probable, and hence, mixed Ta/Mn oxides are formed. It is worthy to mention that the passive film resistance decreases with increasing the applied potential (cf. Table III). This means that incorporation of more Mn species in the passive film lowers the resistance of the film.
At higher applied potentials, the formation of insoluble high oxidation states of Mn species is probable, and hence, mixed Ta/Mn oxides are formed. It is worthy to mention that the passive film resistance decreases with increasing the applied potential (cf. Table III). This means that incorporation of more Mn species in the passive film lowers the resistance of the film.
Figure 4. Bode plots of sputter-deposited Mn-48 Ta alloys in naturally aerated unstirred  solution at different applied potentials.
solution at different applied potentials.
Table III.
Equivalent circuit parameters for Mn-48 Ta sputter-deposited film in 1 M 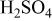 solution at different applied potentials at 30°C. solution at different applied potentials at 30°C. | ||||
|---|---|---|---|---|
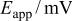 |
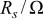 |
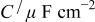 | α |
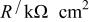 |
| −100 | 7.9 | 7.77 | 0.98 | 2160 |
| 200 | 7.8 | 7.02 | 0.98 | 1999 |
| 500 | 7.8 | 6.34 | 0.98 | 1028 |
| 800 | 7.6 | 5.50 | 0.98 | 735 |
| 1100 | 7.6 | 4.71 | 0.98 | 749 |
| 1400 | 7.6 | 4.20 | 0.98 | 600 |
| 1700 | 7.6 | 3.82 | 0.98 | 418 |
Figure 5. Resistance and thickness of the passive film formed on Mn-48 Ta alloys in naturally aerated  solution as a function of applied potential.
solution as a function of applied potential.
XPS investigation of the Ta-Mn in 1 M  solution
solution
The results of the electrochemical measurements were supported by XPS. The presence of C, O, Mn, and Ta peaks was identified in the XP survey spectra of the alloy. The carbon peak is very small and originates from the residuals of the vapors of the oil pumps.22
