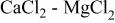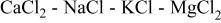Abstract
Electrodeposition of magnesium from calcium chloride-based melts was studied on metallic (tungsten and molybdenum) and glassy carbon electrodes. Pure  melt and different alkali chloride solvents including binary
melt and different alkali chloride solvents including binary  equimolar composition, and ternary
equimolar composition, and ternary  melts with compositions 35:55:10 mol %, containing different
melts with compositions 35:55:10 mol %, containing different  concentrations, were used. In the case of the binary mixture, the operating temperature was both above and below the melting point of metallic magnesium (923 K) so that solid and liquid deposits were obtained. Underpotential deposition of calcium is an important process in melts containing
concentrations, were used. In the case of the binary mixture, the operating temperature was both above and below the melting point of metallic magnesium (923 K) so that solid and liquid deposits were obtained. Underpotential deposition of calcium is an important process in melts containing  due to formation of Ca-Mg alloys, which affects the magnesium electrodeposition process. Three-dimensional magnesium deposits that grow macroscopically on the studied substrates were found. This influences determination of the diffusion coefficient of Mg(II) ions. Formation of a magnesium monolayer on both electrodes, and especially on tungsten, was a stable process in the ternary mixture containing
due to formation of Ca-Mg alloys, which affects the magnesium electrodeposition process. Three-dimensional magnesium deposits that grow macroscopically on the studied substrates were found. This influences determination of the diffusion coefficient of Mg(II) ions. Formation of a magnesium monolayer on both electrodes, and especially on tungsten, was a stable process in the ternary mixture containing  which was related to the presence of 10 mol % KCl. Moreover, in this melt a remarkable pseudopassivation process was found at high overpotentials. © 2004 The Electrochemical Society. All rights reserved.
which was related to the presence of 10 mol % KCl. Moreover, in this melt a remarkable pseudopassivation process was found at high overpotentials. © 2004 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Electrolysis of molten magnesium chloride and thermal reduction of magnesium oxide are the two principal production processes in use today. Although silicothermic reduction has become more important in recent years, currently most of the production on a worldwide scale is still by electrolysis.1
Different electrolytic methods can be distinguished, depending on the cell feed (raw material and preparation of magnesium chloride), design of the cells, and utilization of the by-product, chlorine. The dehydration methods (producing anhydrous magnesium chloride) are all energy intensive and expensive but give many benefits, mainly related to minimal consumption of the graphite electrodes, which permits a fixed cell configuration and recovery of concentrated chlorine as a by-product.2
Good wettability of the cathode substrate by the melt and the magnesium deposit is one of the most important factors in electrolytic production of magnesium. Experimental results have shown that the magnitude of the magnesium losses during the electrolysis is principally determined by the conditions of the deposition of magnesium on the cathode, the coalescence of the magnesium droplets in the electrolyte, and by the design of the electrolyzer.2 These parameters depend on the physicochemical properties of the electrolytes, particularly on their interfacial properties. For example, if the interfacial tension at the electrolyte/cathode is relatively higher than at the cathode/liquid magnesium, liquid magnesium is deposited on the cathode as a continuous layer and readily coalesces into large droplets. Under such conditions the losses of metallic magnesium during the electrolysis are minimal and maximum current yield is attained. Major amounts of dispersed magnesium in the electrolyte, which is difficult to harvest, are then absent.
Cathodes of steel are used in industrial electrolytic production of magnesium due to their stability and low cost, but they have poor wetting properties and in addition, the magnesium produced is contaminated by iron due to corrosion. Thus, alternative cathode materials should be proposed.
Extensive electrochemical studies of magnesium in molten chlorides have been carried out through the years. The effect of the cathode material and impurities in the electrodeposition process were the main subjects of those investigations. Most of these articles are written in Chinese and Russian and only English abstracts are available, thus reducing the scientific dissemination. In addition, complete and systematic works where different experimental parameters are varied (melt composition, working temperature, cathode, etc.) are missing, especially those resembling industrial conditions.
A wide variety of cathode materials has been used, but due to industrial applications, iron and stainless steel are the substrates used by most of the authors,3 4 5 6 7 8 9 10 although tungsten, platinum, and glassy carbon have also been used.10 11 12 13 14 Kannan et al.4 indicated that the current efficiency in the electrolytic cells for magnesium depends much upon the wettability of the cathode surface by the molten metal and the electrolyte, but due to several experimental restrictions in measuring wetting angles, only ex situ visual observations of nine different steel cathodes after electrolysis were carried out.
All authors conclude that magnesium deposition from molten chlorides is a simple diffusion-controlled process involving a two-electron transfer. However, Kisza et al.15 found the magnesium reduction reaction on liquid magnesium electrodes and in pure molten magnesium chloride at temperatures ranging from 750 to 810°C, to follow a two-step electrode mechanism, the high-frequency process being pure charge transfer with the low-frequency process showing mixed charge-transfer-diffusion character.
Some authors also performed nucleation studies during magnesium electrodeposition on foreign substrates. It was found that the magnesium electrodeposition follows an instantaneous nucleation process with three-dimensional growth of the deposit.5 8 16
Many authors studied the effect of impurities, mainly metal17 and oxide ions6 12 18 19 in the electrodeposition process. In hydrous melts a passivation effect in cathodic polarization was found, which was explained by formation of insoluble MgO at the electrode surface.18 It is also pointed out that the MgO adsorbed on magnesium particles interferes with the cohesion of Mg droplets.19
Børresen et al. studied the charge-transfer and diffusion kinetics of the magnesium electrodeposition process from halide melts, mainly pure magnesium chloride melt.20 Authors also studied the influence of oxide ions as well as metal dissolution in the electrodeposition process. Spectroscopic studies of the so-called metal fog were also carried out.21
Because alkali and alkaline-earth fluorides are used as effective agents to prevent passivation of the cathode surface to a considerable extent, many authors also studied the influence of fluoride ions in the magnesium discharge.7 20 22 23 Observations showed that fluorides cause active breakdown of the passivating films, which gradually peels away from the cathode.22 Rao23 concluded that the beneficial effect of sodium fluoride addition is attributed to the fluxing action of fluoride on the surface oxide of the electrolytically produced magnesium droplets. It is also reported that the addition of large amounts of fluoride into magnesium electrolytes may lead to undesirable discharge of alkali and/or alkaline-earth metals at the cathode if the limiting-current region is reached.22
The present study is part of a research project with the goal of understanding the factors that affect the formation of a magnesium deposit with the appropriate characteristics. Different cathode materials were tested: glassy carbon rods and metallic wires of tungsten and molybdenum, although the reported results are mainly focused on the use of tungsten electrodes in calcium chloride-based melts.
Experimental
The experiments were carried out inside a glove box (VAC model HE-43-2) in an inert argon atmosphere. The chloride mixtures were melted in a glassy carbon crucible (Carbone Lorraine) placed in a quartz cell inside a furnace. A Eurotherm model 2404 programmable device controlled the temperature of the furnace (±2°C), which was measured with a thermocouple (Pt/Pt-Rh 10%) protected by an alumina tube inserted into the melt.
