Abstract
Nanocrystalline metal oxides can be prepared with large surface area, electrochemical stability, and pseudocapacitive behavior, being able to be used as supercapacitor electrodes. Among the various metal oxides studied, amorphous and hydrated manganese oxide  is the most promising for supercapacitor electrodes due to the low cost of the raw material. In the present work, amorphous manganese dioxide
is the most promising for supercapacitor electrodes due to the low cost of the raw material. In the present work, amorphous manganese dioxide  is prepared by chemical co-precipitation of Mn(VII) and Mn(II) in water medium, giving small particles of relatively high surface area. Carbon nanotubes (CNTs) are proposed as an alternative additive of carbon black for improving the electrical conductivity of the manganese oxide electrodes used to build capacitors. The results demonstrate that CNTs are effective for increasing the capacitance and improving the electrochemical properties of the
is prepared by chemical co-precipitation of Mn(VII) and Mn(II) in water medium, giving small particles of relatively high surface area. Carbon nanotubes (CNTs) are proposed as an alternative additive of carbon black for improving the electrical conductivity of the manganese oxide electrodes used to build capacitors. The results demonstrate that CNTs are effective for increasing the capacitance and improving the electrochemical properties of the  electrodes which show a better capacitive behavior than with carbon black. This enhancement is due to the high entanglement of CNTs which form a network of open mesopores, allowing the bulk of
electrodes which show a better capacitive behavior than with carbon black. This enhancement is due to the high entanglement of CNTs which form a network of open mesopores, allowing the bulk of  to be easily reached by the ions. The performance optimization requires a careful control of the electrolyte pH in order to avoid the irreversible reactions Mn(IV) to Mn(II) at the negative electrode and Mn(IV) to Mn(VII) at the positive one. © 2004 The Electrochemical Society. All rights reserved.
to be easily reached by the ions. The performance optimization requires a careful control of the electrolyte pH in order to avoid the irreversible reactions Mn(IV) to Mn(II) at the negative electrode and Mn(IV) to Mn(VII) at the positive one. © 2004 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
Recently, various metal oxides have been investigated as possible electrode materials for high-power electrochemical capacitors.1 2 3 4 5 6 The energy storage mechanism with these oxides is mainly based on fast faradaic redox reactions which occur between the oxide and the electrolyte,7 8 giving rise to the so-called pseudocapacitance. Therefore they are potentially able to store more energy than the activated carbons used in the typical double-layer capacitors.8 In the latter case, the electrical energy is stored only in the electric double layer at the electrode/electrolyte interface. This phenomenon is controlled by the surface area of the interface, with a maximum specific capacitance generally ranging between 10 and 30 μF/cm2 depending on the carbon material.9 By contrast, with a pseudo-capacitive material, the capacitance is related to the charge and mass transferred between the electrode and the electrolyte. Hence, it is a process where the bulk of the electrode is working, not only the surface, and capacitances of 100-400 μF/cm2 can be obtained.
The characteristics required for using a metal oxide as a capacitor electrode are a pseudocapacitive behavior, a large surface area, a high conductivity, and electrochemical stability.4 The best example is the amorphous and hydrated ruthenium oxide  which yields remarkably high specific capacitance (720 to 780 F/g) when compared with other oxides, like Co or Ni oxides.1
2
3 The amorphous nature of hydrated ruthenium oxide together with the interaction between the proton of the hydroxyl group and the constitutional water and/or the electrolyte lead to fast proton diffusion rates, which seem to be the reason for the high capacitance obtained with this material.5
7
which yields remarkably high specific capacitance (720 to 780 F/g) when compared with other oxides, like Co or Ni oxides.1
2
3 The amorphous nature of hydrated ruthenium oxide together with the interaction between the proton of the hydroxyl group and the constitutional water and/or the electrolyte lead to fast proton diffusion rates, which seem to be the reason for the high capacitance obtained with this material.5
7
The main disadvantage of  is the high cost of the raw material. Therefore, great effort was recently devoted in order to find new and cheaper materials. The different research trends are either to prepare composite electrodes dispersing ruthenium oxide in very small particles over a high surface area support, like activated carbon or carbon aerogels, which additionally presents a high double-layer capacitance,10
11
12
13
14
15 or to use other metallic oxides.4
5
6 In this sense, manganese oxides are promising supercapacitor materials due to their low cost and the fact that manganese can be considered more environmentally friendly than other transition metals.16
17
18
19
20
21
22
23
24
25 Manganese oxide can be prepared either hydrated and amorphous as ruthenium oxide, however its electrical conductivity is not high enough to use it as an electrode material for supercapacitors. In order to realize an electrode with manganese oxide, one of the most used experimental methods is the electrodeposition of a thin manganese oxide layer over a conductive support such as graphite20
