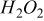Abstract
The electrogeneration of hydroxyl radicals was studied at a synthetic boron-doped diamond (BDD) thin film electrode. Spin trapping was used for detection of hydroxyl radicals with 5,5-dimethyl-1-pyrroline-N-oxide and with salicylic acid using electron spin resonance and liquid chromatography measurements, respectively. The production of hydrogen peroxide and competitive oxidation of formic and oxalic acids were also investigated using bulk electrolysis. The results have shown that oxidation of salicylic acid leads to the production of hydroxylated products (2,3- and 2,5-dihydroxybenzoic acids). These results demonstrate that the oxidation process on BDD electrodes involves hydroxyl radicals as electrogenerated intermediates. © 2003 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Oxidative electrochemical processes promising versatility, environmental compatibility, and cost effectiveness have a continuously growing importance both in selective organic synthesis and in the electrochemical incineration (ECI) of organic pollutants in aqueous media. In case of organic electrosynthesis, the selectivity is to be enhanced, whereas the aim of the ECI process is the mineralization of the toxic and nonbiocompatible pollutants with high current efficiency.1
2
3
4
5
6
7
8
9
10 Recently, a new mechanism for the electrochemical oxidation of organics based on intermediates of oxygen evolution reaction in aqueous media has been proposed by Johnson et al.11
12
13
14
15
16 The process involves anodic oxygen transfer from  to organics via hydroxyl radicals formed by water discharge. A typical example is the oxidation of organic compounds on
to organics via hydroxyl radicals formed by water discharge. A typical example is the oxidation of organic compounds on  or
or  doped
doped  electrodes.11
12
13
14
15
16
electrodes.11
12
13
14
15
16
In our laboratory, we have found that electrochemical oxidation of most organics in aqueous media occurs only at high potentials with concomitant evolution of  without any loss in electrode activity.1
2
3
4
5 Furthermore it has been found that the nature of the electrode material influences strongly both the selectivity and the efficiency of the process.1
6
17
18 In order to interpret these observations, a comprehensive model for anodic oxidation of organics in acid medium, including competition with oxygen evolution, has been proposed.1
6
17
18 This model distinguishes between two limiting cases, active and nonactive anode.
without any loss in electrode activity.1
2
3
4
5 Furthermore it has been found that the nature of the electrode material influences strongly both the selectivity and the efficiency of the process.1
6
17
18 In order to interpret these observations, a comprehensive model for anodic oxidation of organics in acid medium, including competition with oxygen evolution, has been proposed.1
6
17
18 This model distinguishes between two limiting cases, active and nonactive anode.
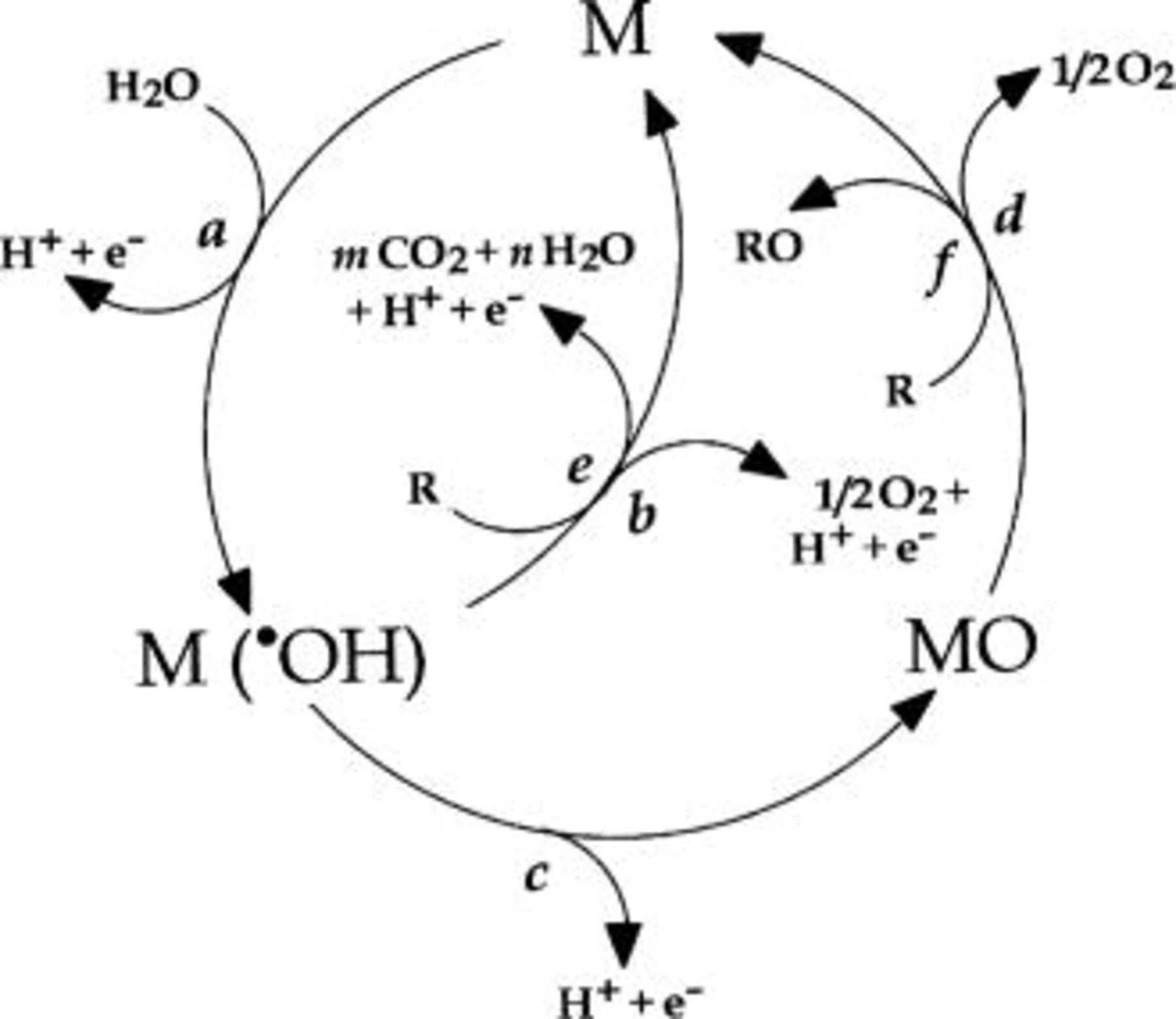
Figure 1 illustrates the reaction scheme of the proposed model in acid medium (1 M  where M designates an active site at the anode surface.17
18
where M designates an active site at the anode surface.17
18
Figure 1. Scheme of the indirect oxidation of organic compounds with simultaneous oxygen evolution (a) water discharge to hydroxyl radicals, (b) oxygen evolution by electrochemical oxydation of hydroxyl radicals, (c) formation of the higher metal oxide, (d) oxygen evolution by chemical decomposition of the higher metal oxide, (e) oxidation of the organic compound, R, via hydroxyl radicals, (f) oxidation of the organic compound via the higher metal oxide.