The working electrodes consisted of glassy carbon rods (3 mm diam, Tokai Carbon Co., grade GC-20S) or tungsten and molybdenum wires (1 mm diam, Alfa, 99.995%). Another glassy carbon rod, the glassy carbon crucible, or a magnesium electrode was used as counter electrode. The reference electrode consisted of a magnesium electrode (solid magnesium rod or magnesium pool, depending on the working temperature, Riedel-DeHaen, 99.8%). In a few experiments, a Ag|AgCl electrode was used. The preparation of such an electrode has been described previously (e.g., Ref. 24). Unless otherwise stated, all potentials are referred to the Mg|Mg(II) system.
The lower end of the glassy carbon and tungsten electrodes was polished thoroughly by using SiC paper and 1 μm diamond paste. They were then cleaned in ethanol using an ultrasonic bath followed by heating under high vacuum.
Purification of NaCl and KCl consisted of heating the salt to 600°C (approximately) under vacuum. Dehydrated  and
and  were prepared from commercial
were prepared from commercial  and
and  respectively, by drying them carefully under HCl atmosphere. The dried
respectively, by drying them carefully under HCl atmosphere. The dried  was subsequently distilled under vacuum. The dried
was subsequently distilled under vacuum. The dried  was used without further purification.
was used without further purification.
The pure salts were stored and handled inside a glove box (M-BRAUN Labmaster 130) with inert argon atmosphere. The moisture and oxygen levels inside the box were kept below 1 ppm.
Results
The  system at 1123 K.—
system at 1123 K.—
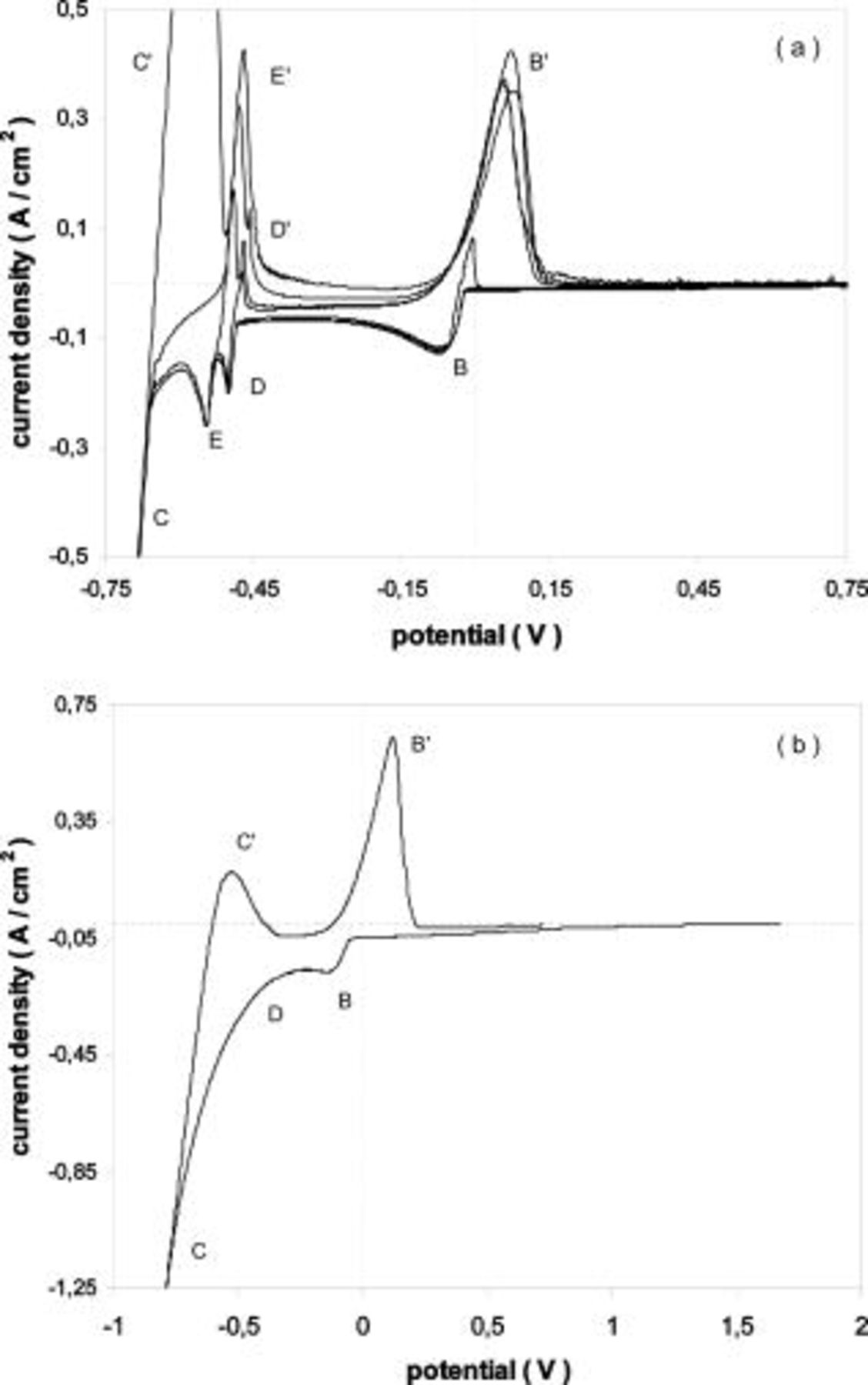
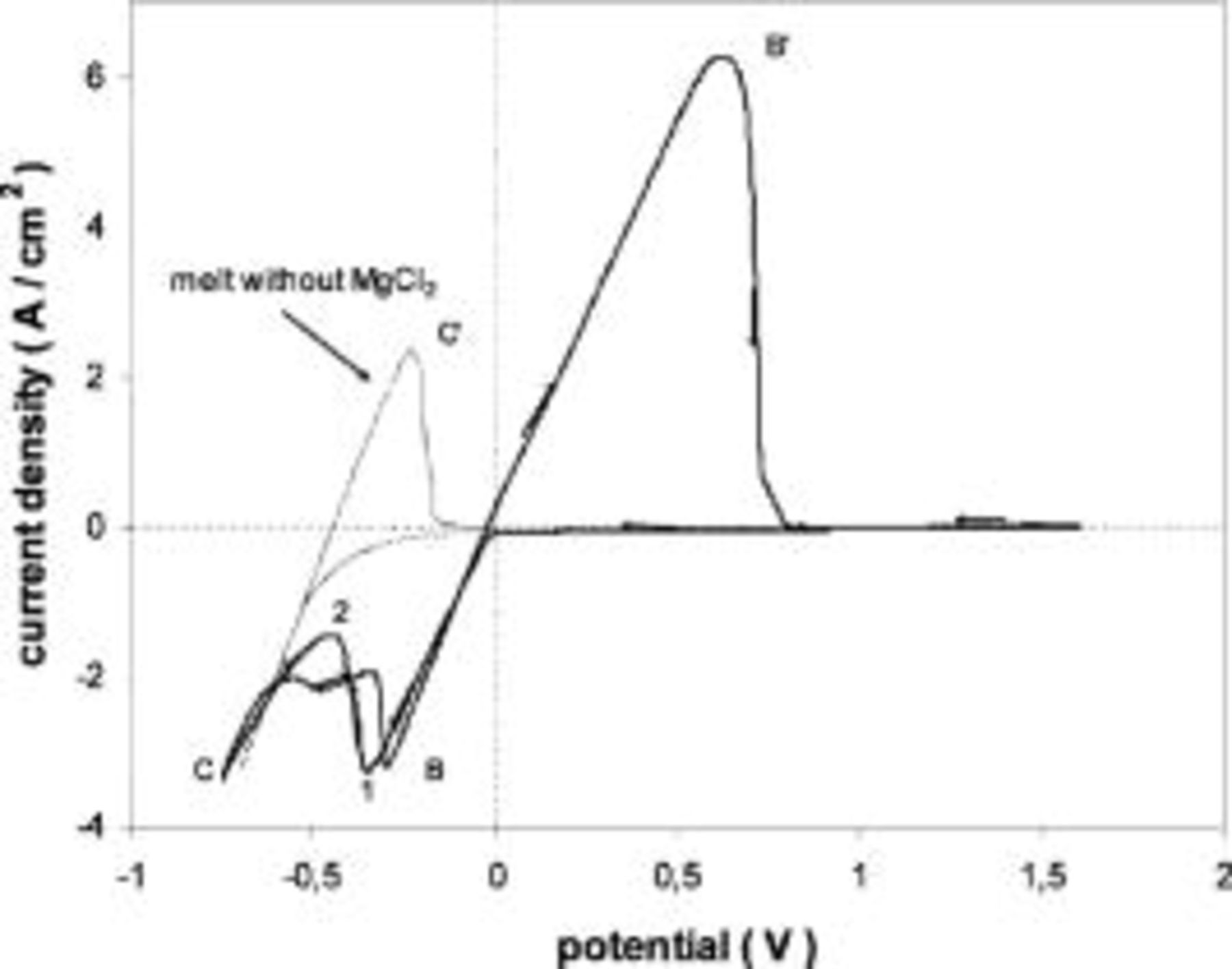
Figure 1 shows some voltammograms obtained on a tungsten electrode when various amounts of  were added to the pure
were added to the pure  melt. The wave
melt. The wave  corresponds to electrodeposition of liquid Mg which is reoxidized in the anodic scan. In this case, and contrary to what has been observed in other molten chloride media20 (discussed later in this paper), no evidence of underpotential deposition (UPD) of Mg was observed on any of the employed substrates.
corresponds to electrodeposition of liquid Mg which is reoxidized in the anodic scan. In this case, and contrary to what has been observed in other molten chloride media20 (discussed later in this paper), no evidence of underpotential deposition (UPD) of Mg was observed on any of the employed substrates.  corresponds to formation and subsequent reoxidation of calcium metal.
