Abstract
© 2002 The Electrochemical Society.
Export citation and abstract BibTeX RIS
In honor of the 100th anniversary of The Electrochemical Society, a retrospective look at the development of fuel cell technology over the past 100 years is presented. The development of fuel cells can be traced back over 160 years to Sir William Grove's invention in 1839. The history of these very early years have been described elsewhere.1 2 3 Additionally, comprehensive technical reviews of fuel cell technology are also available (see, for example, Ref. 4 and 5), as well as recent review articles on the latest developments.6 7 Therefore, this paper will emphasize the progress on fuel cells that has been presented in the Journal of The Electrochemical Society (JES) and other ECS publications throughout the Society's first 100 years.
This historical review includes all the major types of fuel cells, which are named according to the electrolyte employed in the cells: the alkaline fuel cell (AFC), the polymer-electrolyte fuel cell (PEFC), the phosphoric-acid fuel cell (PAFC), the molten-carbonate fuel cell (MCFC), and the solid-oxide fuel cell (SOFC). We will review the significant advances that have occurred and how these developments have been influenced by external factors. Research groups that have made substantial contributions to these developments and the fuel cell literature in ECS publications will be given special emphasis.
Fuel Cell Development in the Last 100 Years
The development of fuel cells over the last century has been heavily influenced by external factors. Initially, fuel cells were seen as an attractive means for the generation of power because the efficiencies of other technologies were very poor. However, as the efficiency of these other technologies rapidly improved, the interest in fuel cells waned. Then, when the "space race" began in the late 1950s fuel cells were rapidly developed for deployment in space. More recently, significant technical progress in fuel cell technology has made fuel cells appear more viable than ever for a variety of applications. Additionally, concerns about energy resources and the environment have elevated interests in generating power with even higher efficiencies and lower emissions, and this has also raised the interest level in fuel cells.
Although some interesting work was done on fuel cells during the first half of the 20th century, it appears that almost nothing was published on this subject in ECS publications during this period. This is not surprising, given that most of the early work on fuel cells was conducted in Europe and the Society was primarily an American institution in the early days.8 In fact, the initial publication of the Society was known as the Transactions of The American Electrochemical Society until 1930 when the word "American" was dropped from the name of the organization to reflect the increasing global composition of the membership and the title was changed to Transactions of The Electrochemical Society.9
Development of Large-Scale Electricity Generation and Distribution
The large-scale distribution of electric power began at the end of the nineteenth century. Although Michael Faraday had discovered electromagnetic induction in 1831 (i.e., the basic principle of a generator), it took a number of other developments to establish large-scale generation of electric power. But, by the mid-1870s electric arcs were illuminating the streets of many major cities in Europe and America. Initially, power transmission was limited to relatively short distances and power-generation stations were relatively small. However, with the development of an ac system, principally due to the contributions of George Westinghouse and Nikola Tesla, the era of large-scale power generation and transmission was born. And, in 1896, power generated by a pair of high-speed turbines at Niagra Falls was transmitted 26 miles to the city of Buffalo, NY.
However, early electric power generators were very inefficient. For example, the coal-burning generation station built by Thomas Edison in 1882 in lower Manhattan converted only about 2.5% of the available energy into electricity. Even in the 1920s the overall thermodynamic efficiencies of reciprocating steam engines was approximately 13-14%, and steam turbines obtained just under 20%. These poor thermal efficiencies provided one of the major motivations for the pioneers of fuel cell development. In fact, in 1894, Ostwald10 pointed out the wastefulness of the steam engine and expressed the hope that the 20th century would become the "Age of Electrochemical Combustion." This still visionary paper also emphasized the reduction of emissions with the elimination of the burning of fuels: "kein Rauch, kein Russ" (no smoke, no soot).
Early Fuel Cell Development Efforts
Given that a major fuel at the turn of the century was coal, it is not surprising that much of the work on fuel cells at this time was focused on using this energy source. Both direct and indirect coal fuel cells were investigated.
Ludwig Mond, who founded the International Nickel company and other chemical industries in England, had developed a process in which coal and coke were used to derive a gas containing a large proportion of hydrogen. With the assistance of Dr. Charles Langer,11 they pursued the dream of scaling-up Grove's gas battery into something that would deliver useful power from converted fuels, whereas Grove had only considered "effecting the decomposition of water by means of its composition."12 Unfortunately, impurities in Mond's industrial gas poisoned the fuel cell's platinum-black catalyst and the high cost of the required loadings of this catalyst made this alternative power-generation technology cost prohibitive. (Both of these problems continue to challenge fuel cell developers to this day.) Mond and Langer did make some interesting advances that significantly increased the power density of the fuel cell by greatly enhancing what Grove referred to as the "notable surface of action." For example, they employed a porous matrix to contain their liquid electrolytes and they introduced the use of powdered electrocatalysts like platinum black.
Other researchers pursued the direct coal fuel cell. W. W. Jacques built relatively large carbon/air batteries capable of delivering up to 1.5 kW in 1896. Unfortunately, the lifetime of these cells was limited because they employed molten alkali electrolytes that are not invariant in the presence of carbon. Although Jacques was an American, he did not describe his work in any scientific journal. Professor Baur, working at the Swiss Federal Institute of Technology in Zurich, also attempted to develop direct-coal fuel cells. He investigated a series of electrolytes capable of operating at increasing elevated temperatures, including molten carbonates. Baur and his students brought good scientific discipline to the development of fuel cells, and they made some impressive advances in cell design and performance. However, they were plagued by numerous practical problems (e.g., ash formation, incomplete oxidation, and the continuous feeding of a solid fuel) that reduced their interest in the direct use of carbon. In a comprehensive review of the technology in 1933, Baur and Tobler conclude that the fuel cell showing the earliest promise of commercial success is one that operates at ordinary temperatures with alkaline electrolyte and hydrogen as a fuel.13 An interesting review of all of this early work, which includes the progress made on electrode structures, can be found elsewhere.1
The Bacon Cell
Sir Francis Bacon began his historical work on fuel cells in 1933, and he obviously agreed with the assessment by Baur and Tobler, given that he set out to develop a hydrogen-oxygen cell that operated at moderate temperatures using alkaline electrolytes and improved catalysts. Bacon's own account of the development of a high power density AFC (e.g.,  at 0.6 V at 240°C and very high pressures) is both interesting and entertaining.14 These cells employed nickel electrodes with a dual-porosity structure that along with differential gas pressures across the cell provided a thin electrolyte film in the larger pores. However, the performance of these cells degraded rapidly due to corrosion of the porous-nickel cathodes. This issue was eventually overcome after extensive experimentation led to the development of a nickel-oxide electrode (doped with lithium for improved electronic conductivity) that was more corrosion resistant. In 1959, this technology was licensed by Pratt and Whitney (now a subsidiary of United Technologies Corporation), and was developed into the fuel cell system employed by the U.S. Apollo space program.
at 0.6 V at 240°C and very high pressures) is both interesting and entertaining.14 These cells employed nickel electrodes with a dual-porosity structure that along with differential gas pressures across the cell provided a thin electrolyte film in the larger pores. However, the performance of these cells degraded rapidly due to corrosion of the porous-nickel cathodes. This issue was eventually overcome after extensive experimentation led to the development of a nickel-oxide electrode (doped with lithium for improved electronic conductivity) that was more corrosion resistant. In 1959, this technology was licensed by Pratt and Whitney (now a subsidiary of United Technologies Corporation), and was developed into the fuel cell system employed by the U.S. Apollo space program.