In any case, the initial step is the discharge of water molecules to form adsorbed hydroxyl radicals (Reaction 1, Fig. 1)
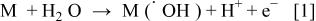
The electrochemical and chemical reactivity of the adsorbed hydroxyl radicals depends strongly on the nature of the electrode material used. Two extreme classes of electrodes can be defined as active and nonactive electrodes:
1. In active electrodes, there is a strong interaction of the hydroxyl radicals with the electrode surface. In that case, the adsorbed hydroxyl radicals may interact with the anode with the possible transition of the oxygen from the hydroxyl radical to the anode surface, forming the so-called higher oxide (Fig. 1, Reaction 2). This may be the case when higher oxidation states on the surface electrode are available above the thermodynamic potential for oxygen evolution (1.23 V/SHE)
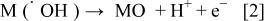
We speculate that at active electrodes the surface redox couple MO/M can act as a mediator in the oxidation of organics (Reaction 3). This reaction is in competition with the side reaction of oxygen evolution due to the chemical decomposition of the higher oxide (Reaction 4)
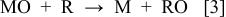
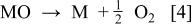
The oxidation reaction via the surface redox couple MO/M (Reaction 3) may be much more selective than the reaction involving hydroxyl radicals (see below, Reaction 5)
2. In nonactive electrodes there is a weak interaction between the hydroxyl radicals and the electrode surface. The oxidation of organics is mediated by hydroxyl radicals (Reaction e), which may result in fully oxidized reaction products such as 
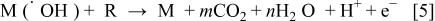
In this schematic reaction equation, R is the fraction of an organic compound containing no heteroatom that needs one atom of oxygen to be transformed to fully oxidized products. (Actual values of m and n depend on the elemental composition of R to be oxidized.) This reaction is in competition with the side reaction of hydroxyl radicals discharge (direct or indirect through formation of  as intermediate) to
as intermediate) to  (Fig. 1, Reaction 6) without any participation of the anode surface
(Fig. 1, Reaction 6) without any participation of the anode surface
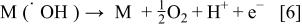
The nonactive anode has been defined as an electrode that does not participate in the anodic reaction and does not provide any catalytically active site for adsorption of reactants and/or products in aqueous medium. In that case the anode can serve only as an inert substrate, which can act as a sink for the removal of electrons. Outer sphere reactions (in which case the reactant and product do not interact strongly with the electrode surface) and water discharge (the electrode is considered to be covered by at least one adsorbed layer of water molecules) are in principle the only possible anode reactions on nonactive anodes. Active intermediates such as hydroxyl radicals produced by water discharge on nonactive anodes (Reaction a in Fig. 1) are considered to be involved in the oxidation of organic compounds in aqueous medium. This can result in the ECI of the organic compounds (Reaction e in Fig. 1).17 18
The electrochemical activity (overpotential for oxygen evolution) and chemical reactivity (rate of organics oxidation with electrogenerated hydroxyl radicals) of adsorbed hydroxyl radicals, are strongly related to the strength of anode (M)-hydroxyl radicals  interaction. As a general rule, weak
interaction. As a general rule, weak  interactions lead to low anode activity toward oxygen evolution (high overvoltage anodes) and high is the anode reactivity for organics oxidation (fast chemical reaction).
interactions lead to low anode activity toward oxygen evolution (high overvoltage anodes) and high is the anode reactivity for organics oxidation (fast chemical reaction).
Boron-doped diamond (BDD) based anode is a new electrode material with high anodic stability and acceptable conductivity.19
20
21 Moreover BDD has an inert character and is well known to have weak adsorption properties. This suggests that this material is an ideal nonactive electrode on which oxidation of organics and oxygen evolution take place via the formation of hydroxyl radicals. Furthermore, diamond-based anodes have very low activity for  evolution reaction (high overpotential), the onset potential for
evolution reaction (high overpotential), the onset potential for  evolution on p-Si/BDD anodes (2.2 V/SHE) being about 1 V higher than the standard redox potential of
evolution on p-Si/BDD anodes (2.2 V/SHE) being about 1 V higher than the standard redox potential of  evolution (1.23 V/SHE).22
23
evolution (1.23 V/SHE).22
23
In this work we report more evidence for the formation of hydroxyl radicals on BDD by means of spin-trapping using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), hydroxylation of salicylic acid (SA), and formation of hydrogen peroxide. Furthermore, the different reactivity between hydroxyl radicals and two carboxylic acids (formic and oxalic) has been used to confirm the formation of free hydroxyl radicals on BDD anodes.
Experimental
Preparation of diamond films.—
Boron-doped diamond films were synthesized by the hot filament chemical vapor deposition technique (HF-CVD) on conducting p-Si substrate (Siltronix). The temperature of the filament was from 2440 to 2560°C and that of the substrate was monitored at 830°C. The reactive gas used was 1% methane in hydrogen containing 1-3 ppm of trimethylboron. The gas mixture was supplied to the reaction chamber at a flow rate of 5 L min−1 to give a growth rate of 0.24 μm h−1 for the diamond layer. The diamond film thickness was about 1 μm and the resistivity 10-30 mΩ cm. This HF-CVD process produces columnar, randomly textured, polycrystalline films.