22
23 or a metal.18
19
25 Another way consists of mixing manganese oxide with a conducting additive.16
17
21
24 Generally, carbon black is used as additive for electrodes due to its conductive nature and chemical inertness.
is the high cost of the raw material. Therefore, great effort was recently devoted in order to find new and cheaper materials. The different research trends are either to prepare composite electrodes dispersing ruthenium oxide in very small particles over a high surface area support, like activated carbon or carbon aerogels, which additionally presents a high double-layer capacitance,10
11
12
13
14
15 or to use other metallic oxides.4
5
6 In this sense, manganese oxides are promising supercapacitor materials due to their low cost and the fact that manganese can be considered more environmentally friendly than other transition metals.16
17
18
19
20
21
22
23
24
25 Manganese oxide can be prepared either hydrated and amorphous as ruthenium oxide, however its electrical conductivity is not high enough to use it as an electrode material for supercapacitors. In order to realize an electrode with manganese oxide, one of the most used experimental methods is the electrodeposition of a thin manganese oxide layer over a conductive support such as graphite20
22
23 or a metal.18
19
25 Another way consists of mixing manganese oxide with a conducting additive.16
17
21
24 Generally, carbon black is used as additive for electrodes due to its conductive nature and chemical inertness.
Recently, it has been demonstrated that carbon nanotubes (CNTs) noticeably enhance the electrical properties of composite materials based on insulating polymers, as epoxy resins.26
27
28 Although added to the polymer matrix in a smaller volume fraction than carbon black, the CNTs provide a better conductivity and lower percolation threshold than carbon black. Therefore, for the realization of real two-electrode capacitors based on  electrodes, it seems very attractive to use CNTs as a conductivity additive.
electrodes, it seems very attractive to use CNTs as a conductivity additive.
CNTs have been already used in supercapacitors as electrode materials,8 29 as additives for improving the performance of conducting polymers,30 31 or for supporting small metal oxide particles in order to increase the capacitance of the carbon nanotubes.32 33
The values of specific capacitance reported for manganese oxide in the literature range between 150 and 250 F/g.16
17
18
19
20
21
22
23
24
25
34 These values are far from the theoretical value, ca. 1000 F/g, which can be obtained in a  alkaline battery, where one electron is involved in the first discharge plateau. Whereas, a bulk process is observed for
alkaline battery, where one electron is involved in the first discharge plateau. Whereas, a bulk process is observed for  it seems that only the surface is involved in the pseudocapacitive behavior of manganese oxide.34 Taking into account that CNTs are strongly entangled, providing a network of open mesopores, some better accessibility to the bulk of
it seems that only the surface is involved in the pseudocapacitive behavior of manganese oxide.34 Taking into account that CNTs are strongly entangled, providing a network of open mesopores, some better accessibility to the bulk of  could be expected by the realization of
could be expected by the realization of  /CNTs composites.
/CNTs composites.
Finally, one must point out that until now manganese oxide has only been studied using a three-electrode cell system, in the positive range of potential,16 17 18 19 20 21 22 23 24 25 but never in a symmetric two-electrode capacitor where the two manganese oxide electrodes operate in different potential ranges.
Taking into account the above remarks, the first objective of this study is to test the performance of CNTs as an alternative additive to carbon black for improving the electrical conductivity of manganese oxide electrodes. In a second step, the electrochemical behavior of the  /carbon composites is investigated in a real two-electrode system. We show that the cyclability of such a device is strongly dependent on the pH of the electrolytic medium.
/carbon composites is investigated in a real two-electrode system. We show that the cyclability of such a device is strongly dependent on the pH of the electrolytic medium.