corresponds to formation and subsequent reoxidation of calcium metal.
Figure 1. Voltammograms obtained in the pure  melt with different
melt with different  contents.
contents.  Tungsten substrate.
Tungsten substrate. 
In addition, a small oxidation wave  related to an inflection of the cathodic wave D, is observed. This is most likely related to the formation of a Ca-Mg alloy (dissolution of liquid calcium in the already deposited liquid magnesium). Dissolution of calcium metal into the melt can also be observed in Fig. 1 by the shape of oxidation wave
related to an inflection of the cathodic wave D, is observed. This is most likely related to the formation of a Ca-Mg alloy (dissolution of liquid calcium in the already deposited liquid magnesium). Dissolution of calcium metal into the melt can also be observed in Fig. 1 by the shape of oxidation wave  which is typical for oxidation of dissolved species rather than a deposit. This is due to the high solubility of calcium in
which is typical for oxidation of dissolved species rather than a deposit. This is due to the high solubility of calcium in  melts, reported as 2.7 mol % at 1093 K.25
melts, reported as 2.7 mol % at 1093 K.25
Deposition processes on foreign substrates may often be studied by potentiostatic current transients. The current tends to increase with time if the deposited phase is conducting. The time dependency of the current increase depends on the morphology or shape of the growth of the deposited nuclei in a microscale. Theoretical current-time behavior for various situations is reported in the literature.26 27 During the growth process the current increases until the diffusion fields of the independent growth centers are overlapping. For longer times, an increasing current indicates a growth of the deposit on a macroscale.
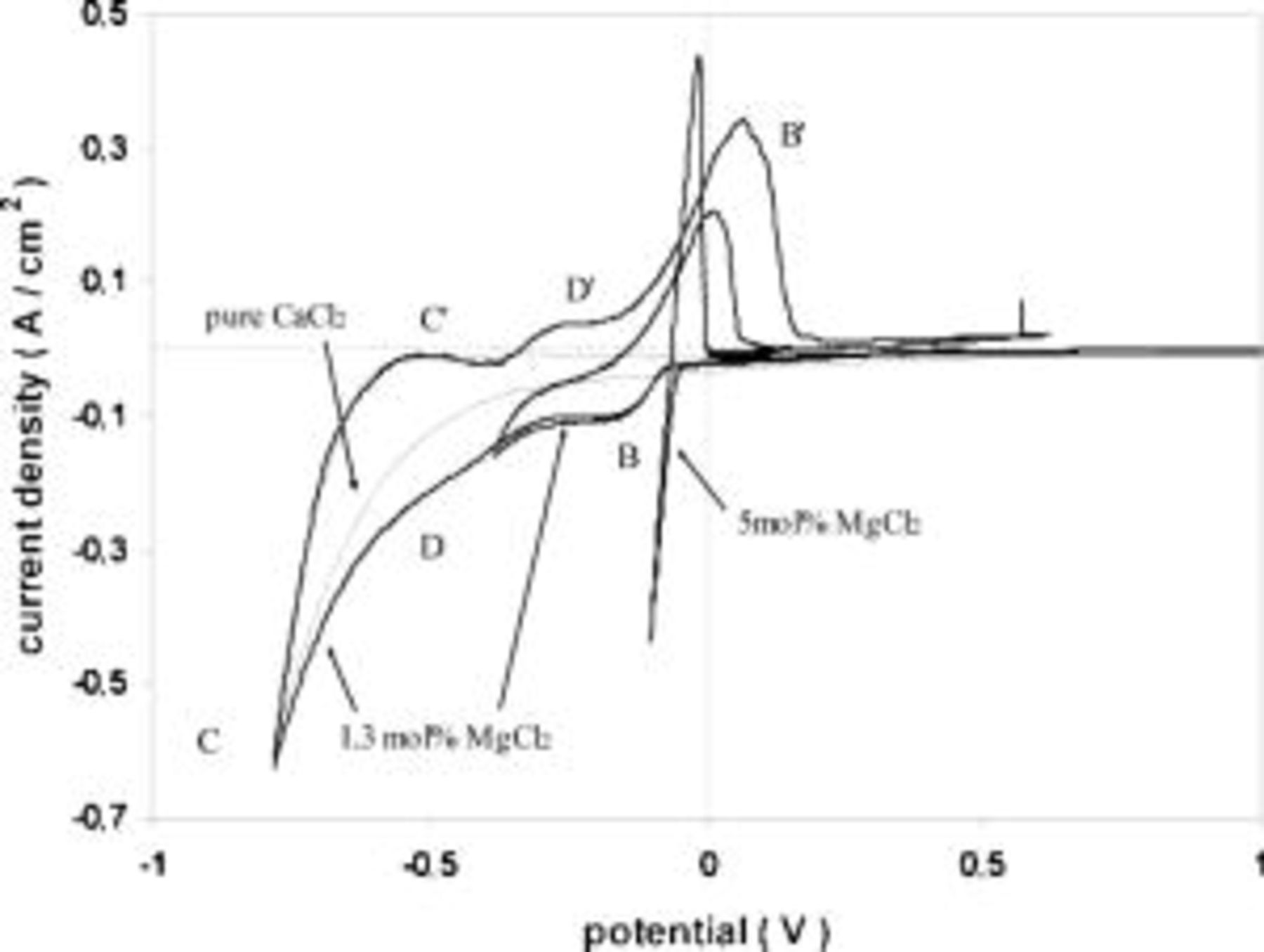
Current transients recorded on tungsten substrates in this melt (see Fig. 2) show characteristic features due to nucleation and growth phenomena of the magnesium deposit at high overpotentials and  concentrations around 5 mol % and higher. The increase in current in the chronoamperometric curves is due to nucleation and growth of the magnesium droplets, which after some time tend to coalesce, giving a steady value of the current.