Detection of hydroxyl radicals by spin-trapping using DMPO as the trapping agent.—
Hydroxyl radicals formed by water discharge at the BDD anode (Eq. 7) are directly detectable by electron spin resonance (ESR) only if the  are produced in a relatively high concentration in the ESR cavity by in situ electrolysis
are produced in a relatively high concentration in the ESR cavity by in situ electrolysis
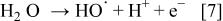
The indirect technique for the detection and the identification of low concentration of  involves trapping of the hydroxyl radicals by an addition reaction (spin trapping) to produce a more stable radical (spin adduct). In this work 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) has been used as a spin trap (Eq. 8)
involves trapping of the hydroxyl radicals by an addition reaction (spin trapping) to produce a more stable radical (spin adduct). In this work 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) has been used as a spin trap (Eq. 8)
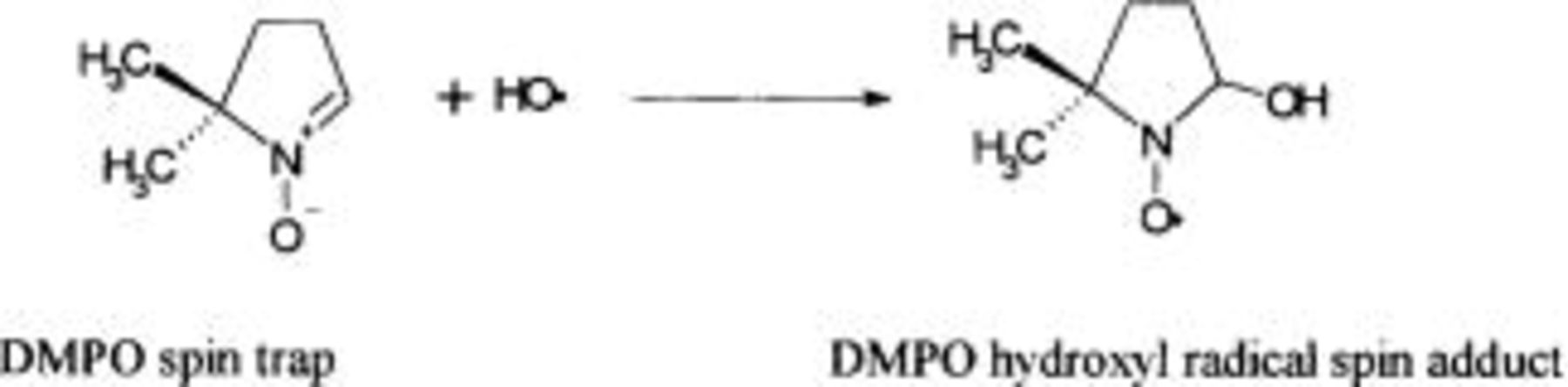
Electrolysis of DMPO solution (10 mM) in perchloric acid was carried out using a one-compartment cell. Diamond was used as the working electrode (exposed apparent area, 1 cm2) and Pt as a counter electrode. The electron spin resonance (ESR) signals of the DMPO spin adduct were recorded at ambient temperature (298 K) with a Brucker ECS 080 spectrometer. The conditions for the measurement were X Band; 100 kHz modulation with 1.5 G application; microwave power, 10 mW; central magnetic field, 3350 G; scan width, 100 G.
The obtained ESR spectrum was compared with the spectrum obtained using the Fenton reaction where a solution of DMPO, 10 mM;  1 M; and
1 M; and  10 mM is allowed to react for 60 min.
10 mM is allowed to react for 60 min.
Hydroxylation of salicylic acid.—
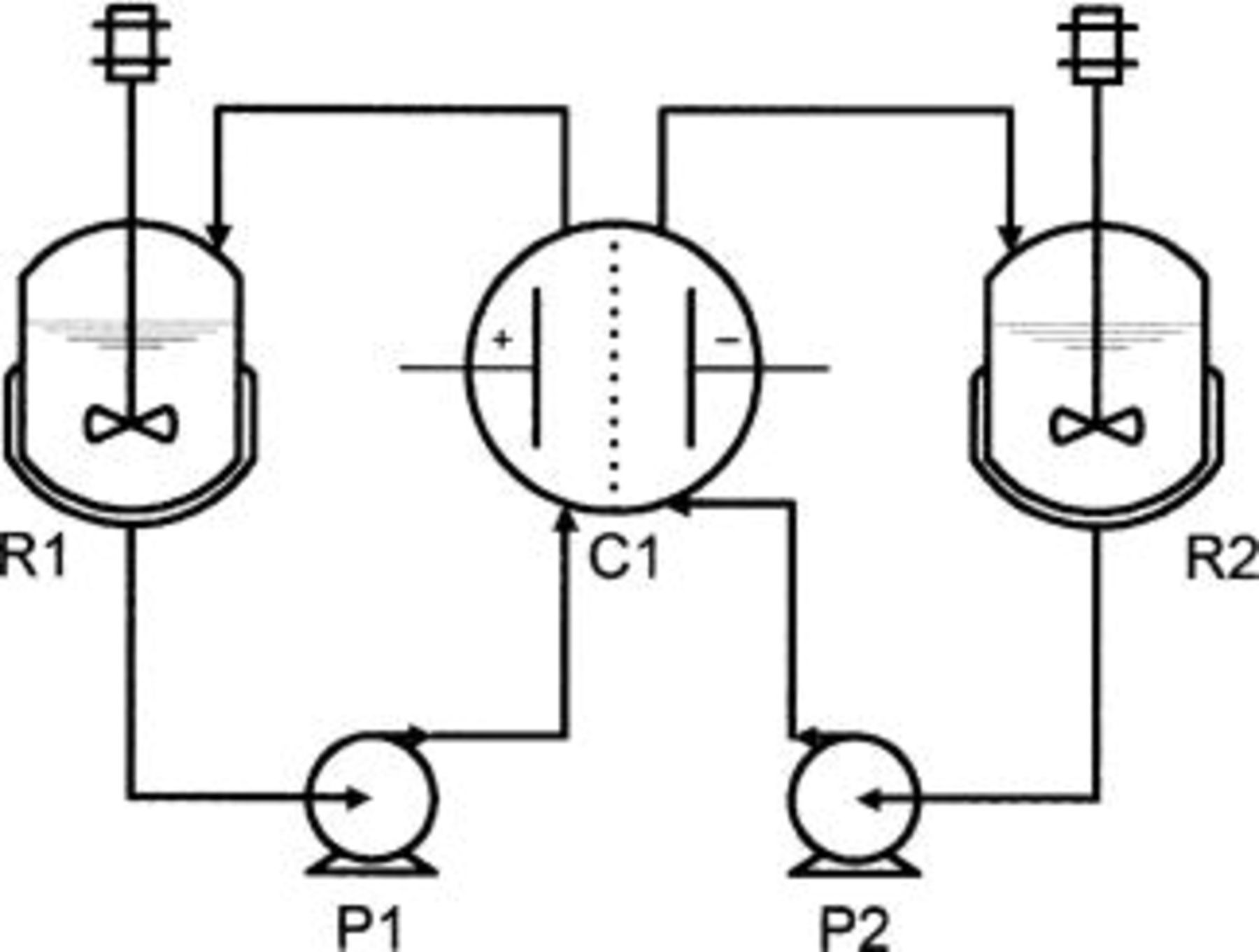
A divided electrolytic flow cell was used for the measurements (Fig. 2). The anode and the cathode were disks of BDD and zirconium, respectively, with a geometric area of 64 cm2. Anolyte (7.25 mM salicylic acid, 1 M  and catholyte (1 M
and catholyte (1 M  were separated by ion exchange membrane (Nafion 117, Dupont).
were separated by ion exchange membrane (Nafion 117, Dupont).