Fuel Cells in Space
The Sputnik launches in 1957 and the ensuing "space race" that followed was undoubtedly one of the most significant historical events to influence the development of fuel cells. The requirements for space applications, namely a lightweight and very high efficiency power plant (to reduce the amount of fuel and oxidant required), are uniquely met by fuel cells, especially given that cost is not an overriding factor. This new application spurred the development of both AFC and PEFC power plants.
It is interesting to note that the very first fuel cell used in a practical application was a PEFC, which is the same fuel cell type that is currently the focus of many of the major development programs attempting to develop fuel cells for terrestrial applications. The PEFC was invented at General Electric (GE) in 1955 by William Grubb,15
16 who was looking for new applications for ion-exchange membranes (See Fig. 1).17 These early PEFCs utilized hydrocarbon-based polymers that have limited lifetimes in a fuel cell environment. The polymer-electrolyte membranes were composed of polystyrene-divinylbenzene sulfonic acid cross-linked with an inert fluorocarbon film. The life-limiting factor of these cells was the oxidative degradation of the  bonds in the membrane, particularly the α-H sites where the functional groups are attached. Despite this limitation, GE developed this technology into the power plant that was successfully deployed in the U.S. Gemini program beginning in 1962. Another drawback of these early PEFC cells were the high loadings of platinum catalysts required. The AFC, operating at elevated temperature and pressure, required less expensive catalysts.
bonds in the membrane, particularly the α-H sites where the functional groups are attached. Despite this limitation, GE developed this technology into the power plant that was successfully deployed in the U.S. Gemini program beginning in 1962. Another drawback of these early PEFC cells were the high loadings of platinum catalysts required. The AFC, operating at elevated temperature and pressure, required less expensive catalysts.
Figure 1. Diesel oil is combined with air in a new General Electric fuel cell, generating electricity directly to power the fan motor at right. Pouring the fuel (commercial 18-cents-a gallon diesel oil, simply purified) is Dr. Thomas Grubb, pioneer with Dr. Leonard Niedrach (left) in the development of the first fuel cell to operate successfully with a broad range of inexpensive hydrocarbon fuels at moderate temperatures. The new cell has been operated with a variety of other common liquid fuels as well as such gaseous hydrocrabon fuels as propane and natural gas. (Photo courtesy of Science Service.)
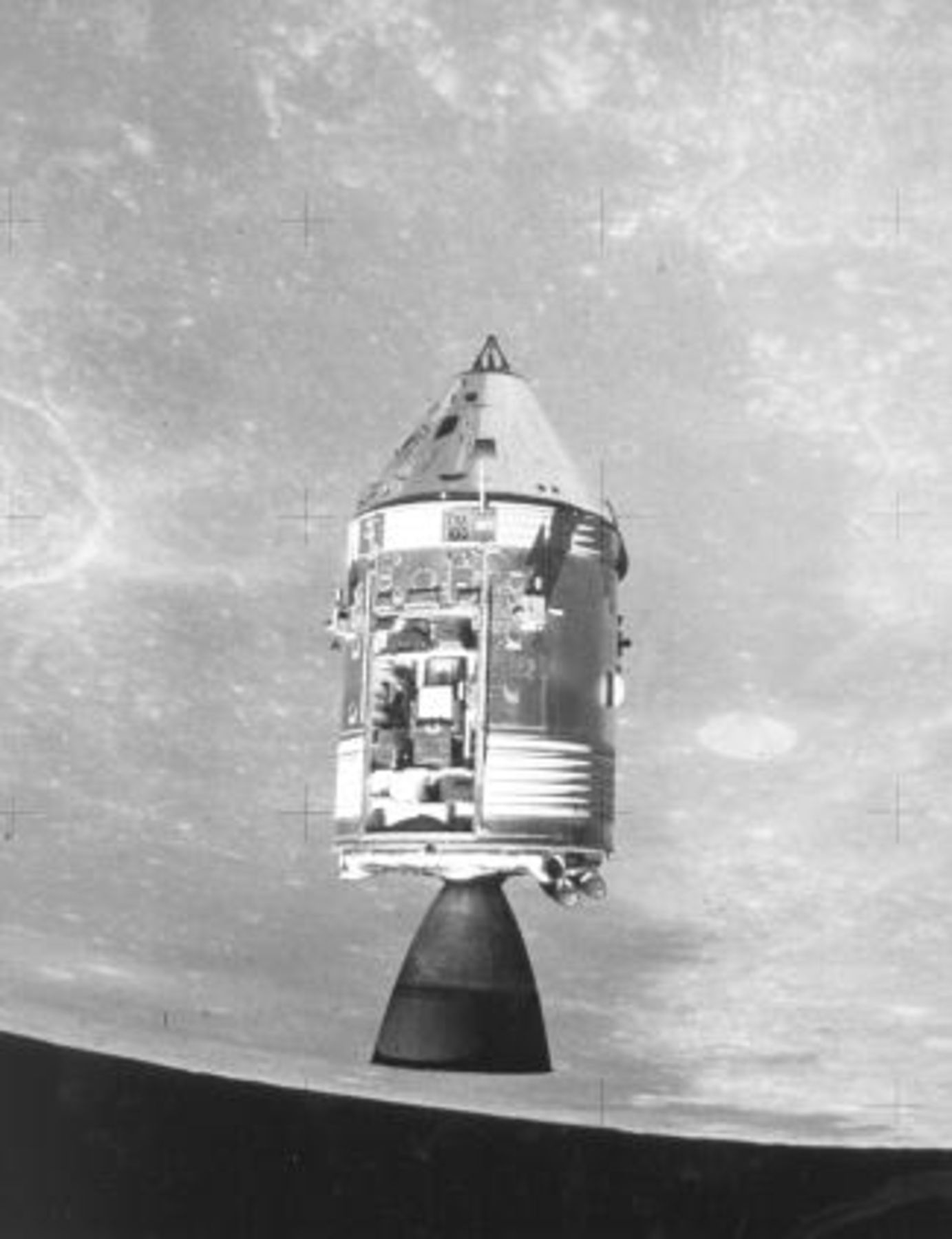
Three 28 V power plants provided all the onboard electrical power to the Apollo Command and Service Modules, Fig. 2. The AFC developed by Pratt and Whitney for the Apollo program operated at a higher temperature (260°C), higher KOH concentration  and lower pressure (near atmospheric) than the Bacon cell. The anodes and cathodes were still porous Ni and lithiated NiO, respectively, but improvements in their structure provided comparable performance at lower operating pressures.1 On the other hand, the electrodes used in the Orbiter AFC, which are still in use on the Space Shuttle missions, contain noble metals (Pt/Pd anodes and Pt/Au cathodes) that are bonded with PTFE to Ag-plated Ni screens. The PTFE (which was not available when the Apollo electrodes were developed) is used to form a more stable three-phase interface (electrode/electrolyte/gas) than could be obtained in previous electrodes.
and lower pressure (near atmospheric) than the Bacon cell. The anodes and cathodes were still porous Ni and lithiated NiO, respectively, but improvements in their structure provided comparable performance at lower operating pressures.1 On the other hand, the electrodes used in the Orbiter AFC, which are still in use on the Space Shuttle missions, contain noble metals (Pt/Pd anodes and Pt/Au cathodes) that are bonded with PTFE to Ag-plated Ni screens. The PTFE (which was not available when the Apollo electrodes were developed) is used to form a more stable three-phase interface (electrode/electrolyte/gas) than could be obtained in previous electrodes.