concentrations around 5 mol % and higher. The increase in current in the chronoamperometric curves is due to nucleation and growth of the magnesium droplets, which after some time tend to coalesce, giving a steady value of the current.
Figure 2. Chronoamperograms obtained in the pure  melt containing
melt containing 
 Tungsten substrate. Applied potentials vs. Mg reference electrode.
Tungsten substrate. Applied potentials vs. Mg reference electrode.
The rising part of the curves was analyzed according to the criteria from the literature,26 27 indicating that the nucleation process is three-dimensional with instantaneous growth of the nuclei (see inserted graph in Fig. 2).
The high surface tension of this type of molten chlorides, which often implies a high interfacial tension between the deposit, Mg, and the electrode, W, makes possible the formation of a deposit formed by magnesium "globules" rather than homogeneous/continuous layers.
The  system at 823 and 1000 K.—
system at 823 and 1000 K.—
Voltammograms on W substrates obtained in the  (45:55 mol %) mixture containing dilute
(45:55 mol %) mixture containing dilute  solutions at a temperature below the melting point of magnesium (see Fig. 3a) showed similar features to those obtained under the same conditions in an equimolar
solutions at a temperature below the melting point of magnesium (see Fig. 3a) showed similar features to those obtained under the same conditions in an equimolar  melt, which were previously reported.28
melt, which were previously reported.28
Figure 3. Voltammograms obtained in molten  (45:55 mol %) melt at (a) 823 and (b) 1000 K containing
(45:55 mol %) melt at (a) 823 and (b) 1000 K containing  at different cathodic limits. Tungsten substrate,
at different cathodic limits. Tungsten substrate, 
The electrochemical reduction of Mg(II) ion also takes place in a single step  The current peaks
The current peaks  and
and  probably correspond to the formation of solid Ca-Mg alloys29
30 due to dissolution of calcium in the solid magnesium already deposited on the tungsten electrode.
probably correspond to the formation of solid Ca-Mg alloys29
30 due to dissolution of calcium in the solid magnesium already deposited on the tungsten electrode.  and
and  could also be due to Na-Mg alloy formation, although the solubility of liquid sodium in solid magnesium is small.29
31 The cathodic limit of the melt
could also be due to Na-Mg alloy formation, although the solubility of liquid sodium in solid magnesium is small.29
31 The cathodic limit of the melt  system) corresponds to liquid sodium and/or solid calcium deposition and subsequent reoxidation. Voltammograms obtained at molybdenum substrates showed similar features.
system) corresponds to liquid sodium and/or solid calcium deposition and subsequent reoxidation. Voltammograms obtained at molybdenum substrates showed similar features.
When the working temperature was increased above the melting point of metallic magnesium (see Fig. 3b), the voltammetric curves show a more pronounced UPD of calcium on the liquid magnesium already deposited at the tungsten substrate, probably due to formation of a Ca-Mg liquid solution.29 The solubility of Ca and/or Na metal in the electrolyte increases with temperature, as seen from the shape of the reoxidation peak 
One should emphasize the absence of magnesium UPD on both metallic substrates. When using carbon substrates a significant depolarization of the reduction of sodium ions is usually observed due to sodium penetration into the carbon lattice to form a very stable sodium-carbon bond. Despite this, it was possible to study magnesium deposition in the molten  (45:55 mol %) containing 0.6 mol %
(45:55 mol %) containing 0.6 mol %  on a glassy carbon electrode (working temperature 823 K). Figure 4 shows a typical voltammogram obtained during electrodeposition of solid magnesium.
on a glassy carbon electrode (working temperature 823 K). Figure 4 shows a typical voltammogram obtained during electrodeposition of solid magnesium.
Figure 4. Voltammogram obtained in molten  (45:55 mol %) melt at 823 K containing
(45:55 mol %) melt at 823 K containing  Glassy carbon substrate.
Glassy carbon substrate. 
Apart from the electrochemical signals corresponding to magnesium electrodeposition/oxidation  one observes a high background current (F). Taking into account the voltammogram obtained in the pure melt (without
one observes a high background current (F). Taking into account the voltammogram obtained in the pure melt (without  , one may assume that it is mainly due to sodium deposition which is enhanced by Na intercalation into the carbon structure. The current wave C is due both to Na-C intercalation compounds and Ca-Mg alloy formation, although the first process is probably predominant.
, one may assume that it is mainly due to sodium deposition which is enhanced by Na intercalation into the carbon structure. The current wave C is due both to Na-C intercalation compounds and Ca-Mg alloy formation, although the first process is probably predominant.
In any case, the shape of the  wave is characteristic of the formation of a new phase. This has been confirmed by examination of the voltammograms, because the ratio of the anodic to the cathodic current was higher than unity, and the ratio of the total anodic to the total cathodic charge was close to unity and independent of scan rate.
wave is characteristic of the formation of a new phase. This has been confirmed by examination of the voltammograms, because the ratio of the anodic to the cathodic current was higher than unity, and the ratio of the total anodic to the total cathodic charge was close to unity and independent of scan rate.
It was possible to calculate the diffusion coefficient of Mg(II) ions by cyclic voltammetry (CV) and convolution of voltammograms by applying Eq. 1 and 2, respectively
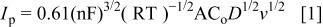
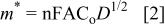
where  is the peak current of the voltammograms,
is the peak current of the voltammograms,  the bulk concentration of the electroactive species, D the diffusion coefficient, A the electrode surface area, and
the bulk concentration of the electroactive species, D the diffusion coefficient, A the electrode surface area, and  the maximum value of the convoluted current of the voltammograms.
the maximum value of the convoluted current of the voltammograms.
The results obtained on the different substrates are gathered in Table I. Results obtained on glassy carbon electrodes are considered to be inconsistent due to the high background current from Na intercalation in the carbon lattice, although the difference is rather small.
Table I.
Diffusion coefficient of Mg(II) species obtained by different electrochemical techniques in the molten  (45:55 mol %) melt at different temperatures. (45:55 mol %) melt at different temperatures. | |||
|---|---|---|---|
| Technique | T (K) | Substrate |
 (cm2 s−1) (cm2 s−1) |
| Voltammetry | 823 | W | 0.7 |
| Mo | 0.5 | ||
| GC | 0.6 | ||
| 1000 | W | 1.5 | |
| Mo | 1.7 | ||
| Convolution | 823 | W | 1.3 |
| Mo | 1.2 | ||
| GC | 1.5 | ||
The diffusion coefficient values obtained directly from CV are lower than those obtained by convolution of the voltammetric curves. This could be attributed to the simultaneous Ca/Na deposition which would influence to a larger extent when the time scale of the measurements is increased.