Experimental
The high purity CNTs used in this study were prepared by decomposition of acetylene over a powdered  solid solution catalyst.35 Amorphous and hydrated manganese oxide
solid solution catalyst.35 Amorphous and hydrated manganese oxide  was prepared by chemical co-precipitation, mixing
was prepared by chemical co-precipitation, mixing  and
and  water solutions.16
17 The mixed solution was stirred for 6 h and the precipitated oxide was thoroughly washed with deionized water before being dried at 120°C for 12 h. Predetermined amounts of carbon nanotubes (0, 10, 15, 20, 25, 30, 40, 50 wt %) were added to the solution before
water solutions.16
17 The mixed solution was stirred for 6 h and the precipitated oxide was thoroughly washed with deionized water before being dried at 120°C for 12 h. Predetermined amounts of carbon nanotubes (0, 10, 15, 20, 25, 30, 40, 50 wt %) were added to the solution before  precipitation in order to obtain a homogeneous mixture. After washing and drying, the
precipitation in order to obtain a homogeneous mixture. After washing and drying, the  /CNTs composite was obtained. Similar experiments, but using carbon black, were realized for comparison purposes.
/CNTs composite was obtained. Similar experiments, but using carbon black, were realized for comparison purposes.
X-ray diffraction (XRD) analyses of the prepared  were carried out using the Cu Kα radiation (INEL CPS120). Thermal analysis with a coupled TG-MS (SDT 2960, TA instruments) was used to determine the weight loss as well as the gases evolved during a thermal treatment of
were carried out using the Cu Kα radiation (INEL CPS120). Thermal analysis with a coupled TG-MS (SDT 2960, TA instruments) was used to determine the weight loss as well as the gases evolved during a thermal treatment of  in a
in a  flow (100 mL/min).
flow (100 mL/min).
The porous texture of the CNTs, oxides and composites was analyzed by  adsorption at 77 K (Autosorb-6, Quantachrome). The specific surface area was calculated by applying the Brunauer-Emmett-Teller (BET) equation to the
adsorption at 77 K (Autosorb-6, Quantachrome). The specific surface area was calculated by applying the Brunauer-Emmett-Teller (BET) equation to the  isotherm data. The texture of the pristine CNTs and the composites was studied by scanning electron microscopy (SEM, Hitachi S 4200). The structure of CNTs was characterized by transmission electron microscopy (TEM, Philips CM 20).
isotherm data. The texture of the pristine CNTs and the composites was studied by scanning electron microscopy (SEM, Hitachi S 4200). The structure of CNTs was characterized by transmission electron microscopy (TEM, Philips CM 20).
The capacitor electrodes were films formed by a mixture of the active material  /carbon composite, 90 wt %) with a binder solution (Teflon, 10%). In a few experiments polyvinylidene (PVDF, Kynar Flex 2801) was used as a binder. The mixture was thoroughly homogenized in an agate mortar and dried at room temperature before being rolled into a thin film of uniform thickness. Electrode pellets were cut with a surface of 1 cm2 which corresponds approximately to a mass of 10 mg. Two electrode capacitors were built with a glassy fibrous separator and gold current collectors, using a Teflon Swagelok® type system. Different aqueous electrolytic solutions have been studied, 1 mol L−1
/carbon composite, 90 wt %) with a binder solution (Teflon, 10%). In a few experiments polyvinylidene (PVDF, Kynar Flex 2801) was used as a binder. The mixture was thoroughly homogenized in an agate mortar and dried at room temperature before being rolled into a thin film of uniform thickness. Electrode pellets were cut with a surface of 1 cm2 which corresponds approximately to a mass of 10 mg. Two electrode capacitors were built with a glassy fibrous separator and gold current collectors, using a Teflon Swagelok® type system. Different aqueous electrolytic solutions have been studied, 1 mol L−1  with a pH of 6.4 or with a modified pH of 10 by the addition of NaOH. Experiments were also done in Teflon three-electrode cells by using Pt and
with a pH of 6.4 or with a modified pH of 10 by the addition of NaOH. Experiments were also done in Teflon three-electrode cells by using Pt and  as the auxiliary and reference electrode, respectively. The values of the specific capacitance (F/g for the total mass of composite electrode material including the conducting additive) were estimated by cyclic voltammetry (scan rate of potential from 2 to 10 mV/s). The specific capacitance (C) was calculated by the general equation
as the auxiliary and reference electrode, respectively. The values of the specific capacitance (F/g for the total mass of composite electrode material including the conducting additive) were estimated by cyclic voltammetry (scan rate of potential from 2 to 10 mV/s). The specific capacitance (C) was calculated by the general equation
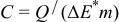
where Q is the charge obtained after integrating the voltammogram, m is the total mass of electrode, and  is the potential window, in the case of using a three-electrode cell, or the polarization potential range of each electrode, considered as half of the applied cell voltage, for a two electrode cell.
is the potential window, in the case of using a three-electrode cell, or the polarization potential range of each electrode, considered as half of the applied cell voltage, for a two electrode cell.