Figure 2. Apollo command and service modules.
Commercialization of Fuel Cells
Unlike some other so-called "spin-off" technologies (i.e., technology developed for space applications that subsequently become the basis of commercial products), fuel cells are not yet a major commercial success. In fact, the only commercially available fuel cell power plant, UTC Fuel Cells' "PC-25," is based on a different technology (PAFC) than the AFC-based power plants UTC makes for NASA.
So, why are fuel cells still considered a relatively exotic technology? Fundamentally, there are no insurmountable technical obstacles that prevent fuel cells from enjoying commercial success. And, the inherent advantages of fuel cells (e.g., high efficiencies and low emissions) relative to other electricity generation methods makes them quite attractive. The main obstacle has undoubtedly been the cost of this technology. Additionally, the requirement of operating on readily available fuels and air (vs. pure  and
and  ) makes terrestrial applications somewhat more challenging and complex than the systems used in space. Fortunately, a lot of technical progress has been made that help address these issues and should enable fuel cells to enjoy widespread use in the near future.18 Additionally, market forces are making the attributes of fuel cells look more attractive. For example, distributed power generation is expected to begin supplanting large centralized power stations due to a variety of factors (e.g., increased power demand, the need for high quality power, deregulation of the power industry, less susceptibility to terrorist attack, and environmental concerns). The automotive industry is also looking to obtain substantially higher efficiencies and lower emissions than can be obtained by internal combustion engines. Fuel cells have been identified as one of the most promising technologies that can deliver these desirable attributes, and almost all the major automakers have recently made substantial investments into fuel cell development.
) makes terrestrial applications somewhat more challenging and complex than the systems used in space. Fortunately, a lot of technical progress has been made that help address these issues and should enable fuel cells to enjoy widespread use in the near future.18 Additionally, market forces are making the attributes of fuel cells look more attractive. For example, distributed power generation is expected to begin supplanting large centralized power stations due to a variety of factors (e.g., increased power demand, the need for high quality power, deregulation of the power industry, less susceptibility to terrorist attack, and environmental concerns). The automotive industry is also looking to obtain substantially higher efficiencies and lower emissions than can be obtained by internal combustion engines. Fuel cells have been identified as one of the most promising technologies that can deliver these desirable attributes, and almost all the major automakers have recently made substantial investments into fuel cell development.
Fuel Cells and ECS
Much of the recent progress in fuel cell technology has been documented in the Journal and other ECS publications. Therefore, the remainder of this article will focus on some of the major advancements that have been presented in ECS publications, with an emphasis on particular groups of researchers who have made substantial contributions to the fuel cell literature. (For more exhaustive reviews of fuel cell technology the reader is referred to the references given in the introduction of this paper.)
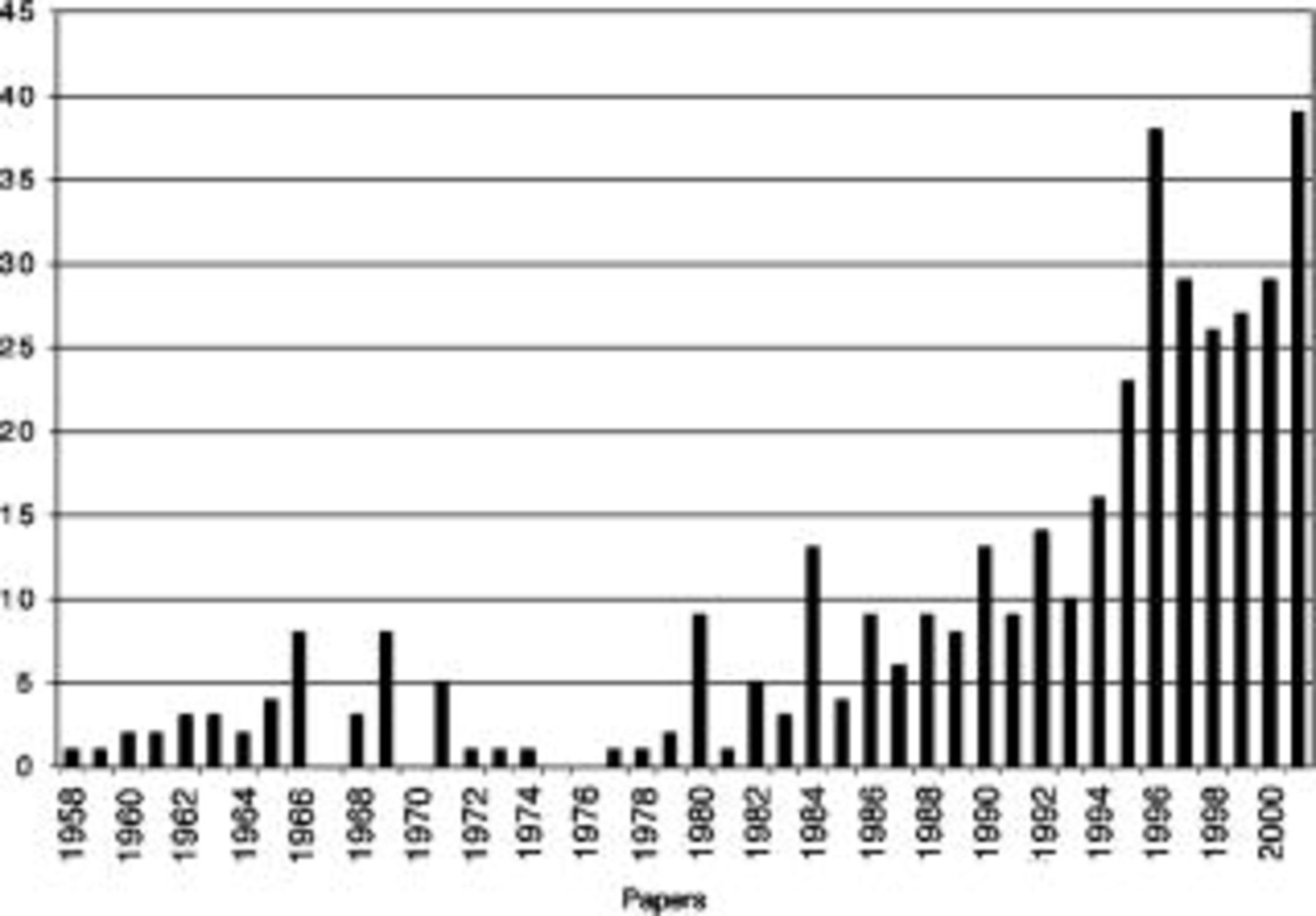
Figure 3 shows the number of paper published in the journal which contain the phrase "fuel cell" in the title. There are undoubtedly many more papers of direct relevance to fuel cells, however, so this data are not precise. They do, however, give us a qualitative picture of the activity in fuel cells in the Society. There is a similar increase in activity in the symposia and proceedings volumes with "fuel cells" in the title. The majority of these symposia have been sponsored by the Battery Division, but many other divisions have been active in this area, e.g., Energy Technology, Corrosion, High Temperature Materials, and Physical Electrochemistry. Several of the key fuel cell technologies (e.g., SOFC, MCFC, and PEM) have established recurring symposia and regularly published proceedings volumes. In each case, these symposia bring together the leading experts from the world and represent the cutting edge of fuel cell technology.