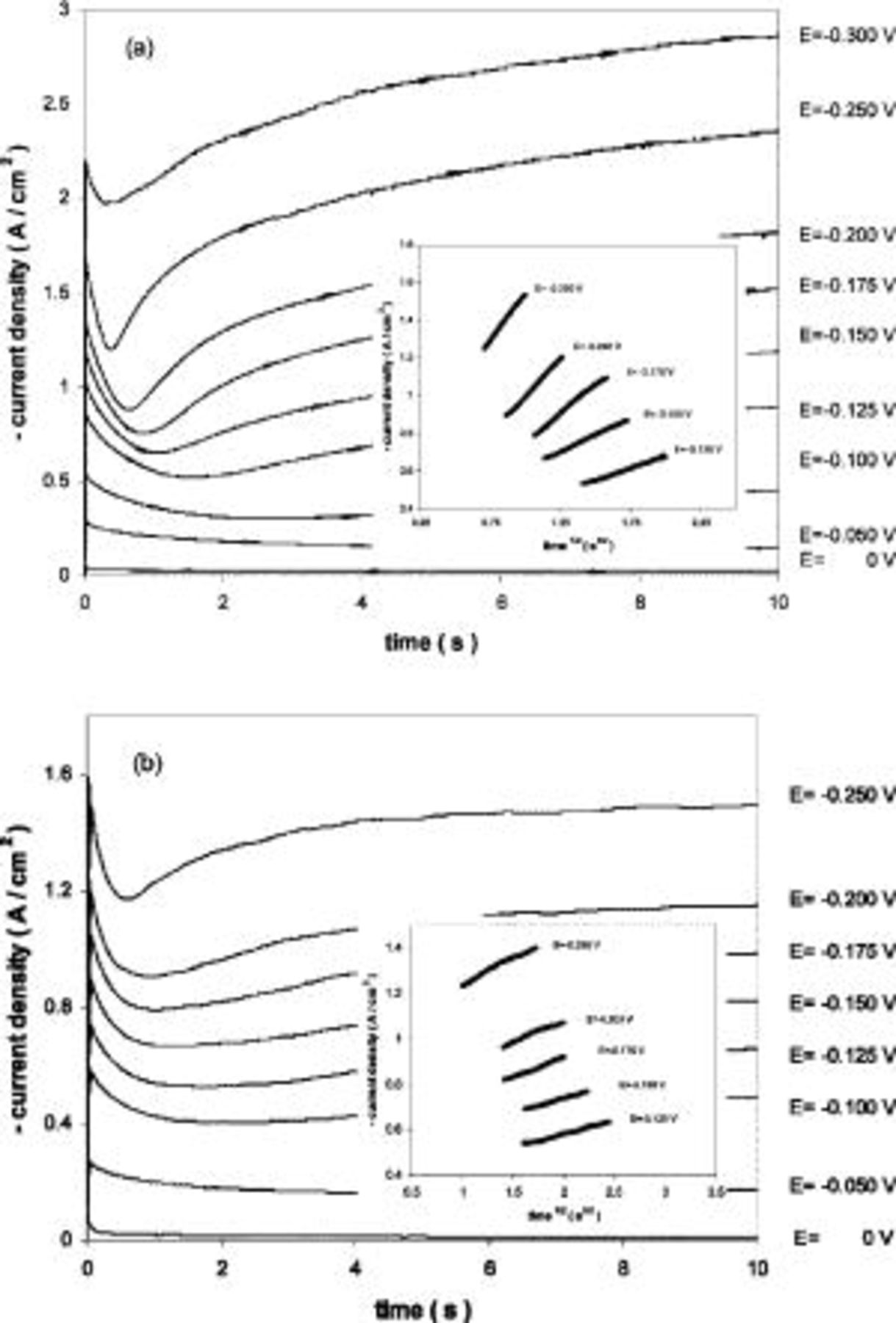
Chronoamperometric curves obtained in the dilute  system (0.6 mol %) showed Cottrell behavior for all the substrates. However, at higher magnesium chloride contents (5-10 mol %), the chronoamperometric curves evidenced nucleation and growth of a liquid or solid Mg deposit on metallic electrodes (W and Mo) at high overpotentials (see Fig. 5a and b), which cause an increase of the electroactive area. It is also expected that the interfacial properties are changing due to Ca-Mg alloy formation.
system (0.6 mol %) showed Cottrell behavior for all the substrates. However, at higher magnesium chloride contents (5-10 mol %), the chronoamperometric curves evidenced nucleation and growth of a liquid or solid Mg deposit on metallic electrodes (W and Mo) at high overpotentials (see Fig. 5a and b), which cause an increase of the electroactive area. It is also expected that the interfacial properties are changing due to Ca-Mg alloy formation.
Figure 5. Chronoamperograms obtained in molten  (45:55 mol %) melt containing
(45:55 mol %) melt containing 
 and (b) 1000 K. Tungsten substrate. Applied potentials vs. Mg reference electrode.
and (b) 1000 K. Tungsten substrate. Applied potentials vs. Mg reference electrode.
The rising part of the curves was analyzed according to the criteria from the literature,26 27 indicating that the nucleation process also here is three-dimensional with instantaneous growth of the nuclei (see inserted graph in Fig. 5a and b).