Galvanostatic charge/discharge cycling at a constant current of 100 mA/g was also performed on the two- and three-electrode devices (VMP-Biologic-France).
Results and Discussion
Samples characterization.—
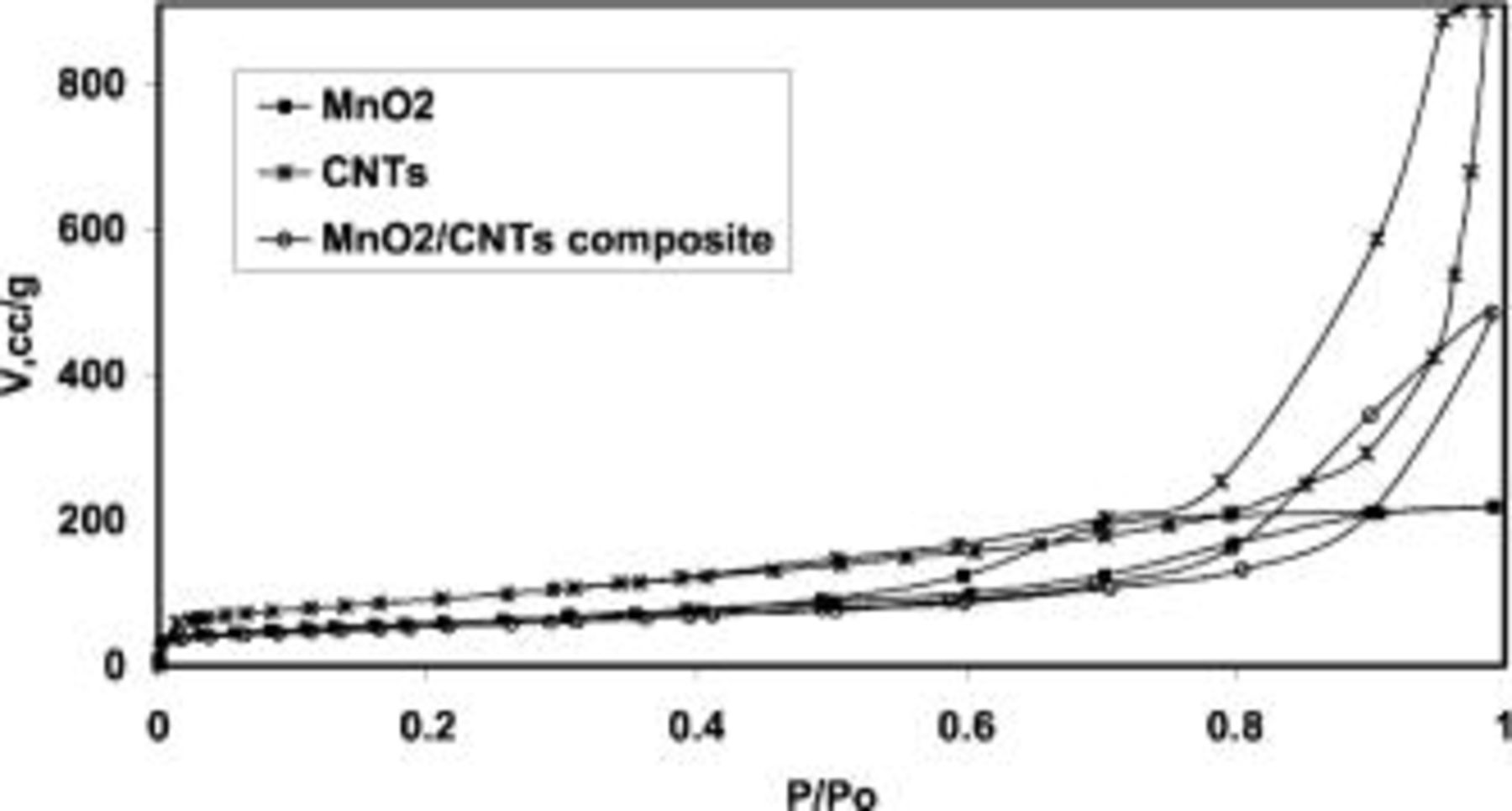
Figure 1 shows the  adsorption isotherm of the prepared manganese oxide. The isotherm is typical for a mesoporous material with a hysteresis loop at high partial pressures. The slight adsorption at low relative pressure indicates some moderate amount of micropores in this material. A surface area of 220 m2/g was calculated by applying the BET equation to the adsorption data, that fits with previous reports.16
17
21 The material obtained has a relatively high surface area, considering that it is a metallic oxide, what is interesting for its further application as the electrode in electrochemical capacitors.