Figure 3. The number of papers appearing in the Journal which include the phrase "fuel cell" in the title.
According to Bacon, the expression "fuel cell" was not coined until after 1937. The first paper with the specific phrase "fuel cell" appeared in the Journal in 1958 "The Fuel Cell Roundtable."19 This was not a technical article; rather it was a report by R. Roberts of a discussion held on fuel cells at the 112th meeting in Buffalo, NY. The battery division organized this roundtable, and about 150 people were in attendance. Roberts reports that the objective of the panel was "to review the status of fuel cell development and potentialities of fuel cells as power sources." The Bacon cell was also reviewed in some detail. One of the key speakers was Ernest Yeager (Fig. 4, President of the Society from 1965-1966) from Case Western Reserve University, where he also served as the director of the Case Center for Electrochemical Sciences from 1976 to 1991. The number of "fuel cell" articles grew slowly with an increase in the late 1960s, presumably driven by the activity spurred by the aforementioned space race. It is interesting to note that few of these articles dealt with the alkaline or PEM technologies that were vying for the space program.
Figure 4. Ernest Yeager, President of the Society from 1965-1966 and Director of the Case Center for Electrochemical Sciences at case Western Reserve University from 1976 to 1991.
One of the more interesting early articles was an editorial from 1966 by V. Gardner entitled "Let's Not Over-Sell the Fuel Cell."20 Even today similar questions are raised about the future of fuel cells. Those of us who are trying to commercialize the fuel cell today were heartened to see that the major concerns identified have been overcome and it is likely that the obstacles facing fuel cells today will not stand. Two issues of note were raised around developing a high voltage output from the low voltage of a single cell, which is less than 1 V. The first concern was the reliability of a large series connected network of cells. To produce high voltage, hundreds of cells must be placed in series, typically in a bipolar configuration. To achieve a high reliability, a high mean time between failures for individual cells or redundant cells are required. It was felt that the only possible approach was the latter, but as we know today the former can be achieved. For example the fleet of PAFC power plants has about 5 million hours of operation in the field in over 200 units. Each power plant has a single stack with over 200 cells in series. The second concern was the conversion of the dc power to high voltage ac. Whereas 40 years ago this was difficult to imagine in anything other than small power applications, today hundreds of kWs are routinely and reliably transformed with solid state inverters.
At the height of the Energy Crisis in the early 1970s publications with the word "fuel cells" almost disappeared. There were, however, frequent symposia focused on fuel cells during this period and the Energy Technology Division was established. We know, for example, that there was significant activity in phosphoric-acid fuel cells, which will be discussed in some detail later. It wasn't until the 1980s that we see a large and steady growth in "fuel cell" publications. Many of these papers were in molten-carbonate technology with fewer in phosphoric-acid and solid-oxide technologies. 1988 brought the renaissance of PEM technology with a paper from S. Srinivasan (2001 Fellow of the Society and 1996 winner of the Energy Technology Research Award) et al. entitled "Methods to Advance Technology of Proton Exchange Membrane Fuel Cells."21 Figure 5 shows a few of the early members of Srinivasan's team. This was followed by a large body of work from the Los Alamos group on PEM fuel cells. At roughly the same time the Ballard company, which was formed in 1979, initiated their work on PEM fuel cells.
Figure 5. LANL's research team in 1986 (from left): W. K. Pai, E. A. Ticianelli, C. R. Derovin, and S. Srinivasan.
Throughout the 1990s we have seen continued growth in the fuel cell publications, to the point where one can find two or three articles on fuel cells in every issue of the Journal. Part of the increase can be attributed to the general increase in the number of articles published in the Journal and larger international participation in the Society, but it is unmistakable that research activity has increased substantially.
Many of the papers that have been published on fuel cells deal specifically with a particular type of fuel cell (e.g., AFC, PEFC, PAFC, MCFC, or SOFC). Before covering each of these fuel cell types separately, it is worth reviewing some of the more general fuel cell topics that have been widely covered by members of ECS over the past 40 years.
General Fuel Cell Topics
In addition to developing the first fuel cell system to be used in a real application, the fuel cell research group at General Electric was also the first to publish a series of technical articles on fuel cells in the Journal. In addition to inventing and developing the PEFC, this group of researchers examined the thermodynamics of fuel cells22 23 and did a considerable amount of work on direct-hydrocarbon fuel cells,24 25 26 27 28 29 as well as direct-ammonia fuel cells.30 31 They also developed new electrode structures32 33 and examined new electrocatalysts for use with reformate fuels.34 35 This group worked with an impressive array of low-temperature electrolytes; in addition to the ion-exchange membrane cells they used a variety of liquid acid electrolytes. An excellent review of this work, as well as a summary of the state of fuel cell technology at the time, is presented by two of the former GE workers, Liebhafsky and Cairns.1 It should be noted that after leaving GE, Elton Cairns (Fig. 6), has continued working on fuel cells at Argonne National Laboratory, Lawrence Berkeley National Laboratory, and at U.C. Berkeley. Elton Cairns is also an ECS Fellow and a past President of the Society.
Figure 6. E. J. Cairns (Society president 1989-1990 and ECS Fellow 1991) was one of the first to publish in the journal on fuel cells and has been active since then. E. J. Cairns worked on the first PEM fuel cells at GE.
The renewed interest in fuel cells inspired a substantial amount of research on the kinetics and mechanisms of the cathodic reduction of oxygen during the 1960s and 1970s. Because most of the performance losses in a  fuel cell are due to the polarization on the cathode it is not surprising that the kinetics of the oxygen-reduction reaction (ORR) received a lot of interest. In fact, the ORR is quite complex and, even after all this work, the detailed reaction mechanisms are still the subject of some controversy. Kinoshita has provided an exhaustive review of this subject in Chapter 2 of his ECS sponsored text on oxygen electrochemistry.4 Although the literature in this area is distributed among a variety of journals on electrochemistry and catalysis, the Journal and Society meetings have certainly been a key forum for discussion on this topic. Some examples of this include: reviews by Yeager and Scherson at Case Western Reserve University (CWRU) on oxygen electrocatalysis in aqueous electrolytes;36
37 ORR kinetic studies in
fuel cell are due to the polarization on the cathode it is not surprising that the kinetics of the oxygen-reduction reaction (ORR) received a lot of interest. In fact, the ORR is quite complex and, even after all this work, the detailed reaction mechanisms are still the subject of some controversy. Kinoshita has provided an exhaustive review of this subject in Chapter 2 of his ECS sponsored text on oxygen electrochemistry.4 Although the literature in this area is distributed among a variety of journals on electrochemistry and catalysis, the Journal and Society meetings have certainly been a key forum for discussion on this topic. Some examples of this include: reviews by Yeager and Scherson at Case Western Reserve University (CWRU) on oxygen electrocatalysis in aqueous electrolytes;36
37 ORR kinetic studies in  and other acids by Appleby at Texas A&M,38
39 Volgol and other researchers at UTC,40
41 and McBreen and O'Grady the Naval Research Laboratory;42 as well the influence of particle-size effects43 and physicochemical properties44 on the kinetics of the ORR.
and other acids by Appleby at Texas A&M,38
39 Volgol and other researchers at UTC,40
41 and McBreen and O'Grady the Naval Research Laboratory;42 as well the influence of particle-size effects43 and physicochemical properties44 on the kinetics of the ORR.