The  system at 1000 K.—
system at 1000 K.—
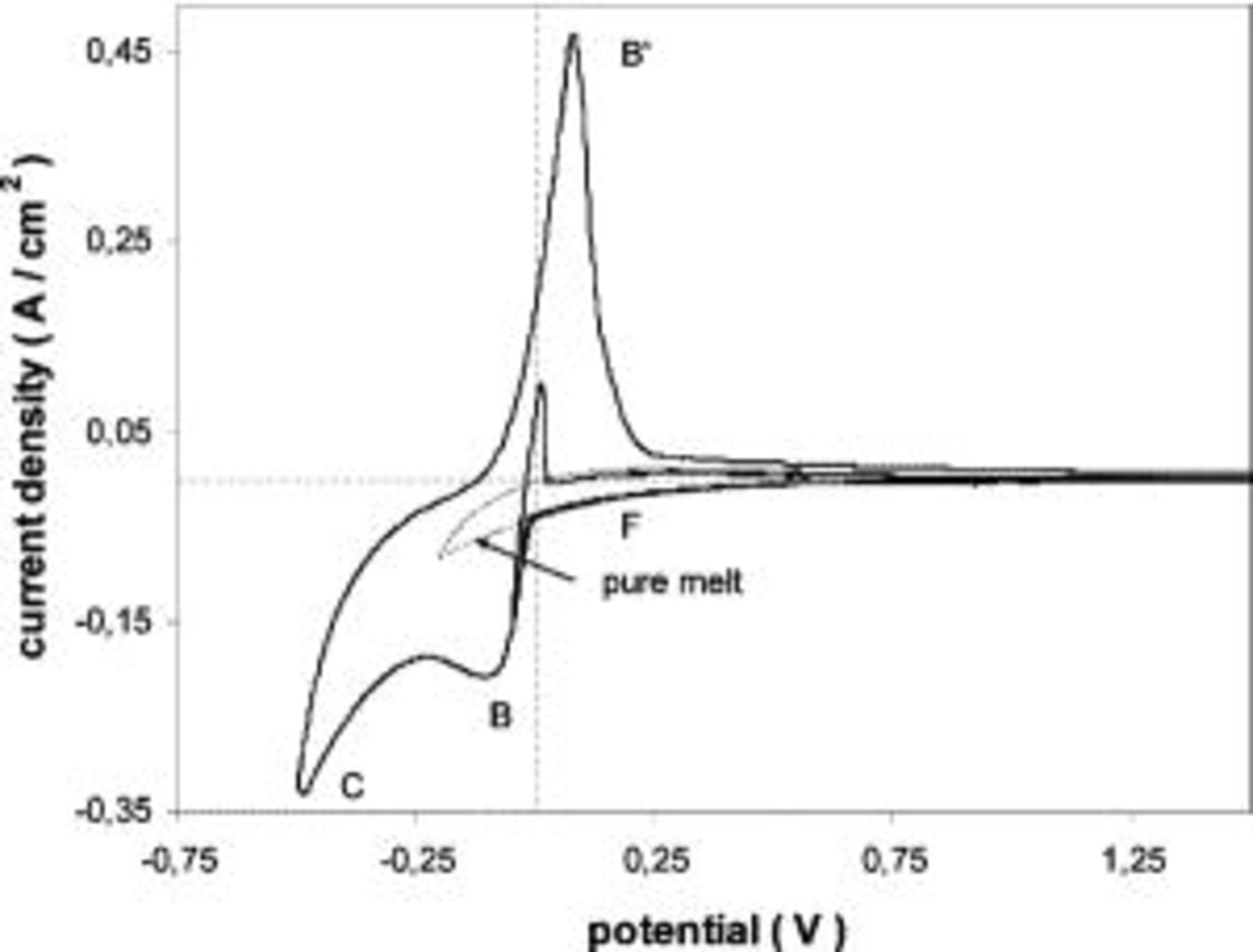
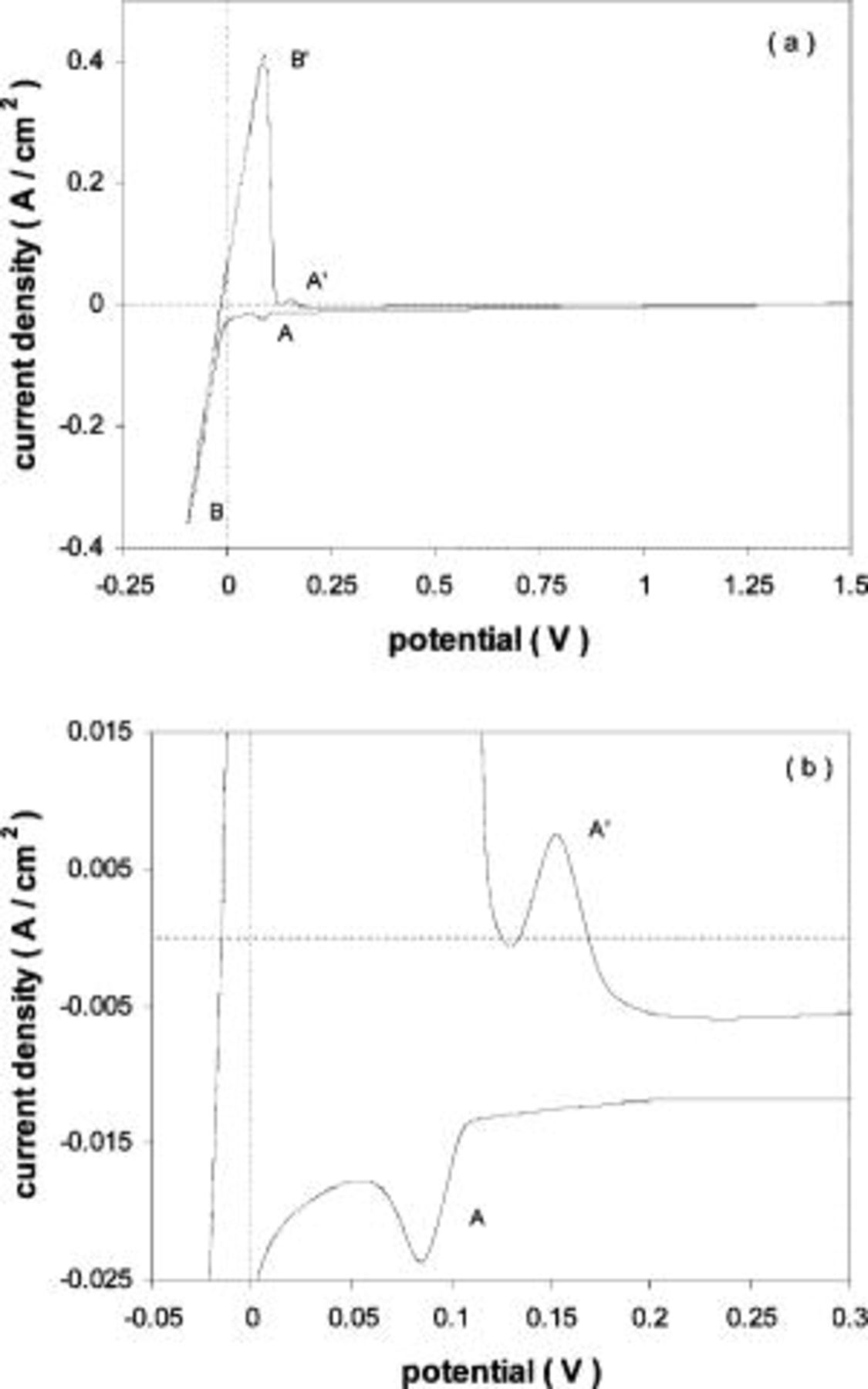
Figure 6 shows a typical voltammogram obtained in  (35:55:10 mol %)-5 mol %
(35:55:10 mol %)-5 mol %  at 1000 K when using a tungsten substrate. Molybdenum electrodes showed a similar behavior.
at 1000 K when using a tungsten substrate. Molybdenum electrodes showed a similar behavior.
Figure 6. (a) Voltammogram obtained in molten  (35:55:10 mol %) melt at 1000 K containing
(35:55:10 mol %) melt at 1000 K containing  Tungsten substrate.
Tungsten substrate.  (b) Detail of the voltammetric curve shown in (a).
(b) Detail of the voltammetric curve shown in (a).
The current peaks  were associated to UPD of magnesium on tungsten. The charge of these current peaks corresponds to the formation of 1-2 monolayers. The reason why UPD appears in this melt and not in the other calcium-based melts previously studied is attributed to the differences in surface tensions of the melts, which affects the interfacial properties.2
were associated to UPD of magnesium on tungsten. The charge of these current peaks corresponds to the formation of 1-2 monolayers. The reason why UPD appears in this melt and not in the other calcium-based melts previously studied is attributed to the differences in surface tensions of the melts, which affects the interfacial properties.2
Figure 7 shows a CV obtained under the same conditions as in Fig. 6, but in this case the reversal potential was much more cathodic. Apart from the current peaks  and
and  (the first one exists but is hidden by the large scale of the figure), a curious effect was observed. The current suddenly decreases at a cathodic potential corresponding to the point labeled 1 up to point 2, where the current increases again (cathodic limit of the melt). In the reverse scan the cathodic current decreases again when reaching point 2 and increases again at the same point as in the forward scan (point 1).