Figure 2. Scheme of the equipment used for bulk oxidation with the two-compartment cell on the diamond anode: (R1) and (R2) thermoregulated reservoirs, (C1) electrochemical cell, (P1) and (P2) pumps.
Galvanostatic assays were performed between 0.4 and 4 mA cm−2. The temperature of the system was kept constant at 15°C. The concentrations of salicylic acid and dihydroxybenzoic acids were monitored by HPLC on a Shimadzu LC-2010A, using a  column, with 10% HPLC-grade acetonitrile, 10% HPLC-grade methanol, 0.03 M citric acid, 0.3 M acetic acid, and water as the mobile phase. The selectivity of the hydroxylated intermediates, relative to the amount of salicylic acid converted, has been defined as (Eq. 9)
column, with 10% HPLC-grade acetonitrile, 10% HPLC-grade methanol, 0.03 M citric acid, 0.3 M acetic acid, and water as the mobile phase. The selectivity of the hydroxylated intermediates, relative to the amount of salicylic acid converted, has been defined as (Eq. 9)
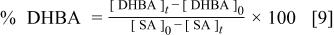
where [DHBA] is the concentration of dihydroxybenzoic acids (2,3- and 2,5-dihydroxybenzoic acids) at times 0 and t (in mmol dm−3) and [SA] is the concentration (in mmol dm−3) of salicylic acid at times 0 and t.
Formation of  .—
.—
The previously explained divided electrolytic flow cell was used for the measurements (Fig. 2). Anolyte and catholyte were 1 M  solutions.
solutions.
Galvanostatic assays were performed between 23 and 160 mA cm−2. Hydrogen peroxide was monitored by titration with permanganate.24 For low  concentrations analysis with Ti(IV) has been used.25
concentrations analysis with Ti(IV) has been used.25
Electrochemical oxidation of formic and oxalic acids.—
The previously explained divided electrolytic flow cell was used for the measurements (Fig. 2). As anolyte, a solution containing 0.5 M formic acid and 0.5 M oxalic acid in 1 M perchloric acid was used. As catholyte, a solution containing 1 M perchloric acid was used. The current density was 23.8 mA cm−2 and the temperature was kept at 40°C. The concentrations of formic and oxalic acids were monitored by HPLC on a Shimadzu LC-6 Series, using a Supelcogel C-610H column, the mobile phase being a solution of 1% 
Results and Discussion
Detection of hydroxyl radicals by spin-trapping using DMPO as trapping agent.—
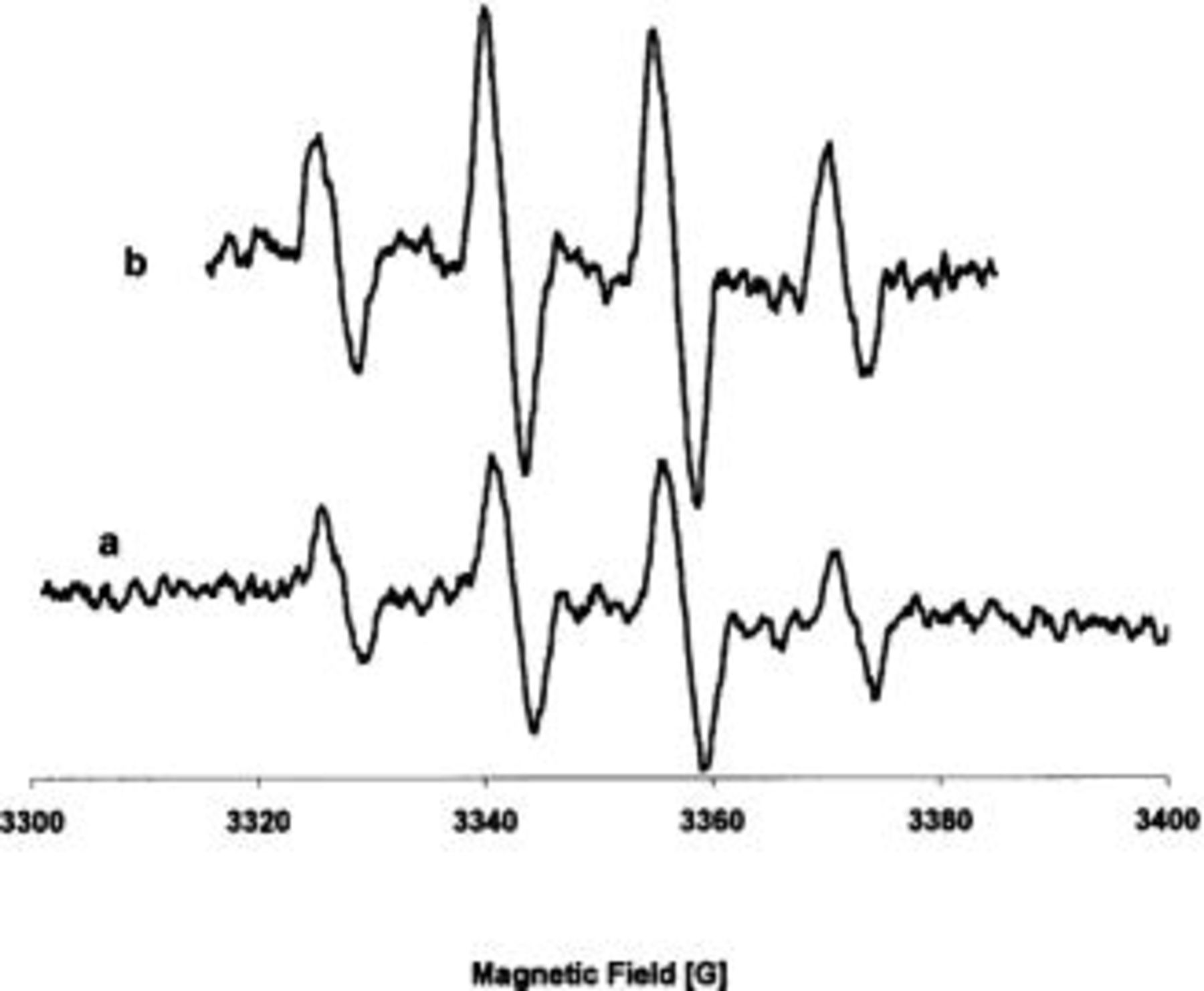
Figure 3 shows ESR spectrum obtained after 120 min of electrolysis of 10 mM DMPO in  1 M at 0.1 mA cm−2 (a) and the spectrum using Fenton reaction (b). The spectra reveal the existence of the DMPO hydroxyl radical spin adduct (Eq. 8). The ESR hyperfine couplings of the DMPO adduct produced by electrolysis (obtained by simulation) are
1 M at 0.1 mA cm−2 (a) and the spectrum using Fenton reaction (b). The spectra reveal the existence of the DMPO hydroxyl radical spin adduct (Eq. 8). The ESR hyperfine couplings of the DMPO adduct produced by electrolysis (obtained by simulation) are  These are in concordance with the parameters obtained with the DMPO adduct produced by Fenton reaction
These are in concordance with the parameters obtained with the DMPO adduct produced by Fenton reaction  These hyperfine coupling constants are typical of the spin adduct DMPO-OH,26
27 meaning that hydroxyl radicals are involved during the oxidation on BDD anodes.