Another topic of fundamental interest to all fuel cell researchers is the preparation and properties of gas-diffusion electrodes (GDEs). In most fuel cells both of the electrodes are GDEs because both of the reactants are typically gases. And, in order to achieve good performance, especially with the slow ORR, a GDE with a very high interfacial contact area is required. Thus, the key to good fuel cell performance is constructing a stable and extensive interface between the required three phases: solid (electrode), liquid (electrolyte), and gas (reactant). (In the case of a true solid electrolyte, such as SOFC, only two phases are required.) This is further complicated by the continuous formation of the product water (as liquid and/or vapor) that must be effectively removed to maintain stable performance. In a GDE, three species must also be transported to or from the electrocatalyst sites in order for the heterogeneous electrochemical reactions to occur on a continuous basis. These species are  the electrons in the solid matrix,
the electrons in the solid matrix,  the ions in the electrolyte, and
the ions in the electrolyte, and  the gases dissolved in the electrolyte, as well as in the gas phase. Given that each of these species must be transported either to or from the catalyst sites, mass transport may control the rate of the reaction, in addition to reaction kinetics; and therefore reaction rates throughout a GDE tend to be nonuniform, especially at high current densities. The design, characterization, and analysis of these complex GDEs have received considerable attention in the Journal. Although much of this work is specific to a particular type of fuel cell, and will therefore be covered in the subsequent section, some of the work that is generally applicable to all fuel types will be reviewed here.
the gases dissolved in the electrolyte, as well as in the gas phase. Given that each of these species must be transported either to or from the catalyst sites, mass transport may control the rate of the reaction, in addition to reaction kinetics; and therefore reaction rates throughout a GDE tend to be nonuniform, especially at high current densities. The design, characterization, and analysis of these complex GDEs have received considerable attention in the Journal. Although much of this work is specific to a particular type of fuel cell, and will therefore be covered in the subsequent section, some of the work that is generally applicable to all fuel types will be reviewed here.
In low-temperature fuel cells, employing aqueous electrolytes, the gas-transport phase is typically provided by using hydrophobic PTFE, which prevents the electrolyte from completely flooding the void spaces of the porous matrix. PTFE-based GDES were first developed in the 1960s (see, for example, Ref. 33, 45, and 46) and were further characterized and optimized in the 1970s (see, for example, Ref. 47 and 48) In addition to providing hydrophobic gas pore within the GDE, the PTFE also serves as the binder for the small particles of supported electrocatalyst, or agglomerates, that comprise the solid matrix of these GDEs. The electrocatalyst in these cells is typically a noble metal (such as Pt or a Pt alloy), and the support (if any) is typically a high-surface-area carbon. Carbon supported catalysts have resulted in dramatic decreases in catalyst loadings, however, because the carbon support will oxidize in a fuel cell environment, special attention must be paid to the preparation of these materials, as well as the operation of the fuel cell to mitigate carbon corrosion. Kinoshita has provided an extensive overview of carbonaceous support materials, their use in fuel cells, and treatments used to improve their stability, as well as their application in a variety of GDEs.49
Theoretical modeling of GDEs has provided considerable insight into what limits the performance of fuel cells. A GDE is a very complicated structure that is difficult to characterize, even on a macroscopic level, and therefore a model of this structure can become very complex. One way to simplify this problem is to specify the size of the porous solid particles, and this is the basis of the "flooded-agglomerate model" first introduced by UTC researchers in the late 1960s.50 They used this model of a modern PTFE-bonded cathode to show that a Tafel slope twice the normal Tafel slope will arise due to mass-transfer limitations of oxygen in the electrolyte at high current densities. Other modelers have since employed the relatively simple flooded-agglomerate model to treat various fuel cell electrodes. For example, Iczkowski and Cutlip51 used this type of model, which included the diffusion of oxygen in the hydrophobic gas pores as well as in the electrolyte phase, to show that ohmic losses in the electrolyte may be responsible for a major portion of the potential losses at moderate current densities. However, these early models employed Ohm's law to treat ionic conduction in the electrolyte, which is not applicable when concentration gradients exist.52 More recent models have accounted for the effects of both ionic migration and diffusion in the solution phase (see, for example, Ref. 53 and 54). These models predict that a double Tafel slope may also arise when the current distribution is dominated by ohmic losses in the GDE. In this case, the ORR exhibits half-order dependence on the oxygen partial pressure, and this fact may be used to differentiate between cathodes that are limited by gas transport and those limited by ohmic losses.
Fuel Cell Development by Type
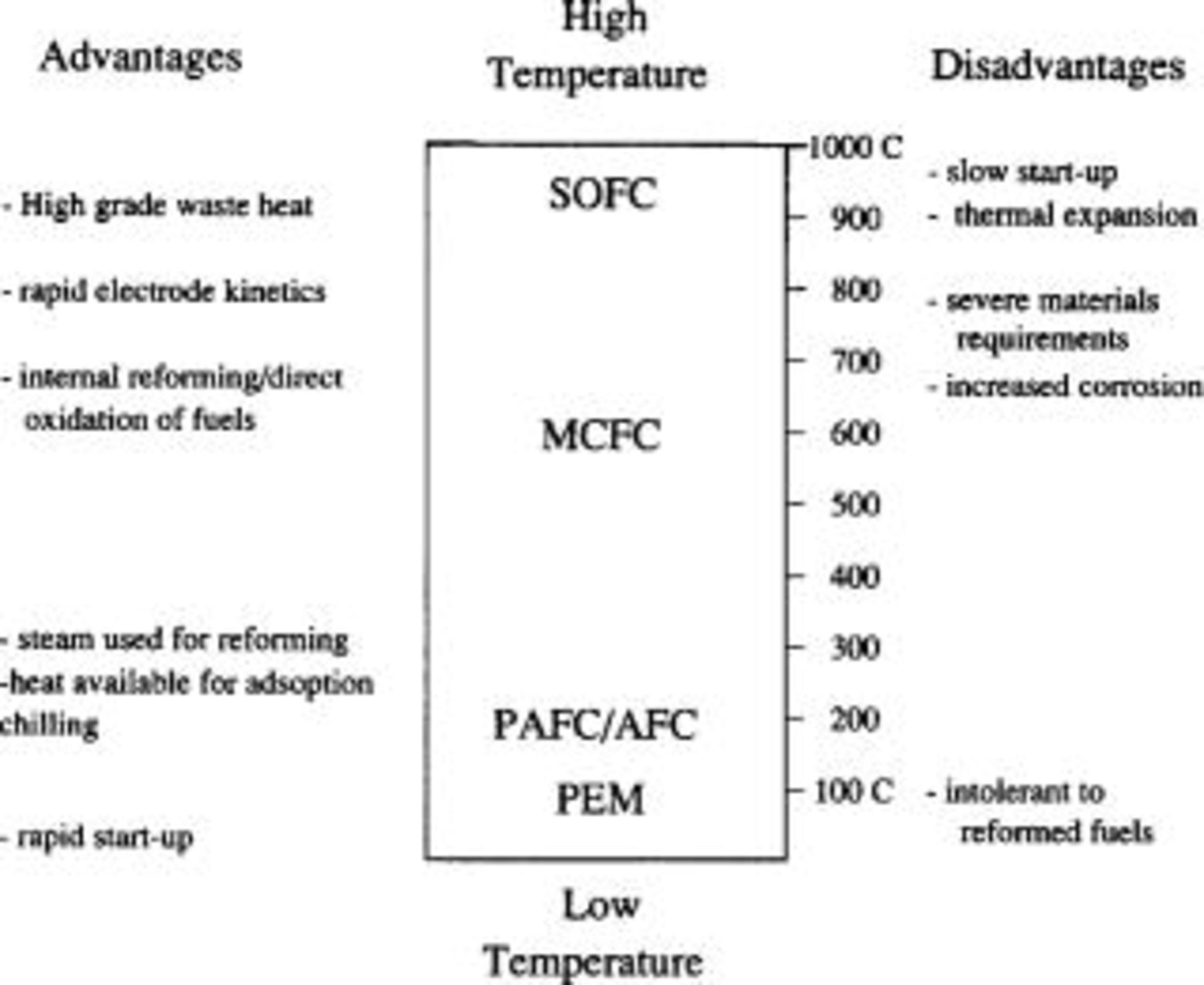
The temperature of operation and the type of electrolyte used distinguishes the various types of fuel cells. As shown in Fig. 7, fuel cells with a wide range of operating temperatures are being developed. The major advantages and disadvantages of each type are also summarized in the figure.