23 The presence of the clear oxygen peak (O 1s) was composed of two overlapping peaks that were attributed to OM and OH oxygen peaks. The OM oxygen corresponds to  ions in the oxide in the surface film. The other oxygen peak initiated from
ions in the oxide in the surface film. The other oxygen peak initiated from  ions and the hydration of the surface film.
ions and the hydration of the surface film.
The XP spectra from the alloy constituents indicate the presence of oxidized species coming from the surface and metallic species coming from the underlying alloy surface. The measured spectra of Ta 4f and Mn  were separated into
were separated into  and
and 
 and
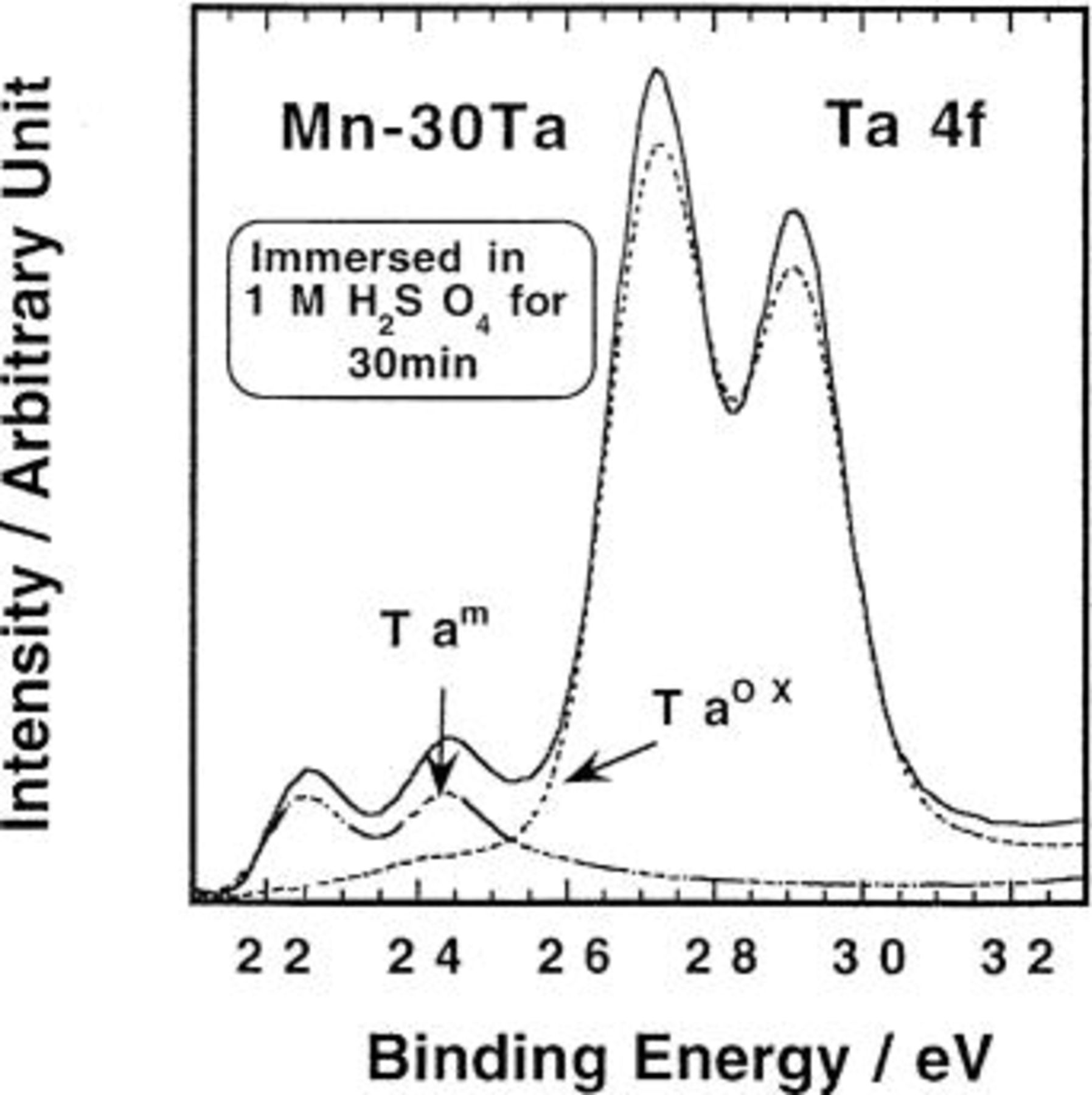
and  spectra, respectively. An example of the deconvolution of the Ta 4f spectrum measured for Mn-30 Ta specimen after immersion in 1 M
spectra, respectively. An example of the deconvolution of the Ta 4f spectrum measured for Mn-30 Ta specimen after immersion in 1 M  for 30 min is presented in Fig. 6. The measured spectrum of the Ta 4f consists of two doublet peaks corresponding to the
for 30 min is presented in Fig. 6. The measured spectrum of the Ta 4f consists of two doublet peaks corresponding to the  state at 26.3 and 28.1 eV and to the metallic
state at 26.3 and 28.1 eV and to the metallic  state at 21.5 and 23.4 eV. The measured spectrum of the Mn
state at 21.5 and 23.4 eV. The measured spectrum of the Mn  consists of a peak at 638.4 eV corresponding to metallic state,
consists of a peak at 638.4 eV corresponding to metallic state,  and a major broad peak for the oxidized state. The oxidized peak consists of two overlapped peaks corresponding to
and a major broad peak for the oxidized state. The oxidized peak consists of two overlapped peaks corresponding to  and
and  8
9 The thickness and the composition of the surface film and the composition of the underlying alloy surface were determined quantitatively by integrating the intensities of the spectra for each species using the same method described elsewhere.15