These hyperfine coupling constants are typical of the spin adduct DMPO-OH,26
27 meaning that hydroxyl radicals are involved during the oxidation on BDD anodes.
Figure 3. ESR spectrum of DMPO adduct obtained (a) after electrolysis of a 8.8 mM DMPO solution in  1 M for 2 h on BDD electrode at 0.1 mA cm−2 and (b) by Fenton reaction.
1 M for 2 h on BDD electrode at 0.1 mA cm−2 and (b) by Fenton reaction.
Hydroxylation of salicylic acid.—
Salicylic acid (SA) is usually used for trapping free hydroxyl radicals in biological systems. In fact, SA reacts with hydroxyl radicals to form dihyroxylated aromatic compounds. The main products of the hydroxylation of salicylic acid with hydroxyl radicals (Fenton reaction) are 2,3- and 2,5-dihydroxybenzoic acids (DHBA) (Eq. 10)28 29 30 31 32
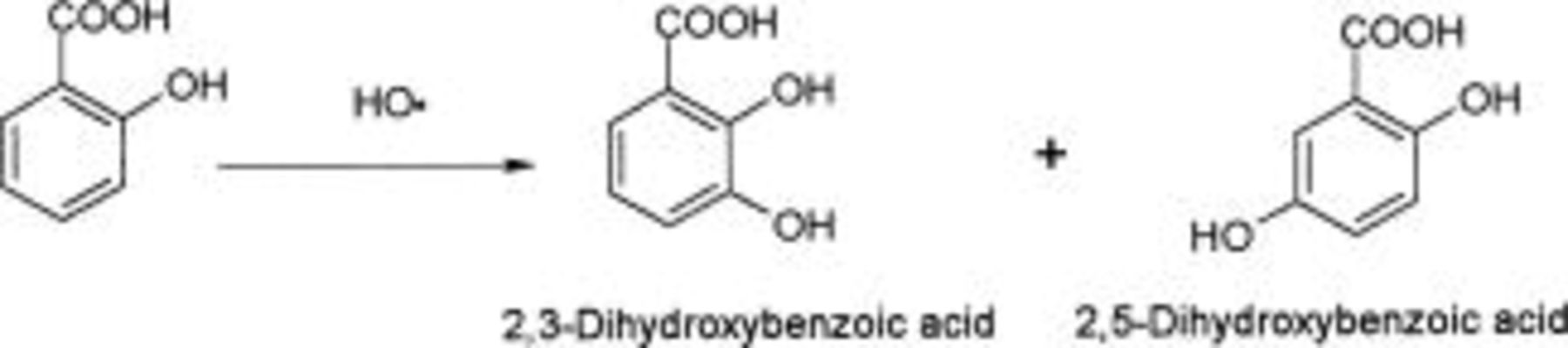
Later reactions imply sequential formation of catechol, quinones, aliphatic acids (after ring opening), and finally 
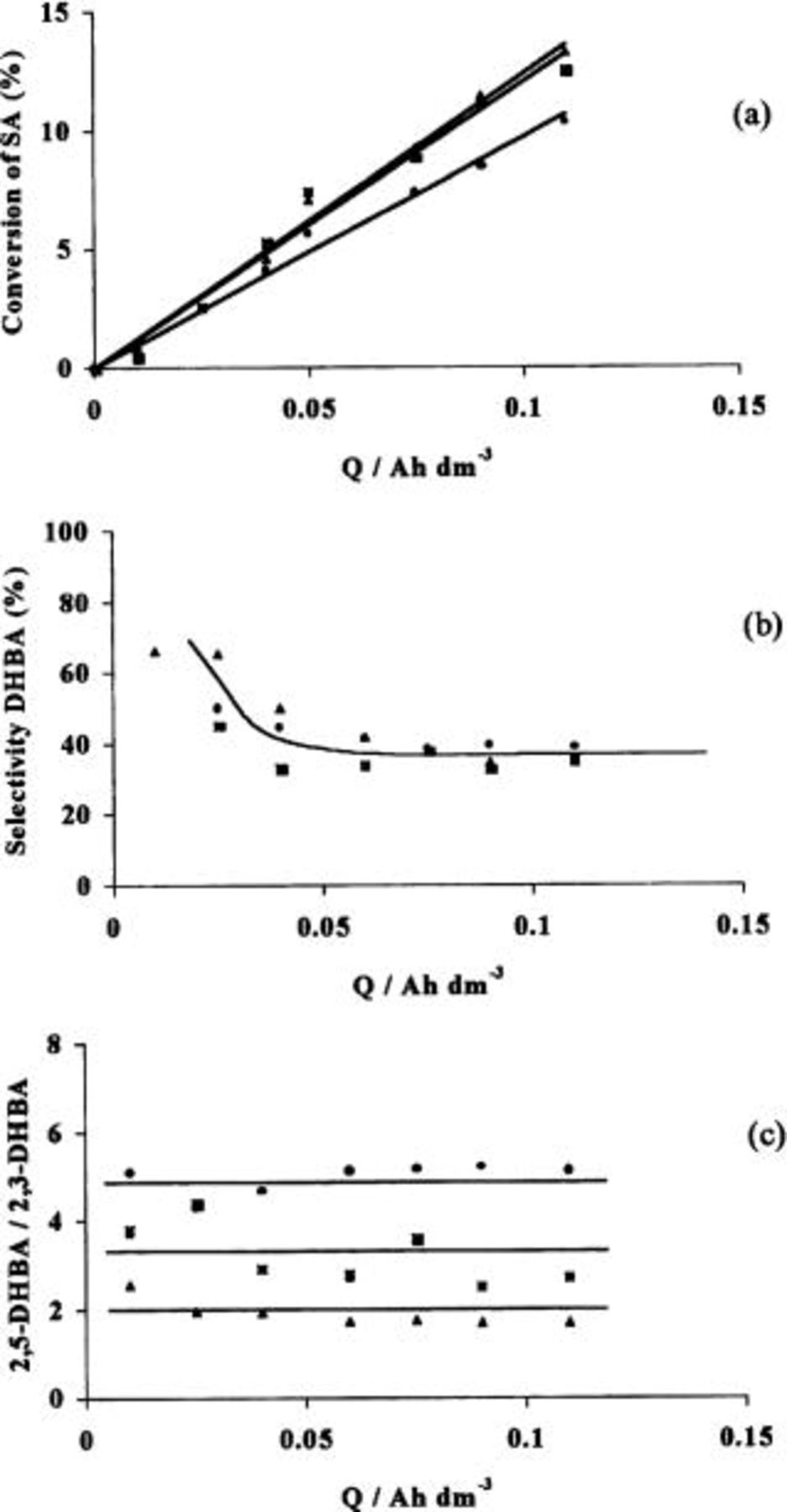
In order to study the electrochemical hydroxylation process at BDD anode, several galvanostatic assays were carried out for current intensities ranging from 0.4 to 4 mA cm−2. Figure 4 shows the results obtained for salicylic acid conversion (a), selectivity to dihydroxybenzoic acids formation (b), and the molar ratio between 2,5- and 2,3-DHBA (c). High 2,5-and 2,3-DHBA formation yields cannot be achieved because of its further oxidation. In fact, HPLC measurements have shown the presence of catechol, maleic, and muconic acids, among others. It can also be observed from Fig. 4 that current density has almost no influence on the conversion of SA and the DHBA selectivity meaning that the chemical reaction of hydroxylation takes place. However, high values of current density favors the formation of 2,5-DHBA acid over 2,3-DHBA.