Figure 7. Types of fuel cells and advantages and disadvantages of higher temperatures.
Alkaline Fuel Cells (AFC)
Oxygen reduction kinetics is more facile in alkaline electrolytes than in acid electrolytes, and therefore AFCs have been developed that have impressive specific power and energy density even at low operating temperatures. However, a critical disadvantage of alkaline electrolytes (e.g., KOH, NaOH) is that they do not reject  therefore applications are restricted to operation on fuel and oxidants that do not contain
therefore applications are restricted to operation on fuel and oxidants that do not contain  (such as reformed fuels or air). Fortunately, for very remote operations where pressurized hydrogen and oxygen are considered viable reactants (e.g., space, undersea, military), cost is usually not a constraining factor and AFCs have therefore been competitive in these niche applications. However, unless the carbonation issue in AFCs is somehow resolved, the recent developments in PEM fuel cells appear to make PEM the technology of choice for a low-temperature cell that will operate on air and/or reformed fuels.
(such as reformed fuels or air). Fortunately, for very remote operations where pressurized hydrogen and oxygen are considered viable reactants (e.g., space, undersea, military), cost is usually not a constraining factor and AFCs have therefore been competitive in these niche applications. However, unless the carbonation issue in AFCs is somehow resolved, the recent developments in PEM fuel cells appear to make PEM the technology of choice for a low-temperature cell that will operate on air and/or reformed fuels.
Polymer Electrolyte Fuel Cells (PEFC)
Two major improvements have been made in PEFC technology since the Gemini program. The first was the development of perfluorosulfonic-acid membranes that are more stable than the hydrocarbon-based membranes used in the early PEFCs. These membranes, such as Nafion®, consist of a PTFE backbone with perfluorinated-vinyl-polyether side chains terminated by a sulfonate group. Nafion, and similar derivatives, are essentially fully fluorinated and therefore they do not suffer from rapid degradation in a fuel cell since  bonds are more stable than
bonds are more stable than  bonds.
bonds.
DuPont originally developed perfluorosulfonic-acid membranes in the early 1960s for use in the chlor-alkali industry, although it was readily recognized that they are well suited for PEFCs, and the first Nafion-based PEFC was tested in 1966. Yeo55 and Eisman56 provided excellent reviews of the properties of these membranes at ECS meetings in the mid-1980s. Thinner membranes (e.g., N112 and composite membranes from W. L. Gore) have also greatly enhanced the performance of PEFCs.
The second major improvement was the dramatic reduction of catalyst loadings in PEFCs first demonstrated by Ian Raistrick at Los Alamos National Laboratory (LANL).57 By impregnating the electrodes with perfluorinated ionomers in liquid form (polymer dissolved in alcohol), a thin coating of the polymer electrolyte can be deposited on the electrocatalysts. The resulting GDE has much greater interfacial area than the previous conventional method of simply hot-pressing the electrocatalysts to the membrane. In fact, Raistrick demonstrated improved performance with an order-of-magnitude less catalyst loading than the conventional PEFC electrodes. Researchers at LANL subsequently refined these electrode preparation techniques and developed the modern membrane electrode assembly (MEA) that is the heart of today's PEFCs (see, for example, Ref. 58).
The fuel cell research conducted at LANL during the mid-1980s and early 1990s laid the groundwork for the renaissance in PEFCs that is still ongoing today. In addition to developing the state-of-the-art MEA, LANL researchers conducted some very useful fundamental research on the kinetics of PEFCs,59 60 61 longer-term testing,62 and developed PEFC models (see, for example, Ref. 63). Supramaniam Srinivasan, one of the original members of the LANL group, continued his PEFC kinetic studies at Texas A&M (see, for example, Ref. 64 and 65). LANL was also the first to propose using an air bleed on the anode to improve the relatively poor tolerance of PEFC to carbon-monoxide poisoning.66 Shimshon Gottesfeld (ECS Fellow, 1999) and Tom Zawodzinski have provided an excellent review of PEFC technology.5
A biennial series of ECS symposia on Proton Conducting Membrane Fuel Cells has been organized. This symposium is presently organized by S. Gottesfeld, T. Fuller, and others. The third in the series will take place in Salt Lake City at the Fall 2002 meeting of the Society. The development of PEFCs is currently an active area of research with numerous companies striving to develop commercial products for use in stationary power generation, automotive/transportation applications, and as an alternative for batteries in portable electronics.