adsorption isotherm of the prepared manganese oxide. The isotherm is typical for a mesoporous material with a hysteresis loop at high partial pressures. The slight adsorption at low relative pressure indicates some moderate amount of micropores in this material. A surface area of 220 m2/g was calculated by applying the BET equation to the adsorption data, that fits with previous reports.16
17
21 The material obtained has a relatively high surface area, considering that it is a metallic oxide, what is interesting for its further application as the electrode in electrochemical capacitors.
Figure 1. Nitrogen adsorption isotherms at 77 K of  pristine nanotubes (CNTs) and of the
pristine nanotubes (CNTs) and of the  /CNTs composite with 15 wt % of nanotubes
/CNTs composite with 15 wt % of nanotubes  /CNTs composite).
/CNTs composite).
The XRD Cu Kα pattern of manganese oxide did not show any discernible reflection, which confirms its amorphous nature. The amorphous structure will favor the fast proton/ions diffusion rates. Thus, an important characteristic needed for a metallic oxide to be used as electrode materials is fulfilled.4
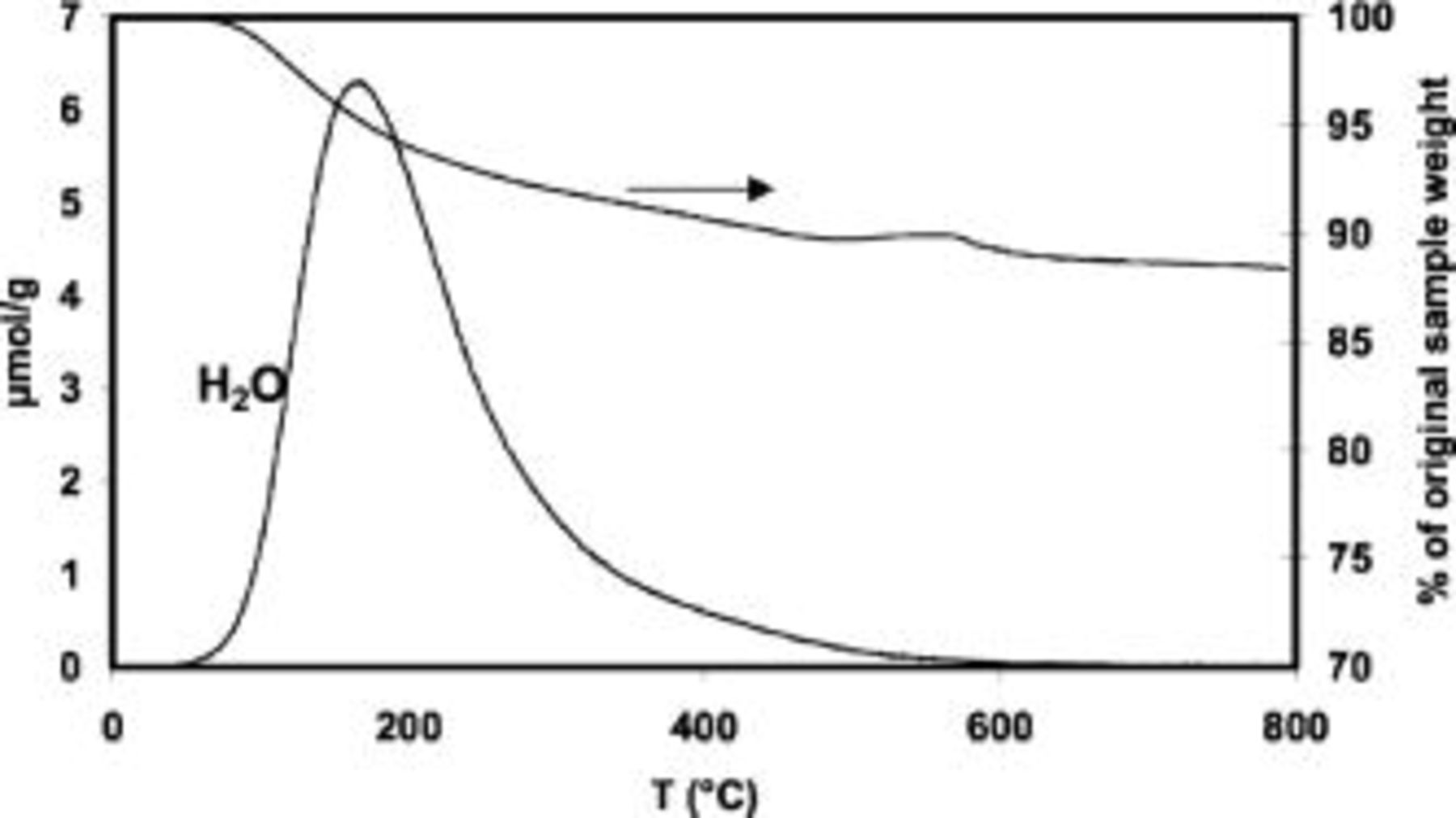
Thermal analysis was also performed on the oxide in order to characterize the chemical nature of water. Figure 2 shows the weight loss during a heat-treatment from room temperature up to 800°C in a nitrogen atmosphere. The gases evolved during the treatment were analyzed by mass spectroscopy and only water was detected (Fig. 2). The weight loss of the material, ca. 11%, occurs in a single step between 80 and 400°C. Since water evolution is continuous and mostly above 100°C, that indicates that it is not adsorbed water, but water in the oxide structure. Hence, the prepared oxide is hydrated, which will favor a pseudocapacitive behavior.5
7 These different analyses confirm that the material prepared is amorphous and hydrated manganese oxide  with a high surface area.
with a high surface area.
Figure 2. Thermogravimetric analysis (TG) of the prepared  and mass spectrometry analysis (MS) of the water evolved.