Figure 4. Oxidation of salicylic acid (SA) on BDD anode at different current densities: (a) conversion of salicylic acid (SA), (b) selectivity to DHBA formation (Eq. 9), (c) ratio between 2,5-DHBA and 2,3-DHBA: (▴) 0.4, (▪) 1.6, and (•) 4 mA cm−2. Electrolyte, 1 M 


Formation of  .—
.—
If the intermediates of the oxidation processes on BDD electrodes are hydroxyl radicals, significant quantities of hydrogen peroxide are expected to be formed by combination of two hydroxyl radicals (Eq. 11)
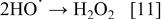
Therefore, in order to verify the generation of hydrogen peroxide using BDD electrodes, several galvanostatic assays of a 1 M  solution were performed (current density, 23-160 mA cm−2).33
solution were performed (current density, 23-160 mA cm−2).33
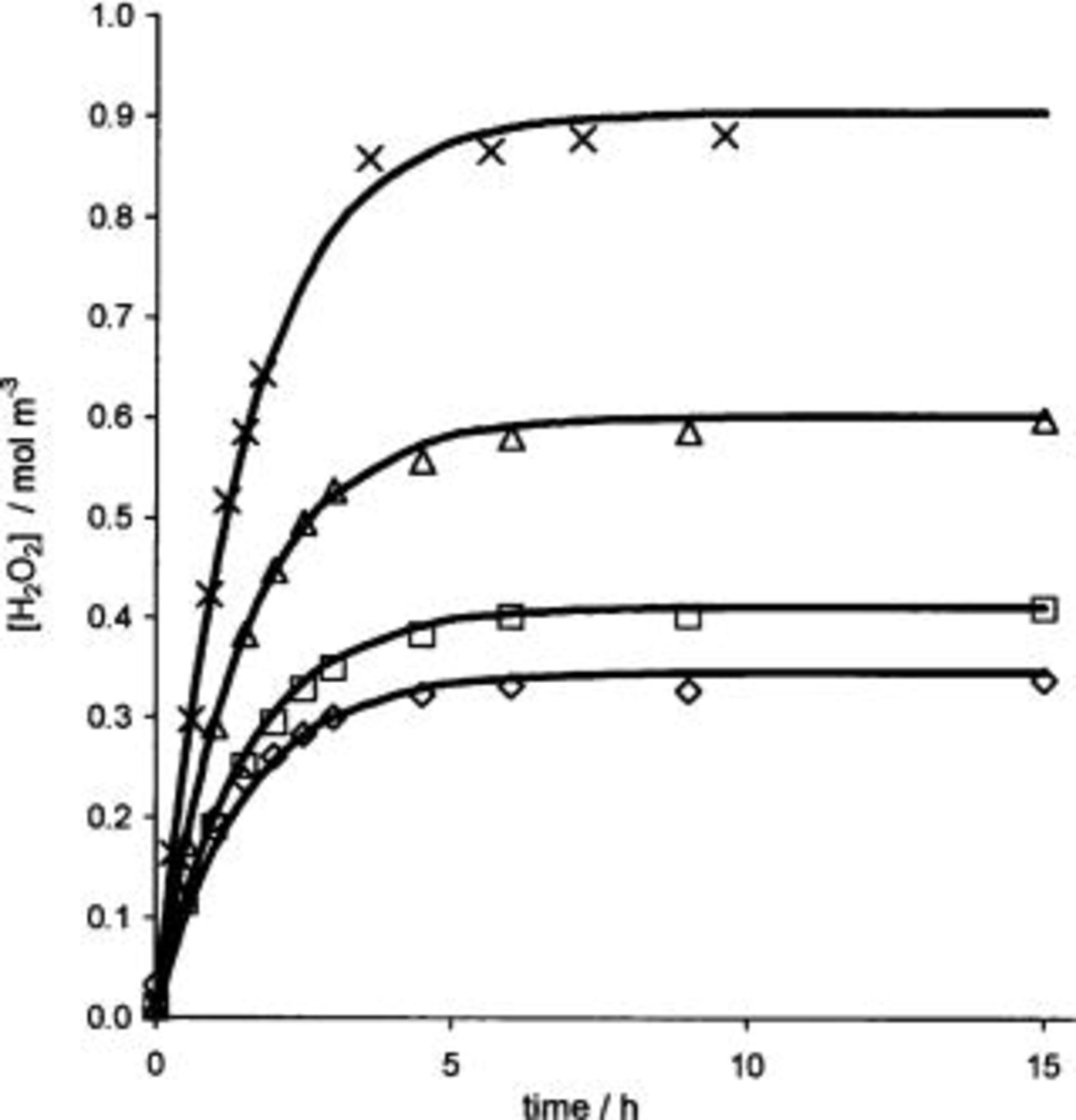
Figure 5 shows the evolution of the hydrogen peroxide concentration for different current densities applied as a function of time. Depending on the applied current density, the concentration of hydrogen peroxide after 1 h can reach a plateau ranging from 0.3 to 1 mmol dm−3. The obtained steady-state  concentration (plateau) is due to the further anodic oxidation of hydrogen peroxide to oxygen (Eq. 12)
concentration (plateau) is due to the further anodic oxidation of hydrogen peroxide to oxygen (Eq. 12)
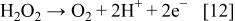
Figure 5. Production of  at different current densities: (◊) 230, (□) 470, (▵) 950, and (×) 1600 A cm−2 during electrolysis of 1 M
at different current densities: (◊) 230, (□) 470, (▵) 950, and (×) 1600 A cm−2 during electrolysis of 1 M  on BDD electrode.