Phosphoric Acid Fuel Cells (PAFC)
In spite of all the recent activity in fuel cells over the last decade, the phosphoric acid technology is still the only one used in commercial product. Kinoshita4 provides a good review of the phosphoric-acid technology; we will take time to review only the significant contributions made through the Society. The technical challenges with this technology are well known. The higher operating temperatures (150-200°C) reduce the complexity of power plants and make the waste heat more valuable, but they also introduce materials challenges that are not nearly so difficult to meet as in PEM technology, for example. In addition, the solubility of oxygen in phosphoric acid is low and there is anion adsorption on the cathode catalysts, which reduces the activity of the electrodes. Two areas are briefly reviewed here:  improved materials, particularly, carbon supports and improved cathode catalysts, and
improved materials, particularly, carbon supports and improved cathode catalysts, and  postmortem analysis of cells. Fabrication of electrodes in phosphoric acid fuel cells borrowed heavily from the alkaline technology, with the early electrodes using metal blacks. Platinum supported on carbon was used to reduce the loadings of the electrodes by about an order of magnitude. (It is interesting that we saw a similar path taken with PEM.) Early on, the technology focused on high loaded platinum blacks and then the transition was quickly made to supported catalysts. Putting the catalysts on carbon supports allowed for a large improvement in performance and reduction in catalysts loading, but issues with the stability of the high surface area carbon arose. Much of the contributions centered on understanding and development of new carbon supports. There are too many significant contributions to go into detail, but a few are worth mentioning here. Some of the key contributors were J. Bett, K. Kinoshita, H. R. Kunz (1998 winner of the research award of the Energy Technology Division), and G. A. Gruver. A second area that was reported on relative to phosphoric acid fuel cells was post mortem analysis of cells. As these fuel cell power plants approach commercialization, we saw a number of articles related to corrosion resistance67 and platinum dissolution and migration,68 post mortem analysis,69 and effects of impurities.70
71
postmortem analysis of cells. Fabrication of electrodes in phosphoric acid fuel cells borrowed heavily from the alkaline technology, with the early electrodes using metal blacks. Platinum supported on carbon was used to reduce the loadings of the electrodes by about an order of magnitude. (It is interesting that we saw a similar path taken with PEM.) Early on, the technology focused on high loaded platinum blacks and then the transition was quickly made to supported catalysts. Putting the catalysts on carbon supports allowed for a large improvement in performance and reduction in catalysts loading, but issues with the stability of the high surface area carbon arose. Much of the contributions centered on understanding and development of new carbon supports. There are too many significant contributions to go into detail, but a few are worth mentioning here. Some of the key contributors were J. Bett, K. Kinoshita, H. R. Kunz (1998 winner of the research award of the Energy Technology Division), and G. A. Gruver. A second area that was reported on relative to phosphoric acid fuel cells was post mortem analysis of cells. As these fuel cell power plants approach commercialization, we saw a number of articles related to corrosion resistance67 and platinum dissolution and migration,68 post mortem analysis,69 and effects of impurities.70
71
UTC began commercial production of 200 kW PAFC power plants that operate on natural gas in 1991 (shown in Fig. 8). Over 200 units have been sold and installed in 15 countries around the world. Individual units have achieved over 40,000 hours of continuous operation without a major overhaul, meeting the stated lifetime goal of these power plants. The total accumulated hours of UTC's PAFC fleet is now over 5 million hours, with a fleet availability of over 95 percent.
Figure 8. PAFC Power Plant—To the First National Bank of Omaha just a few milliseconds without electricity can mean hours of headaches and millions of dollars of lost revenues. Four UTC Fuel Cells 200 kW power plants now serve as the bank's primary source of power. Heat from the fuel cell installation also provides energy for space heating, increasing the overall efficiency of the fuel cell system to more than 80 percent.
Molten Carbonate Fuel Cells (MCFC)
As mentioned earlier, Baur and co-workers started using molten carbonate electrolytes in their attempts to develop a direct coal fuel cell at the beginning of the last century. However, after almost three decades of work, Baur became disillusioned with molten salts and took the position that solid electrolytes would be the best candidate for high temperature fuel cells.72 However, the desire to develop a fuel cell with a greater flexibility to use readily available hydrocarbon fuels continued to motivate others to pursue the development of MCFCs to the present day. In fact, soon after Baur decided to abandon melts, H. Greger made a significant contribution to MCFC technology by showing that when carbon dioxide from the anode is recycled to the cathode that the electrolyte could be kept invariant.73 Then, in the 1950s and 1960s, a lot of development work was done on MCFCs that is well summarized in a 1965 book edited by B. S. Baker;74 important contributors during this period included researchers from Holland (G. H. J. Broers), France (A. Salvadori), England (H. H. Chambers and A. D. S. Tantram), and the United States (E. Gorin, H. Recht, D. L. Douglas, I. Trachtenberg, and B. S. Baker).
The relatively high operating temperature  of MCFCs provides several key advantages:
of MCFCs provides several key advantages:  the opportunity to achieve very high efficiencies with co-generation cycles,
the opportunity to achieve very high efficiencies with co-generation cycles,  noble metal catalysts are not required, and
noble metal catalysts are not required, and  better tolerance to different fuels. However, the higher temperature also limits the materials that can be used. For example, whereas PTFE is used in PEFCs and PAFCs to help establish a stable gas/liquid interface, in MCFCs controlling the pore size is the only method available to maintain this key interface (akin to the Bacon AFC). Electrolyte management in MCFCs is definitely key to long-term performance, and the various processes that contribute to the redistribution of molten carbonate in MCFC stacks has been discussed in the ECS publications by both H. Maru75 and R. Kunz.76 Additional issues that are still being addressed in MCFCs are hardware corrosion, cathode dissolution, and low power density. However, companies in Europe, Japan, and the United States have programs dedicated to developing stationary power plants (typically 100-250 kW) using MCFCs with improved performance and endurance.
better tolerance to different fuels. However, the higher temperature also limits the materials that can be used. For example, whereas PTFE is used in PEFCs and PAFCs to help establish a stable gas/liquid interface, in MCFCs controlling the pore size is the only method available to maintain this key interface (akin to the Bacon AFC). Electrolyte management in MCFCs is definitely key to long-term performance, and the various processes that contribute to the redistribution of molten carbonate in MCFC stacks has been discussed in the ECS publications by both H. Maru75 and R. Kunz.76 Additional issues that are still being addressed in MCFCs are hardware corrosion, cathode dissolution, and low power density. However, companies in Europe, Japan, and the United States have programs dedicated to developing stationary power plants (typically 100-250 kW) using MCFCs with improved performance and endurance.
J. R. Selman, I. Uchida, and others have organized a recurring symposia on Molten Carbonate Technology. Selman and Uchida were awarded the 2001 Research Award of the Energy Technology Division. Five proceedings volume dedicated to this technology have resulted.
Solid Oxide Fuel Cells (SOFC)
The "Nernst Glower" was the first use of a solid ionic conductor, demonstrated in the end of the nineteenth century.77 In the 1940s Carl Wagner (winner of the Society's Olin Palladium Medal, 1951) explained the conduction process, and the first article on a SOFC appeared in the Journal in 1962.78 This early work by Weissbart and Ruka was done at Westinghouse, which has continued to be a leader in SOFC technology to the present day.