16
8
9 The thickness and the composition of the surface film and the composition of the underlying alloy surface were determined quantitatively by integrating the intensities of the spectra for each species using the same method described elsewhere.15
16
Figure 6. The  spectrum measured for amorphous Mn-30 Ta alloy after immersion in naturally aerated unstirred
spectrum measured for amorphous Mn-30 Ta alloy after immersion in naturally aerated unstirred  for 30 min.
for 30 min.
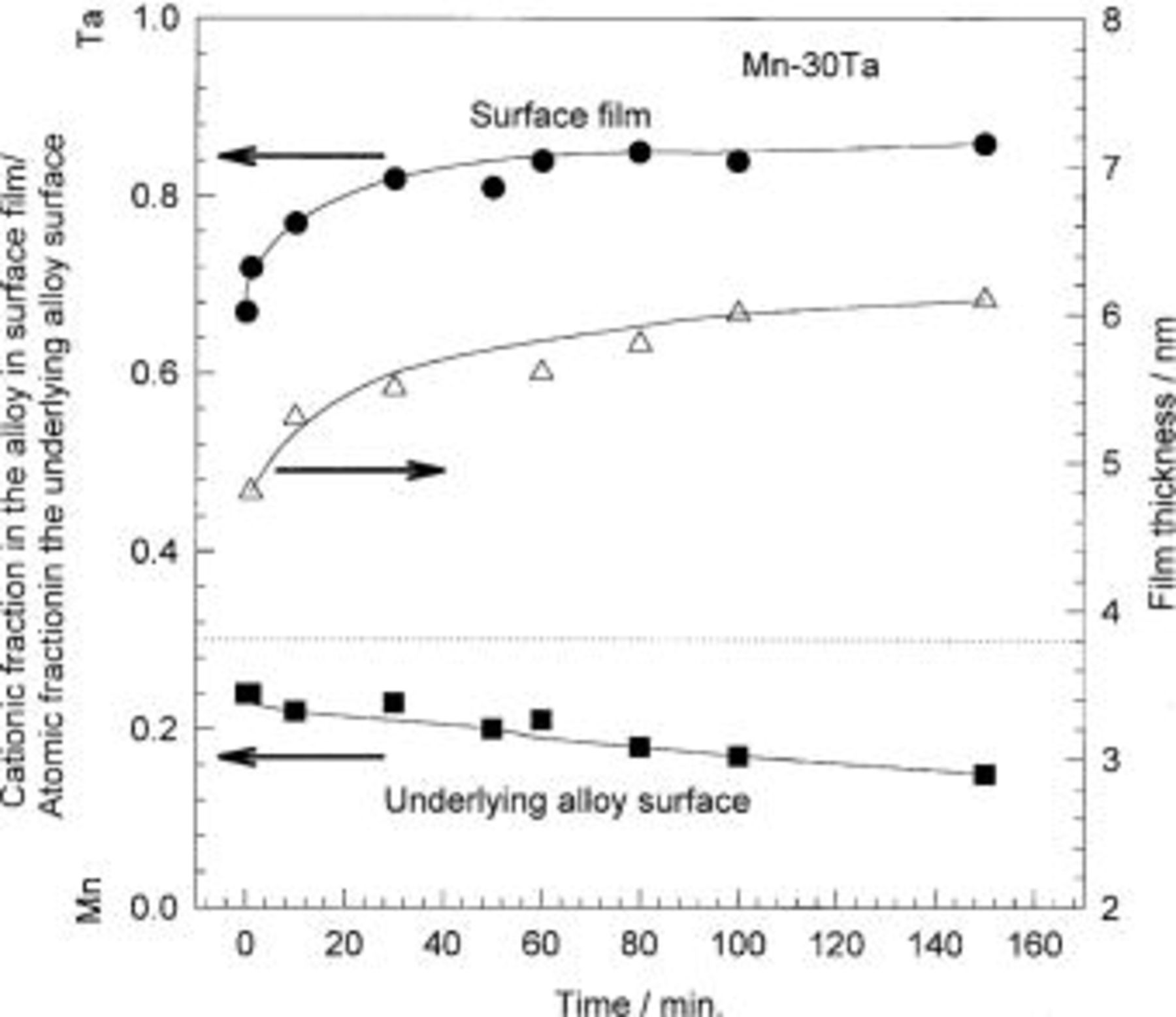
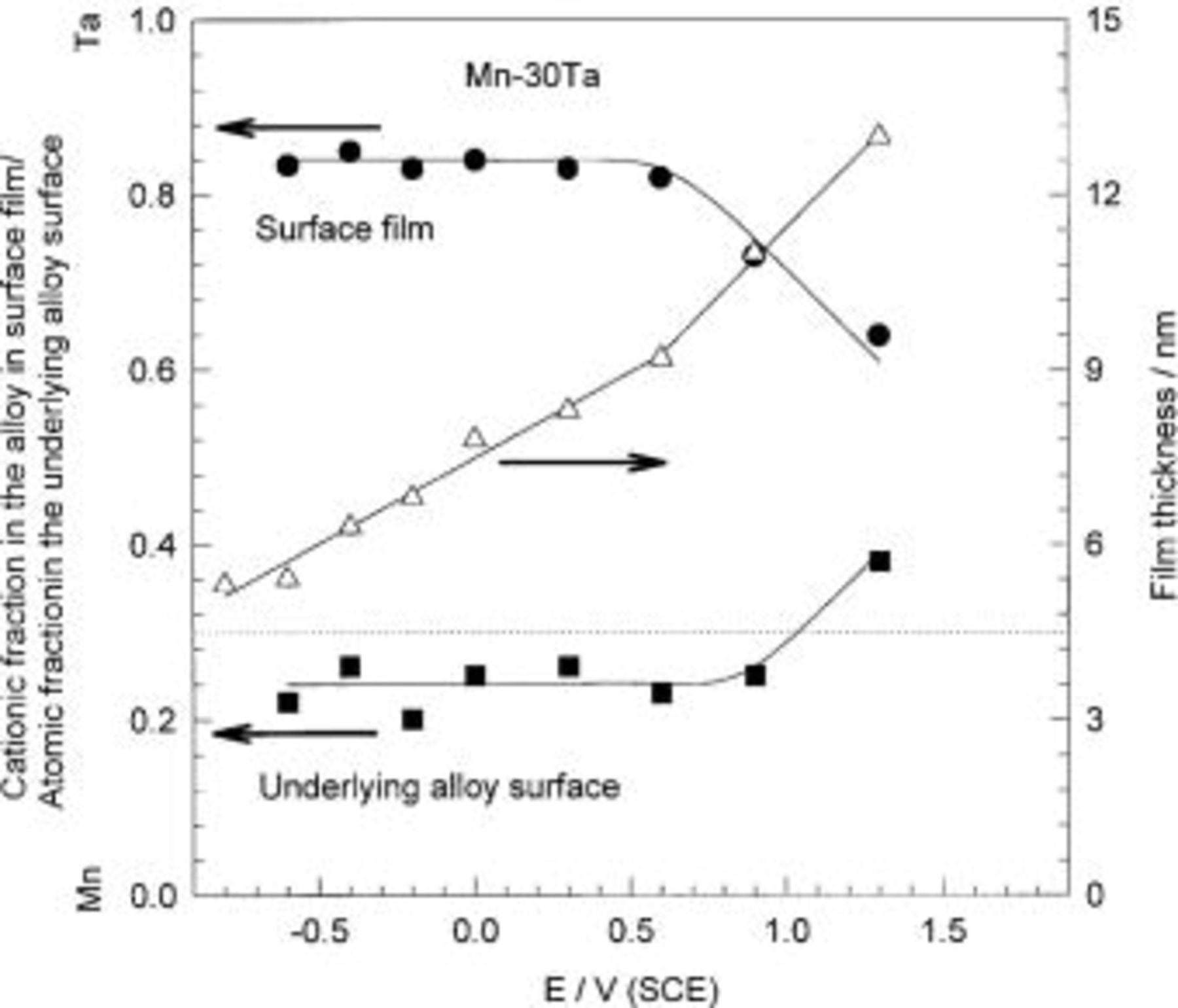
The changes in cationic fraction in the surface film and atomic fraction in the underlying surface and the passive film thickness for Mn-30 Ta alloys as a function of the immersion time are presented in Fig. 7. XPS analysis of the alloy surface after mechanical polishing shows that the air-formed film is rich in tantalum, while the manganese is segregated in the underlying alloy surface. This result reveals that the air exposure leads to preferential oxidation of tantalum. For Mn-30 Ta, the fraction of Ta cations in the surface film continuously increases with immersion time, while the reverse is observed in the underlying alloy surface. Therefore, the longer alloy immersion time in 1 M  the more preferential oxidation of Ta was observed. Using integrated intensities of X-ray photoelectron spectra, the passive film thickness was calculated. The thickness of the film formed on the Mn-30 Ta increases continuously with immersion time, in a similar pattern to the fraction of Ta cations in the surface film.
the more preferential oxidation of Ta was observed. Using integrated intensities of X-ray photoelectron spectra, the passive film thickness was calculated. The thickness of the film formed on the Mn-30 Ta increases continuously with immersion time, in a similar pattern to the fraction of Ta cations in the surface film.