and mass spectrometry analysis (MS) of the water evolved.
The resistance of amorphous  was estimated from the ohmic drop between galvanostatic charge and discharge36
37 of a symmetric capacitor built with two manganese oxide electrodes (film
was estimated from the ohmic drop between galvanostatic charge and discharge36
37 of a symmetric capacitor built with two manganese oxide electrodes (film  Teflon) of 1 cm2 surface in 1 mol L−1
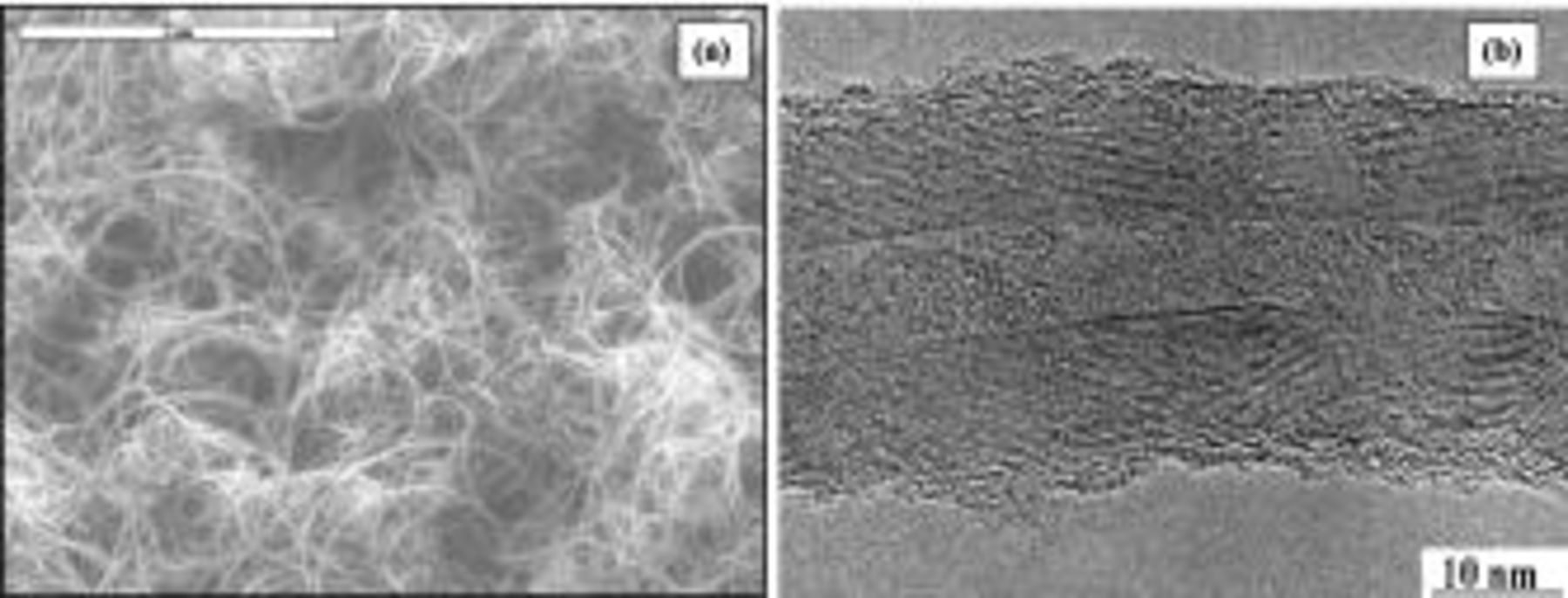
Teflon) of 1 cm2 surface in 1 mol L−1  as the electrolyte. The resistance value found was around 2000 Ω cm2. Thus, the electric conductivity of this material is not high enough to be used as a pure electrode component of an electrochemical capacitor. Generally, carbon black is used in electrochemical cells to enhance the conductivity of the electrodes. In the present work, we want to determine if multiwalled CNTs could be more efficient than carbon black for this purpose. Indeed, the SEM image in Fig. 3a shows long and flexible nanotubes, highly entangled. Moreover, as shown by the TEM picture in Fig. 3b, the CNTs are formed by continuous layers surrounding the central canal, that is favorable for a good electrical conductivity of each nanotube. Therefore, we can predict that this kind of network of conducting CNTs should be able to perfectly connect the
as the electrolyte. The resistance value found was around 2000 Ω cm2. Thus, the electric conductivity of this material is not high enough to be used as a pure electrode component of an electrochemical capacitor. Generally, carbon black is used in electrochemical cells to enhance the conductivity of the electrodes. In the present work, we want to determine if multiwalled CNTs could be more efficient than carbon black for this purpose. Indeed, the SEM image in Fig. 3a shows long and flexible nanotubes, highly entangled. Moreover, as shown by the TEM picture in Fig. 3b, the CNTs are formed by continuous layers surrounding the central canal, that is favorable for a good electrical conductivity of each nanotube. Therefore, we can predict that this kind of network of conducting CNTs should be able to perfectly connect the  particles in the