SOFCs are the only fuel cells that utilize a true solid electrolyte, and therefore electrolyte-management problems that are common to other fuel cell types are not a concern for SOFCs. (Note that the membranes used in PEFCs must be hydrated to function well and therefore require careful management of the water within the cell.) Additionally, the high operating temperature, typically about 1000°C, makes the SOFC much more tolerant of reformed fuels and, because water is generated on the anode, in-cell reformation of hydrocarbon fuels is feasible. SOFCs are also potentially capable of very high electrical efficiencies in a co-generation system (e.g., integrated with gas microturbines). However, much like the MCFC, the high operating temperature also creates a host of durability issues, such as very limited selection of robust sealant materials (e.g., glasses). To address the negative effects of glass-based seals, a variety of stack designs other than the traditional bipolar-plate stack design (e.g., tubular or monolithic) have been pursued. The most severe challenge in planar SOFC stacks is the poor lifetime of coefficient of thermal expansion matching ferritic stainless steels that are being used as interconnects and the requirement for sealants, mostly based on glass ceramics, in cross-flow stack configuration. The seal-less tubular design of Siemens-Westinghouse has been demonstrated with 150 kW power plants for up to 16,000 h with acceptably low degradation rates, which proves the robustness of the set of SOFC materials in this tubular stack configuration. Although significant demonstrations of solid oxide cells have been made,79 several challenges must be overcome for commercialization of planar configurations. N. Minh has provided an excellent technical review of SOFC technology.80
Today, the SOFC is an area of active research in academia, industry, and governmental laboratories. Over the last decade, nearly half of the papers published in the Journal with "fuel cell" in the title were devoted to SOFC technology. Although the advantages of lower operating temperatures were recognized very early on,81 reducing the temperature from 1000° to 700°C, or even 500°C, is presently one of the key areas of research. This reduction in temperature is accomplished by using alternate ionic conductors to yttria-stabilized zirconia (YSZ), e.g., ceria and doped lanthanum gallates. A lower temperature allows the use of stainless steel in cell stack construction, which would greatly reduce the cost. At the same time, these temperatures are not so low as to eliminate the advantages of SOFC technology. The primary challenges with lowering the temperature are reducing the ohmic losses, electrolyte stability, and cathode polarization. The conductivity of the solid electrolyte is strongly dependent on the temperature. Acceptable conductance of the separator can be achieved by using thinner films or doping to increase the conductivity.
The Society has been one of the primary forums for communicating results in SOFC development. S. Singhal (ECS Fellow, 1996) has co-organized a biennial series of symposia in conjunction with the SOFC Society of Japan since 1989. These have been some of the best attended international meetings and most successful proceedings volumes on fuel cells. Seven proceedings volumes have been published by the International Symposia on Solid Oxide Fuel Cells. This volume contains papers dealing with the materials for cell components, fabrication methods for components, and complete cells. Also contained in this volume are cell electrochemical performance and modeling, stacks and systems, and field testing of SOFC demonstration units. Companies in Europe, Japan, and the U.S. are presently working on various SOFC systems in various stages of development. For example, both Siemens-Westinghouse and Sulzer/Hexis are presently developing prototypes of small  SOFC power plants.
SOFC power plants.
Future Directions
More work is being done on fuel cell development than ever before. Multiple companies around the world are focused on the commercialization of fuel cells for stationary power, and almost all of the major automotive companies have either internal fuel cell development programs and/or they are working closely with other companies to develop power plants for transportation applications.
The widespread commercialization of stationary fuel cell power plants appears inevitable, with the only major technological barriers remaining being cost and proven lifetimes. However, because PAFC power plants with suitable lifetimes  for stationary applications have already been developed, it appears reasonable to expect that the lifetime requirements will be met by fuel cells that operate at even lower temperatures (e.g., PEFC). In regard to cost, a major advantage of PEFC is that all the repeat parts (e.g., MEAs and bipolar plates) can be manufactured by high-volume processes that are already being demonstrated by multiple companies. Undoubtedly, improving the lifetime and cost of fuel cells will be a major focus for many years to come given that continuous improvement in these two critical areas will increase the number of applications where fuel cells are competitive with other power-generation technologies.
for stationary applications have already been developed, it appears reasonable to expect that the lifetime requirements will be met by fuel cells that operate at even lower temperatures (e.g., PEFC). In regard to cost, a major advantage of PEFC is that all the repeat parts (e.g., MEAs and bipolar plates) can be manufactured by high-volume processes that are already being demonstrated by multiple companies. Undoubtedly, improving the lifetime and cost of fuel cells will be a major focus for many years to come given that continuous improvement in these two critical areas will increase the number of applications where fuel cells are competitive with other power-generation technologies.
The cost targets for automotive applications are roughly an order-of-magnitude more demanding than those for stationary applications. This, and other technical challenges, will inevitably delay the widespread introduction of fuel cell powered automobiles. However, demonstration vehicles have already been introduced and many more are in development (Fig. 9). The resources being dedicated to these efforts are impressive and will undoubtedly accelerate the commercialization of fuel cells in both stationary and transportation sectors. Another issue that must be resolved before fuel cells find widespread acceptance in transportation applications is the availability of a suitable fuel infrastructure. Hydrogen would be the fuel of choice, but the volume and weight of the various hydrogen-storage technologies still do not compare favorably with liquid fuels. Therefore, efforts to develop fuel cell vehicles that operate on gasoline, methanol, or other fuels are ongoing. Because of the fuel infrastructure issue, fleet vehicles that operate on hydrogen and refuel at a central station will be introduced first. In particular, buses are an attractive application for fuel cells because the cost, volume, and range requirements are not typically as challenging as those for the automotive targets.
Figure 9. Honda's newest fuel cell powered vehicle, the FCX-V4.
The development of new materials will continue to have a major influence on the development of fuel cells. Just as the introduction of PTFE enabled new GDEs for aqueous-electrolyte fuel cells and the availability of ion-exchange membranes resulted in the creation of PEFCs, the introduction of new materials will enable both improved designs and new types of fuel cells. Currently, an active area of research is the search for new polymer-electrolyte membranes. Ideally, these membranes will possess high ionic conductivity without the presence of liquid water, which will enable them to operate at higher temperatures, resulting in simpler PEFC systems due to improved heat rejection and CO tolerance. At the other end of the temperature spectrum, the development of solid oxide conductors that have suitable conductivity at lower temperatures would be desirable because this might allow alternative sealing materials to be employed in SOFCs and should help mitigate material degradation issues. Other examples of new materials that would aid in the commercialization of fuel cells are advanced electrocatalysts (e.g., with higher activity, and/or lower cost, and/or improved stability), low-cost composites with excellent stability and electrical conductivity that could be used to construct bipolar plates, and robust sealing materials (especially for high-temperature fuel cells). Finally, the development of advanced hydrogen-storage materials would greatly aid in the introduction of hydrogen-powered vehicles.
Another need that is clearly evident to those of us who are working towards the commercialization of fuel cells is a workforce of engineers that have a good understanding of electrochemistry and the design of electrochemical systems. Currently, there are very few engineering programs that offer courses in these subjects, especially at an undergraduate level. However, the development, design, and manufacture of a complete fuel cell power plant involves a lot of engineering work that requires at least a working knowledge of electrochemical principles. Universities that recognize this need and provide quality programs in electrochemical engineering, at both the undergraduate and graduate level, will produce graduates who are in high demand as electrochemistry-based industries, including fuel cells, continue to grow. For example, many chemical engineering departments now offer the opportunity for undergraduates to emphasize a particular area by taking several optional courses in that field (e.g., biochemical engineering); a similar program in electrochemical engineering would also appear to be well justified and potentially very beneficial for both industry and the new graduates.
Although one cannot project the future of fuel cells over the next hundred years, it can be stated with some certainty that The Electrochemical Society will continue to play a prominent role in the development of fuel cells. Society members will undoubtedly continue to lead research in fuel cell technologies as well as train others in the field. And the meetings and publications sponsored by the Society will continue to be an important forum for the discussion of these developments. Hopefully, when the 200th anniversary of the Society arrives fuel cells will be as ubiquitous as the internal combustion engine is today.