Figure 7. The film thickness and cationic fractions in the surface film and atomic fractions in the underlying alloy surface for Mn-30 Ta alloy as a function of immersion time in naturally aerated unstirred  solution at 30°C.
solution at 30°C.
In order to get further information about the nature of the passive film formed on the Mn-Ta alloys and to characterize the in-depth distribution of different species in the surface film and underlying alloy surface, angle-resolved measurements were performed. In such experiments, the surface sensitivity is varied by changing the angle of detection of the specimen. At low angles of detection with respect to the surface (low take-off angle), the signal from the species located in the external part of the film is enhanced. At high angles, the signal located in the inner part of the film is enhanced. The Mn-30 Ta alloy surface immersed in 1 M  shows clear concentration gradient in both the surface film as well as the underlying alloy surface as shown in Table IV. Tantalum cations are significantly concentrated in the exterior part of the passive film, while manganese tends to be concentrated just underneath the passive film. Table V shows the change in the ratios of
shows clear concentration gradient in both the surface film as well as the underlying alloy surface as shown in Table IV. Tantalum cations are significantly concentrated in the exterior part of the passive film, while manganese tends to be concentrated just underneath the passive film. Table V shows the change in the ratios of  and
and  for amorphous Mn-30 Ta alloy after immersion in 1 M
for amorphous Mn-30 Ta alloy after immersion in 1 M  solution as a function of photoelectron take-off angle. The Mn-30 Ta alloy shows high ratios of
solution as a function of photoelectron take-off angle. The Mn-30 Ta alloy shows high ratios of  and
and  ions throughout the passive film due to the high concentration of
ions throughout the passive film due to the high concentration of  ions in the film. Also the ratios of
ions in the film. Also the ratios of  in the film is dependent on the take-off angle of photoelectron, whereas the ratio is one at take-off angle 10° indicating highly hydrated or oxyhydroxide passive film.
in the film is dependent on the take-off angle of photoelectron, whereas the ratio is one at take-off angle 10° indicating highly hydrated or oxyhydroxide passive film.
Table IV.
Changes in cationic fractions in the alloy surface film and atomic fractions in the underlying alloy surface for Mn-30 Ta alloy after immersion in 1 M 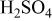 solution for 30 min as a function of the photoelectron take-off angle. solution for 30 min as a function of the photoelectron take-off angle. | ||
|---|---|---|
| Photoelectron take-off angle | Cationic fraction in the alloy surface film | Atomic fraction in the underlyingalloy surface |
| 15.0000 | 0.8600 | 0.1400 |
| 30.0000 | 0.8500 | 0.1500 |
| 45.0000 | 0.8400 | 0.1600 |
| 60.0000 | 0.7800 | 0.2200 |
| 90.0000 | 0.7500 | 0.2500 |
Table V.
Changes in the ratios of 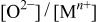 and and 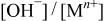 in the surface films and for Mn-30 Ta alloy after immersion in 1 M in the surface films and for Mn-30 Ta alloy after immersion in 1 M 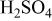 solution for 30 min as a function of the photoelectron take-off angle. solution for 30 min as a function of the photoelectron take-off angle. | ||
|---|---|---|
| Photoelectron take-off angle |
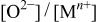 |
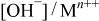 |
| 15.0000 | 1.8000 | 1.0000 |
| 30.0000 | 2.0500 | 0.6500 |
| 45.0000 | 2.0000 | 0.5500 |
| 60.0000 | 2.0700 | 0.4100 |
| 90.0000 | 2.2000 | 0.3000 |
XPS analysis was also performed after electrode immersion and potentiostatic polarization of the amorphous Mn-30 Ta alloy in 1 M  solution for 30 min. The changes in cationic fraction in the surface film and atomic fraction in the underlying surface and the film thickness, calculated from XPS data, as a function of polarization potential were presented in Fig. 8. Thickness of sputter-deposited Mn-30 Ta alloy increases linearly with the applied potential. The linear increase in the film thickness is a typical behavior of valve metals and refers to the barrier nature of the passive film developed on the alloy. The cationic fractions of tantalum in the passive film show no significant change with anodic polarization up to +0.7 V standard calomel electrode (SCE). This means that in spite of the linear increase in the film thickness with applied potential, the passive film composition is nearly unchanged. This suggests that the passive film acts as the barrier layer similar to passive films developed on the valve metals by anodic polarization.10 Above +0.7 V (SCE), anodic polarization leads to significant decay in the fraction of tantalum in the surface film. This remarkable change in the film composition at this potential is attributed to the formation of thermodynamically stable and sparingly soluble Mn species of higher oxidation states such as
solution for 30 min. The changes in cationic fraction in the surface film and atomic fraction in the underlying surface and the film thickness, calculated from XPS data, as a function of polarization potential were presented in Fig. 8. Thickness of sputter-deposited Mn-30 Ta alloy increases linearly with the applied potential. The linear increase in the film thickness is a typical behavior of valve metals and refers to the barrier nature of the passive film developed on the alloy. The cationic fractions of tantalum in the passive film show no significant change with anodic polarization up to +0.7 V standard calomel electrode (SCE). This means that in spite of the linear increase in the film thickness with applied potential, the passive film composition is nearly unchanged. This suggests that the passive film acts as the barrier layer similar to passive films developed on the valve metals by anodic polarization.10 Above +0.7 V (SCE), anodic polarization leads to significant decay in the fraction of tantalum in the surface film. This remarkable change in the film composition at this potential is attributed to the formation of thermodynamically stable and sparingly soluble Mn species of higher oxidation states such as 
Figure 8. The film thickness and cationic fractions in the surface film and atomic fractions in the underlying alloy surface for Mn-30 Ta alloy as a function of applied potentials in naturally aerated unstirred  solution at 30°C.
solution at 30°C.