on BDD electrode. 
Electrochemical oxidation of formic and oxalic acids.—
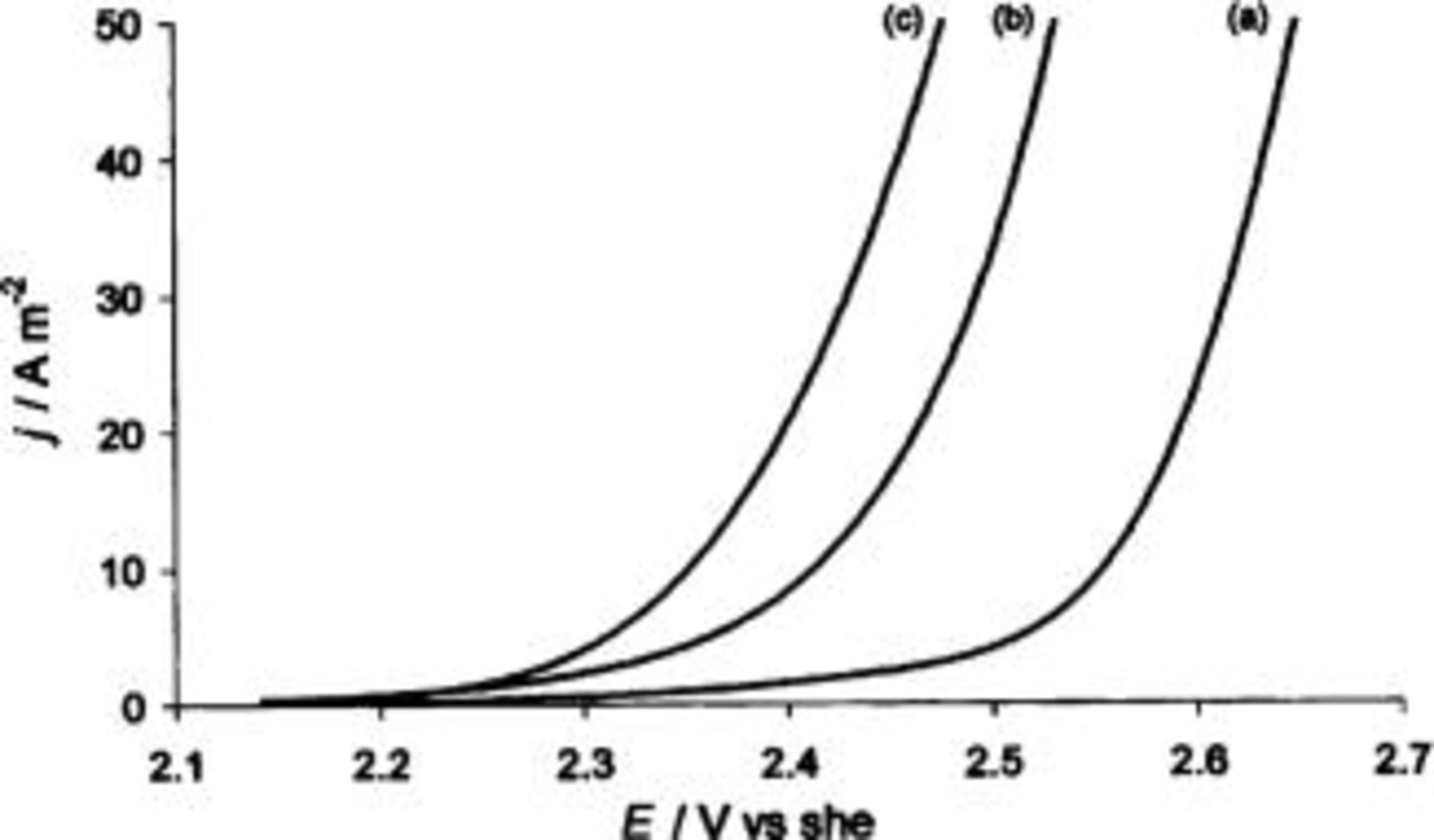
Figure 6 shows voltammetric curves obtained at BDD electrodes in  (supporting electrolyte) for 0.5 M oxalic acid and 0.5 M formic acid. At a given anode potential, the current density for formic acid oxidation is higher than the one for oxalic acid oxidation, indicating a higher activity of BDD anode toward formic acid.
(supporting electrolyte) for 0.5 M oxalic acid and 0.5 M formic acid. At a given anode potential, the current density for formic acid oxidation is higher than the one for oxalic acid oxidation, indicating a higher activity of BDD anode toward formic acid.