(the first one exists but is hidden by the large scale of the figure), a curious effect was observed. The current suddenly decreases at a cathodic potential corresponding to the point labeled 1 up to point 2, where the current increases again (cathodic limit of the melt). In the reverse scan the cathodic current decreases again when reaching point 2 and increases again at the same point as in the forward scan (point 1).
Figure 7. Voltammograms obtained in pure molten  (35:55:10 mol %) melt at 1000 K and containing
(35:55:10 mol %) melt at 1000 K and containing  Tungsten substrate.
Tungsten substrate. 
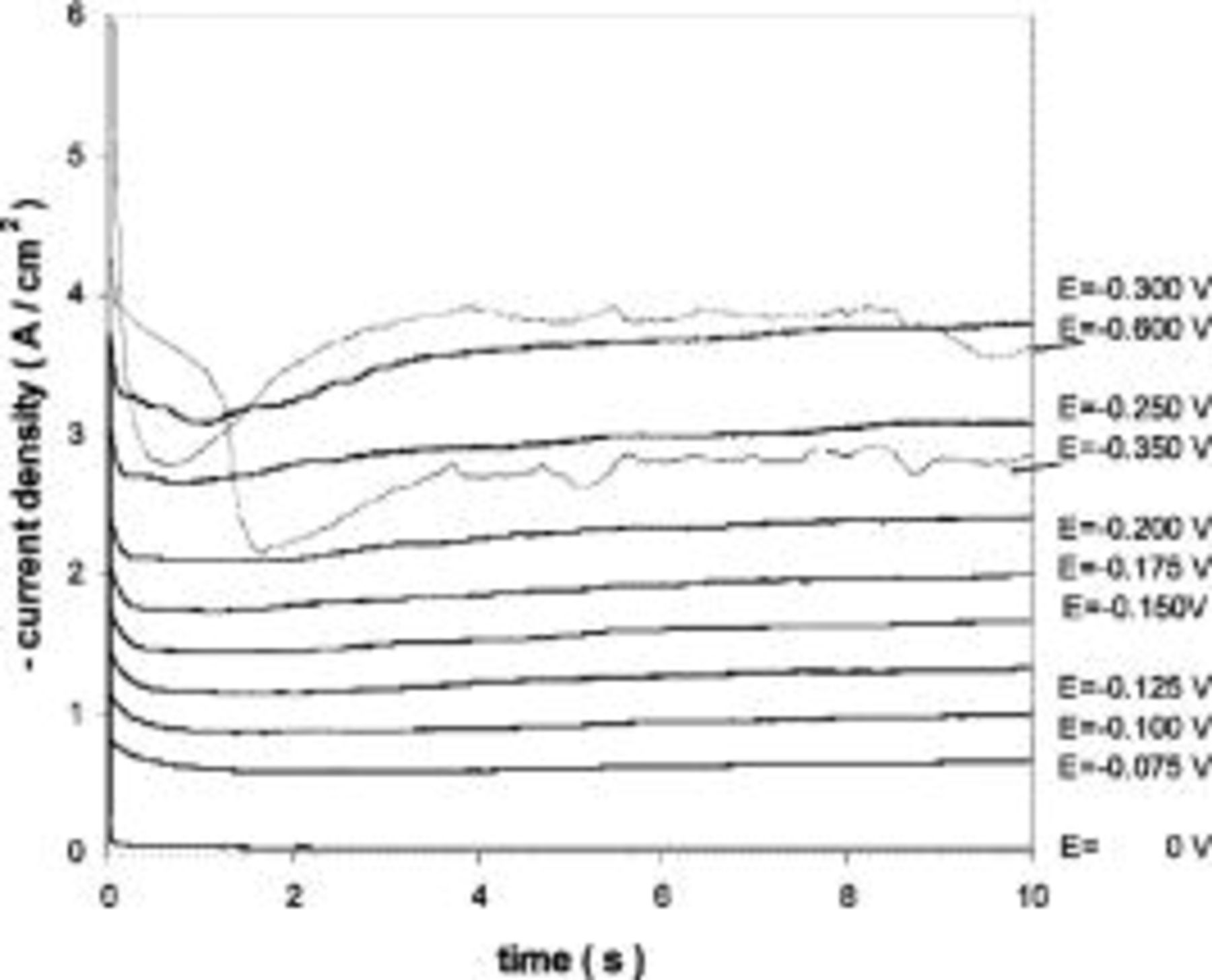
Chronoamperometric curves showed that this behavior could be due to a passivation process. From Fig. 8 one observes that the curves have the expected features down to −0.300 V. At applied cathodic potentials ranging from −0.350 to −0.600 V, the curves have the typical habits of a pseudo-passivation process.27 These potential values correspond to the potential region from point 1 to 2 in the voltammogram from Fig. 7.
Figure 8. Chronoamperograms obtained in molten  (35:55:10 mol %) melt containing
(35:55:10 mol %) melt containing 
 Tungsten substrate. Applied potentials vs. Mg reference electrode.
Tungsten substrate. Applied potentials vs. Mg reference electrode.
This behavior could be explained by the formation of a discontinuous passivation layer the nature of which could have two explanations: (i) local formation of a solid compound (e.g.,  melting point 749°C) at some points of the electrode surface, and (ii) formation of a discontinuous passivating oxide layer (MgO) on top of the already deposited metallic magnesium.
melting point 749°C) at some points of the electrode surface, and (ii) formation of a discontinuous passivating oxide layer (MgO) on top of the already deposited metallic magnesium.
Discussion
Interfacial properties of the molten media are important to consider regarding interpretation of the results obtained in the electrodeposition studies of magnesium from calcium chloride melts. Other phenomena that could affect the electrodeposition process of magnesium could be metal solubility and formation of metal fog or interactions with the substrate, which are also related to interfacial properties. However, it is difficult to obtain information about all those properties and then extract the proper conclusions.
Surface tension values of different molten chlorides at the gas interface are found in the literature.2 According to some authors the surface tension at the gas interface of molten magnesium, calcium, potassium, and sodium chlorides and their mixtures corresponds to the surface tension of these mixtures at a solid interface. Then, if the surface tension of the chlorides in the studied molten systems is known at the gas interface, an approximate idea of their surface tensions at a solid cathode interface may be obtained. Accordingly, the variation of the surface tension of the electrolyte with its composition and temperature must be known. However, it is expected that different values will be obtained depending on the solid cathode material. Most of the data found in the literature correspond to iron, graphite, and  substrates.32 Moreover, it is unsatisfactory to consider the wetting angle of the cathode surface by the electrolyte as the only criterion of wettability of the cathode by the liquid magnesium, although experimental results support this theory in the case of iron substrates.2
substrates.32 Moreover, it is unsatisfactory to consider the wetting angle of the cathode surface by the electrolyte as the only criterion of wettability of the cathode by the liquid magnesium, although experimental results support this theory in the case of iron substrates.2
According to data obtained in the literature32 there is no clear correlation between surface tension and interfacial tension of several molten chlorides. It is then difficult to predict interfacial properties by simply considering the surface tension values usually listed in the literature.