Effect of chloride ions on the behavior of Mn-Ta amorphous alloy
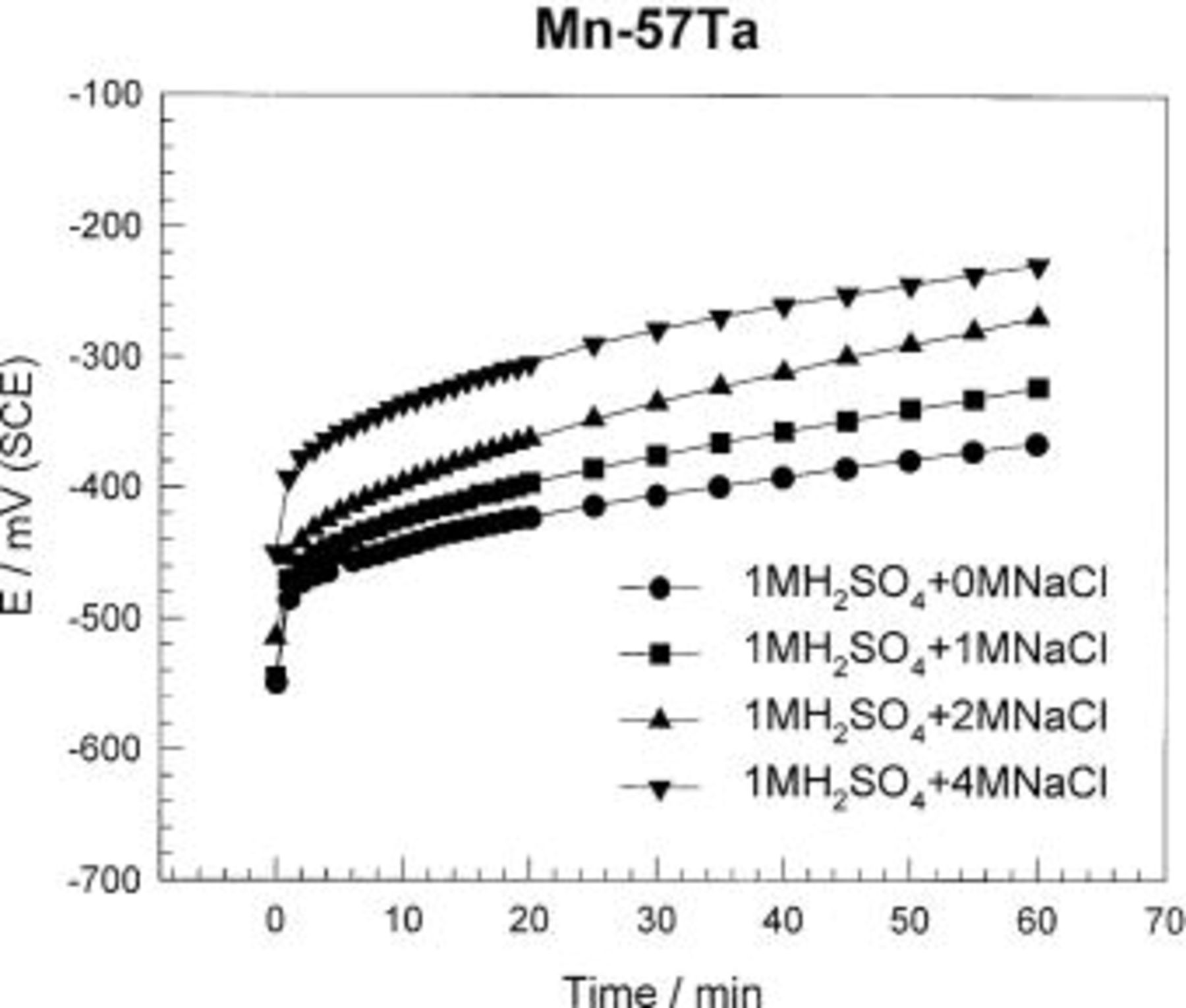
The effect of addition of chloride ions with various concentrations (1-4 M) on the electrochemical behavior of different Mn-Ta alloy compositions in naturally aerated 1 M  solution was investigated. Figure 9 shows the open-circuit potential behavior of the Mn-57 Ta alloy over a period of 60 min in 1 M
solution was investigated. Figure 9 shows the open-circuit potential behavior of the Mn-57 Ta alloy over a period of 60 min in 1 M  solutions containing up to 4 M NaCl. The open-circuit potential of the Mn-57 Ta electrode shows continuous shift toward more positive direction indicating a continuous passivation of the electrode surface over the period of measurement (60 min). The higher the concentration of the
solutions containing up to 4 M NaCl. The open-circuit potential of the Mn-57 Ta electrode shows continuous shift toward more positive direction indicating a continuous passivation of the electrode surface over the period of measurement (60 min). The higher the concentration of the  ions in the 1 M
ions in the 1 M  solution, the more noble potential is attained. It seems that the presence of chloride ions increases the dissolution of Mn metal leading to the formation of a passive film similar to that formed on pure Ta.
solution, the more noble potential is attained. It seems that the presence of chloride ions increases the dissolution of Mn metal leading to the formation of a passive film similar to that formed on pure Ta.