Figure 6. Voltammetric  curve obtained at BDD electrode in (a) 1 M
curve obtained at BDD electrode in (a) 1 M  (b) 1 M
(b) 1 M  oxalic acid, (c) 1 M
oxalic acid, (c) 1 M  formic acid. Scan rate, 20 mV s−1;
formic acid. Scan rate, 20 mV s−1; 
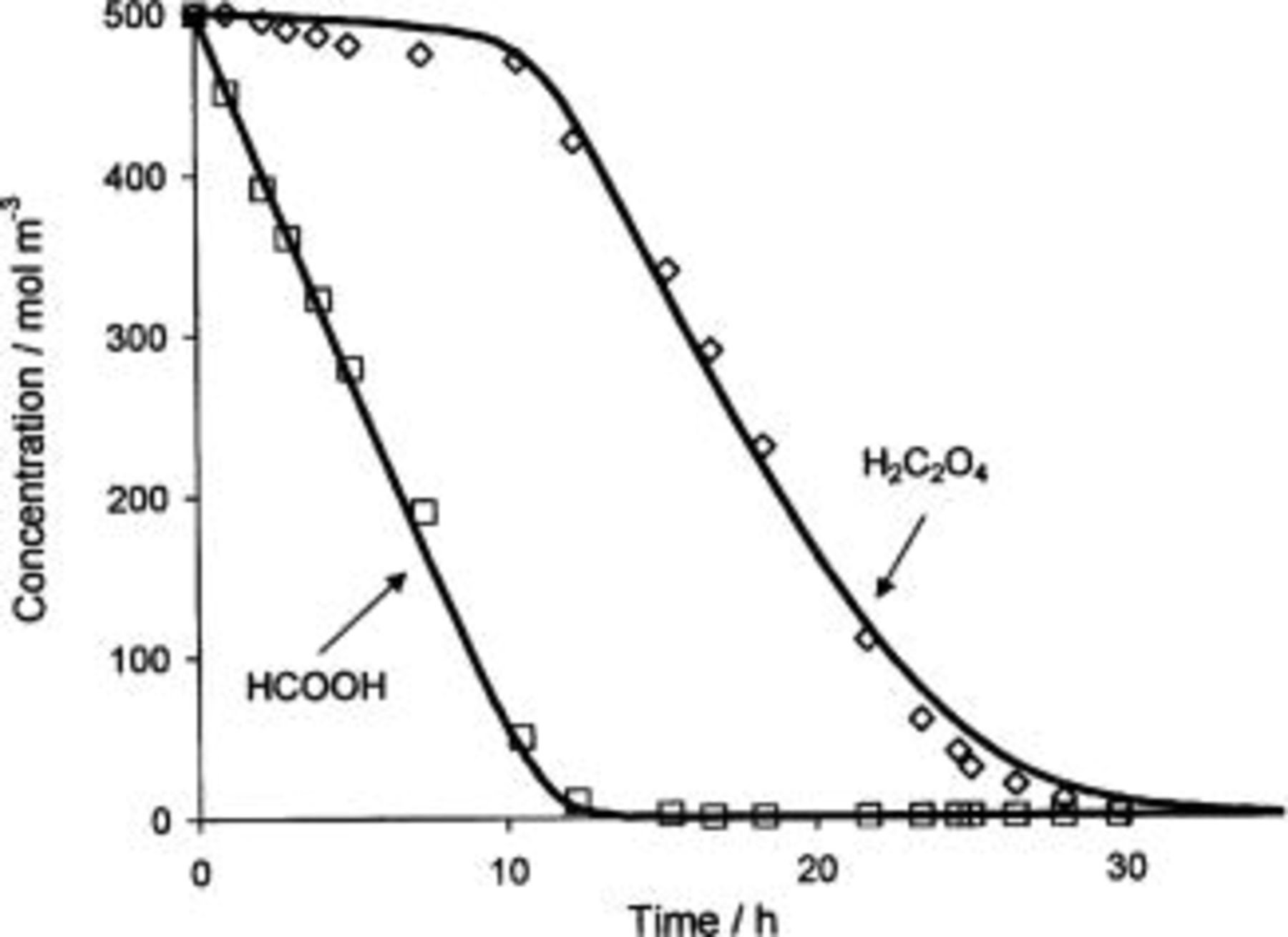
Figure 7 shows the evolution of formic and oxalic acids concentrations during the oxidation of a mixture of 0.5 M oxalic acid and 0.5 M formic acid in  using BDD electrode. The oxidation of formic acid starts at the beginning of the electrolysis while the concentration of oxalic acid remains unchanged. When the formic acid concentration decreases and its oxidation is limited by mass transport (after approximately 12 h of electrolysis), the oxidation of oxalic acid can occur. These results can be explained by the difference of reactivity between the electrogenerated free hydroxyl radicals and the carboxylic acids. In fact, during the oxidation both formic and oxalic acids are in simple competition reaction for
using BDD electrode. The oxidation of formic acid starts at the beginning of the electrolysis while the concentration of oxalic acid remains unchanged. When the formic acid concentration decreases and its oxidation is limited by mass transport (after approximately 12 h of electrolysis), the oxidation of oxalic acid can occur. These results can be explained by the difference of reactivity between the electrogenerated free hydroxyl radicals and the carboxylic acids. In fact, during the oxidation both formic and oxalic acids are in simple competition reaction for  (Eq. 13 and 14)
(Eq. 13 and 14)
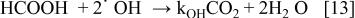
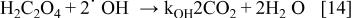
Figure 7. Trend of (□) formic acid concentration and (◊) oxalic acid concentration during formic and oxalic acids electrolysis on boron-doped diamond anode. Electrolyte, 1 M  formic acid + 0.5 M oxalic acid.
formic acid + 0.5 M oxalic acid. 

The oxidation of these two organic compounds with hydroxyl radicals occurs with different rate constants,  and
and 
 for formic and oxalic acid, respectively.34 Since oxalic acid is less reactive towards hydroxyl radicals than formic acid (by two orders of magnitude), its oxidation is expected to occur later.
for formic and oxalic acid, respectively.34 Since oxalic acid is less reactive towards hydroxyl radicals than formic acid (by two orders of magnitude), its oxidation is expected to occur later.
Conclusions
In this paper, we have demonstrated that hydroxyl radicals produced chemically (by the Fenton reaction) as well as electrochemically (at the BDD anode) show the same ESR spectra in presence of the spin trap DMPO, thus indicating the formation of free OH radicals during electrolysis at BDD electrode.
Furthermore, the anodic oxidation of salicylic acid at the BDD anode leads to the formation of dihydroxylated products (2,3- and 2,5-dihydroxybenzoic acids). The same reaction products have been reported using OH radicals produced by  activation (Fenton reaction). Finally, the production of hydrogen peroxide and the competitive oxidation of formic and oxalic acids confirm the role of OH radicals as intermediates during oxidation at the BDD electrode.
activation (Fenton reaction). Finally, the production of hydrogen peroxide and the competitive oxidation of formic and oxalic acids confirm the role of OH radicals as intermediates during oxidation at the BDD electrode.
Acknowledgments
The authors thank the Swiss Center for Electronics and Microtechnology (CSEM), Neucha⁁tel, Switzerland, for preparing the boron-doped diamond electrodes and the Laboratory of Inorganic and Bioinorganic Chemistry (LCIB) of the Swiss Federal Institute of Technology for ESR measurements.
The Swiss Federal Institute of Technology assisted in meeting the publication costs of this article.