In addition, it is well known that the intrinsic interfacial properties of the molten chlorides can change because of the presence of moisture in the electrolyte2 being a cause of inferior wetting of the cathode by the melt. It is known from industrial practice that if the electrolysis cell is not sufficiently dry, which is difficult to achieve due to the hygroscopic nature of the chlorides used, the moisture present during the electrolysis prevents metallic magnesium from wetting the cathode properly, irrespective of the composition of the electrolyte.2
All these points make it difficult to extract definitive conclusions supported by literature data.
Interfacial properties of  melts may be responsible for forming three-dimensional deposits (magnesium spheres) rather than layers. It is expected that the magnesium spheres will coalesce to form a uniform deposit if there is a good wetting of the cathode by metallic magnesium. The results obtained in this type of melt have basically shown no existence of monolayers of magnesium but nucleation and growth phenomena of the magnesium deposits on the metallic substrates. Nucleation and growth phenomena are more important when increasing the content of
melts may be responsible for forming three-dimensional deposits (magnesium spheres) rather than layers. It is expected that the magnesium spheres will coalesce to form a uniform deposit if there is a good wetting of the cathode by metallic magnesium. The results obtained in this type of melt have basically shown no existence of monolayers of magnesium but nucleation and growth phenomena of the magnesium deposits on the metallic substrates. Nucleation and growth phenomena are more important when increasing the content of  in the melts due to an increase in the growth rate of the nuclei.33 There is a significant solubility of calcium metal in the magnesium deposit, giving a Mg-Ca liquid solution. This leads to an important UPD of calcium, which is more pronounced at lower contents of
in the melts due to an increase in the growth rate of the nuclei.33 There is a significant solubility of calcium metal in the magnesium deposit, giving a Mg-Ca liquid solution. This leads to an important UPD of calcium, which is more pronounced at lower contents of  in the melt due to an increase in the calcium chloride activity.31
in the melt due to an increase in the calcium chloride activity.31
In the case of the  melts the results are similar, showing that nucleation and growth are important phenomena in the formation of both solid and liquid magnesium deposits, although continuous layers are formed easier when depositing magnesium in the liquid state (steady currents in the chronoamperometric curves are reached earlier). Due to solubility of calcium metal in magnesium, UPD of calcium also takes place in this kind of melt. In the case of a dilute solution of
melts the results are similar, showing that nucleation and growth are important phenomena in the formation of both solid and liquid magnesium deposits, although continuous layers are formed easier when depositing magnesium in the liquid state (steady currents in the chronoamperometric curves are reached earlier). Due to solubility of calcium metal in magnesium, UPD of calcium also takes place in this kind of melt. In the case of a dilute solution of  in the molten mixture, current waves corresponding to the formation of solid Ca-Mg alloys or formation of a liquid Ca-Mg solution at temperature below or above the melting point of magnesium, respectively, are present. It is less likely that the signals are due to Na-Mg alloy formation due to low solubility of sodium in magnesium.
in the molten mixture, current waves corresponding to the formation of solid Ca-Mg alloys or formation of a liquid Ca-Mg solution at temperature below or above the melting point of magnesium, respectively, are present. It is less likely that the signals are due to Na-Mg alloy formation due to low solubility of sodium in magnesium.
The results were somewhat different when using  (35:55:10 mol %) melt containing 5 mol %
(35:55:10 mol %) melt containing 5 mol %  at 1000 K as electrolyte. KCl has a positive effect on the wetting angle,2 thus enabling the formation of a stable magnesium monolayer on tungsten and molybdenum substrates. There is another important feature in this type of melt and that is the passivation process that takes place at high cathodic overpotentials (around −0.350 V) and which has been observed specially in the chronoamperometric curves. The nature of the passivating layer is uncertain, but one can think of two possible explanations:
at 1000 K as electrolyte. KCl has a positive effect on the wetting angle,2 thus enabling the formation of a stable magnesium monolayer on tungsten and molybdenum substrates. There is another important feature in this type of melt and that is the passivation process that takes place at high cathodic overpotentials (around −0.350 V) and which has been observed specially in the chronoamperometric curves. The nature of the passivating layer is uncertain, but one can think of two possible explanations:
1. The local concentration of the different molten chlorides at some points in the electrode interface is such that the formation of the stable compound  takes place. This compound is solid at the working temperature (melting point 749°C) and its precipitation could cause a pseudopassivation of the electroactive surface.
takes place. This compound is solid at the working temperature (melting point 749°C) and its precipitation could cause a pseudopassivation of the electroactive surface.
2. Formation of a discontinuous MgO passivating layer on top of the previous deposited drops of liquid magnesium. The formation of MgO can be explained by the dramatic decrease of magnesium oxide solubility at the electrode surface, where magnesium chloride concentration drops to zero.34
Conclusion
The formation of monolayers of magnesium on the solid substrates, tungsten and molybdenum, was found to become stable by increasing the content of KCl and decreasing  in the molten chloride. Homogeneous layers of deposits that cover the whole cathode surface were then obtained.
in the molten chloride. Homogeneous layers of deposits that cover the whole cathode surface were then obtained.
The currently studied calcium chloride-based melts without any potassium chloride give rise to three-dimensional magnesium deposits that grow macroscopically on the substrates. This also affects the calculation of the diffusion coefficient of electroactive species by convolution of the voltammetric curves.
Mg-Ca alloy formation leads to an important UPD of calcium, which affects the electrodeposition of magnesium.
In a melt of industrial composition, a remarkable phenomenon was observed. This was related to the formation of a passivating MgO layer on top of the already deposited metallic magnesium, or alternatively, to the precipitation of solid  at some points on the electrode surface.
at some points on the electrode surface.
From the obtained results one could give some recommendations about the most appropriate solvent and cathode material of the ones studied for electrolytic production of magnesium. When it comes to substrates, glassy carbon does not seem to be a good choice due to formation of intermetallic compounds with the cations present in any of the studied molten chlorides. Tungsten and molybdenum could very well be used because both of them have a similar behavior and possess good wetting properties.
Moreover, the obtained results showed that in the studied calcium chloride-based melts it is better to avoid the presence of KCl due to the existing passivation phenomena.  and
and  mixtures possess similar characteristics. However, when dealing with industrial processes there are some other aspects one should consider in order to choose the proper solvent, i.e., conductivity, density, working temperature, availability of raw materials, and cost.
mixtures possess similar characteristics. However, when dealing with industrial processes there are some other aspects one should consider in order to choose the proper solvent, i.e., conductivity, density, working temperature, availability of raw materials, and cost.
Acknowledgments
The Research Council of Norway and Norsk Hydro ASA are acknowledged for financial support. A.M.M. also thanks the "Secretarı́a de Educación, Universidades, Investigación y Desarrollo" (Spain) for a postdoctoral grant. Oddmund Wallevik and Dr. Christian Rosenkilde, Norsk Hydro ASA, are acknowledged for fruitful discussions.
Norwegian University of Science and Technology assisted in meeting the publication costs of this article.