Figure 9. Variations of the potential of sputter-deposited Mn-Ta alloys with time in  solution containing different chloride ion concentrations at 30°C.
solution containing different chloride ion concentrations at 30°C.
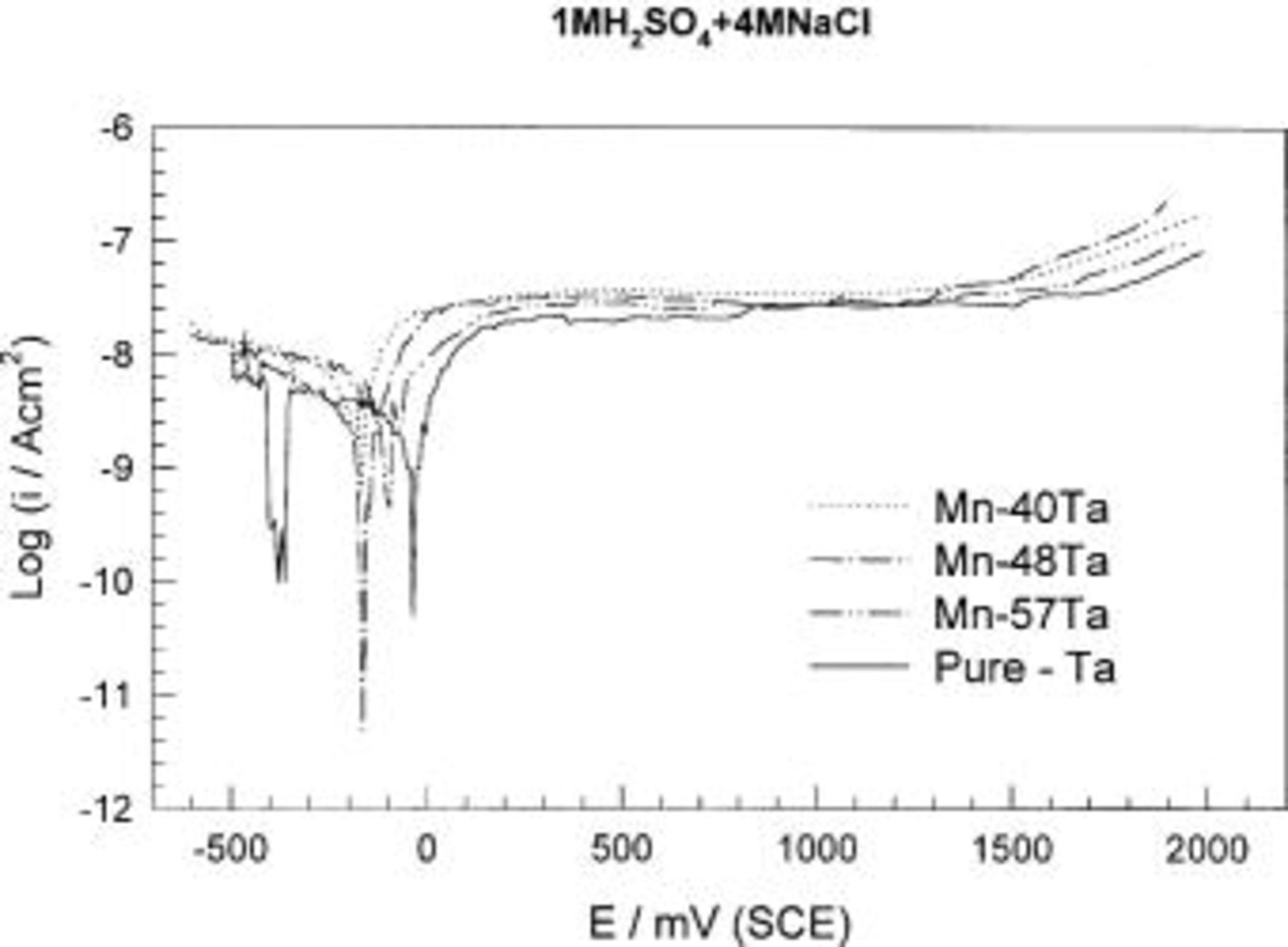
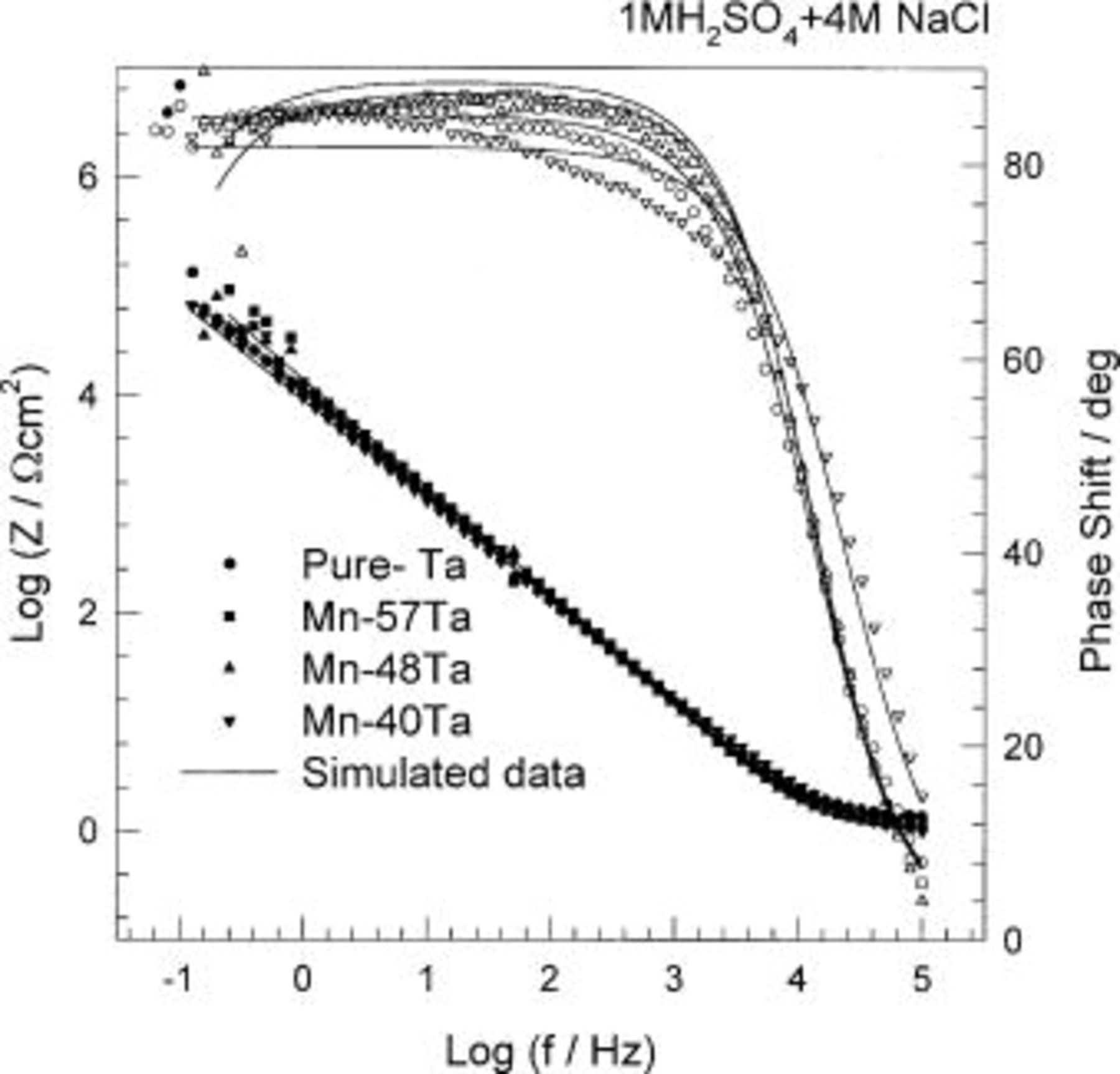
The effect of chloride ions was further investigated using potentiodynamic measurements. In general, the alloys are spontaneously passivated and no signs of pitting corrosion were recorded. The anodic current decreases with increasing the tantalum content. Longer immersion of the alloys in the medium containing chloride ions leads to significant decreases in the anodic current, e.g., after 2 h of immersion, the anodic current decreases by three decades. Figure 10 shows the potentiodynamic curves of the sputter-deposited amorphous Mn-(40-57) Ta in 1 M  solution containing 4 M NaCl and up to 2.0 V (SCE). In spite of the aggressiveness of this medium, these alloys show high stability with very low anodic current density (35 nA cm−2) and no pitting or transpassive dissolution of manganese was observed.
solution containing 4 M NaCl and up to 2.0 V (SCE). In spite of the aggressiveness of this medium, these alloys show high stability with very low anodic current density (35 nA cm−2) and no pitting or transpassive dissolution of manganese was observed.
Figure 10. Potentiodynamic polarization curves of sputter-deposited Mn-Ta alloys in naturally aerated unstirred  solution containing 4 M NaCl at 30°C.
solution containing 4 M NaCl at 30°C.
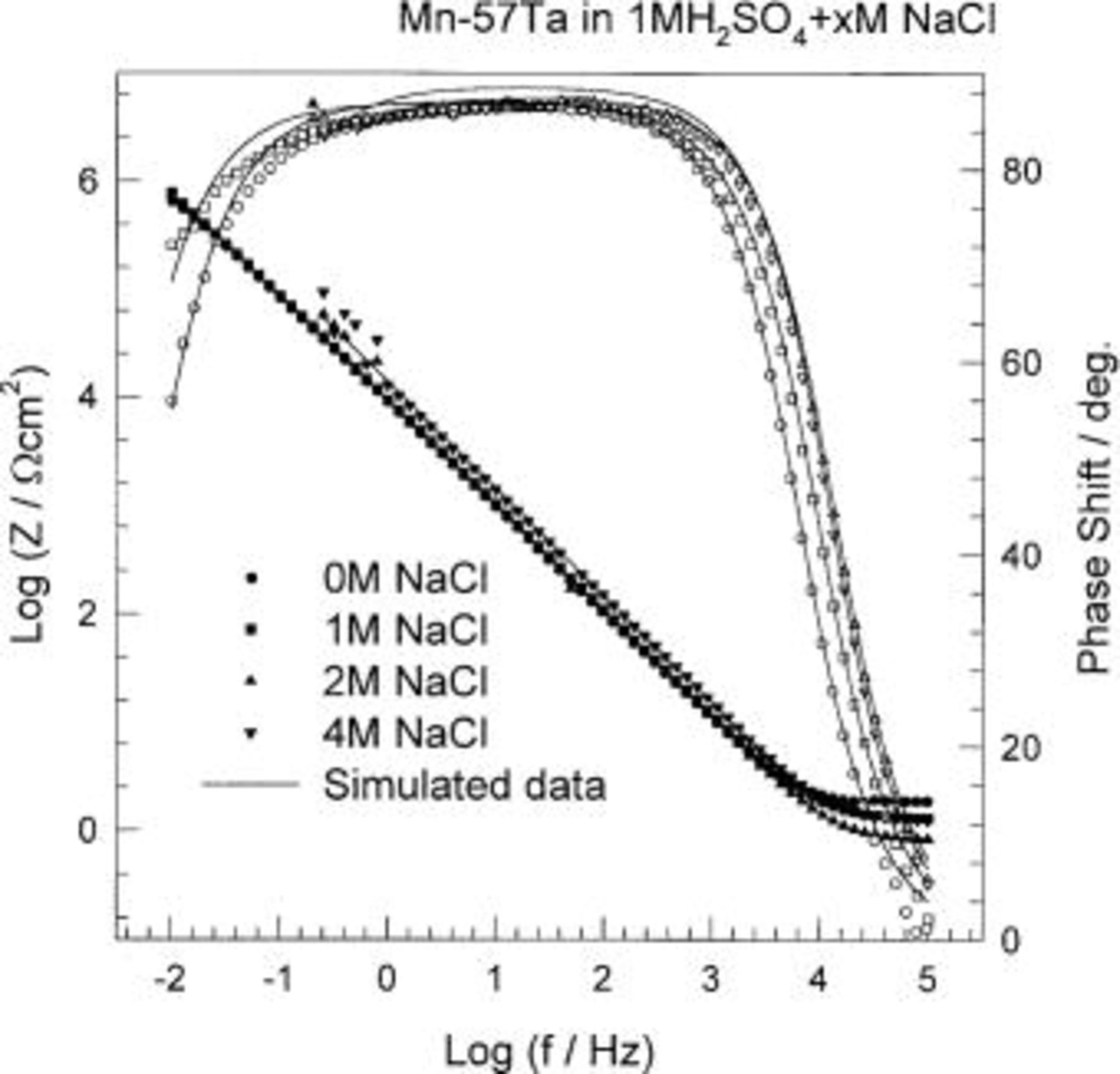
Further investigations of the effect of chloride ions on the electrochemical behavior of different alloy compositions, Mn-(40-57) Ta, have been carried out using EIS. The impedance results are presented as Bode plots in Fig. 11 and 12. It is worthwhile to mention that in the presence of chloride ions, the electrochemical impedance measurements are considerably distorted. Fluctuations, or "noise", were observed in the low frequency range (10 mHz-1 Hz). This phenomenon may be due to the attack of the passive film by the  ions. The impedance data were analyzed using the same equivalent circuit described above. In the presence of 4 M
ions. The impedance data were analyzed using the same equivalent circuit described above. In the presence of 4 M  the passive film resistance was 427 kΩ cm2 compared to 242 kΩ cm2 in 1 M
the passive film resistance was 427 kΩ cm2 compared to 242 kΩ cm2 in 1 M  The increase of the passive film resistance, in this case, can be attributed to the presence of the
The increase of the passive film resistance, in this case, can be attributed to the presence of the  film after the dissolution of the active corrosion centers at high concentration of
film after the dissolution of the active corrosion centers at high concentration of  ions. Tantalum passivated in halide solution, consistent with what has been reported before in literature.24 Fitting of the experimental impedance data to the equivalent circuit model gave a value of 0.97 for the empirical parameter, α, which shows that the presence of chloride ions does not change the passive film roughness. The passive film capacitance, which is inversely proportional to the film thickness, was found to decrease with increasing the
ions. Tantalum passivated in halide solution, consistent with what has been reported before in literature.24 Fitting of the experimental impedance data to the equivalent circuit model gave a value of 0.97 for the empirical parameter, α, which shows that the presence of chloride ions does not change the passive film roughness. The passive film capacitance, which is inversely proportional to the film thickness, was found to decrease with increasing the  ion concentration. This means that the passive film thickness increases with increasing the
ion concentration. This means that the passive film thickness increases with increasing the  ion concentration.
ion concentration.
Figure 11. Bode plots of sputter-deposited Mn-57 Ta alloys in naturally aerated unstirred  solution containing different chloride ion concentrations at 30°C.
solution containing different chloride ion concentrations at 30°C.
Figure 12. Bode plots of sputter-deposited Mn-Ta alloys in naturally aerated unstirred  solution containing 4 M NaCl at 30°C.
solution containing 4 M NaCl at 30°C.
Conclusions
1. The addition of Ta remarkably reduces the rate of dissolution of Mn and the alloys passivate spontaneously.
2. The thickness of the passive film formed on the sputter-deposited Mn-Ta alloy increases linearly with the anodic potential.
3. The alloys show high stability and no pitting or transpassive dissolution of manganese was observed even in presence of 4 M  ions.
ions.
4. At high applied potentials, a thicker and less protective passive film is formed.
5. Although tantalum improves the corrosion resistance of the alloy, remarkable corrosion resistant alloys were only obtained at high Ta content (⩾40 atom %).
Acknowledgments
The sputtered-deposited alloys used in this study were prepared by Dr. A. A. El-Moneim in The Institute for Materials Research, Tohoku University, Sendai, Japan. The authors are grateful for the facilities provided by Professor K. Hashimoto for the characterization of the prepared materials. The AvH foundation (Bonn, Germany) is gratefully acknowledged for providing the impedance system.