particles in the  /CNTs composite.
/CNTs composite.
Figure 3. (a) SEM and (b) TEM images of multiwalled carbon nanotubes prepared by catalytic decomposition of acetylene on a  solid solution.
solid solution.
Figure 1 presents the  adsorption isotherm for the pristine carbon nanotubes. The shape of the curve corresponds to a mesoporous material and, considering that the tips of these nanotubes are closed, the mesopores are due to the open network of entangled nanotubes. The calculated BET specific surface area is 320 m2/g. Figure 1 also includes the
adsorption isotherm for the pristine carbon nanotubes. The shape of the curve corresponds to a mesoporous material and, considering that the tips of these nanotubes are closed, the mesopores are due to the open network of entangled nanotubes. The calculated BET specific surface area is 320 m2/g. Figure 1 also includes the  isotherm for the
isotherm for the  /CNTs composite prepared with 15 wt % of nanotubes. The BET specific surface area of the composite is similar to that of pure manganese oxide, ca. 210 m2/g. However, although the carbon nanotubes content is only 15 wt %, the isotherm of the composite is not similar to that of raw manganese oxide (Fig. 1). Actually, the shapes of both
/CNTs composite prepared with 15 wt % of nanotubes. The BET specific surface area of the composite is similar to that of pure manganese oxide, ca. 210 m2/g. However, although the carbon nanotubes content is only 15 wt %, the isotherm of the composite is not similar to that of raw manganese oxide (Fig. 1). Actually, the shapes of both  adsorption isotherms for the composite and the pristine nanotubes are identical, indicating that the open mesoporous network formed by the nanotubes is maintained after the addition of manganese oxide.
adsorption isotherms for the composite and the pristine nanotubes are identical, indicating that the open mesoporous network formed by the nanotubes is maintained after the addition of manganese oxide.
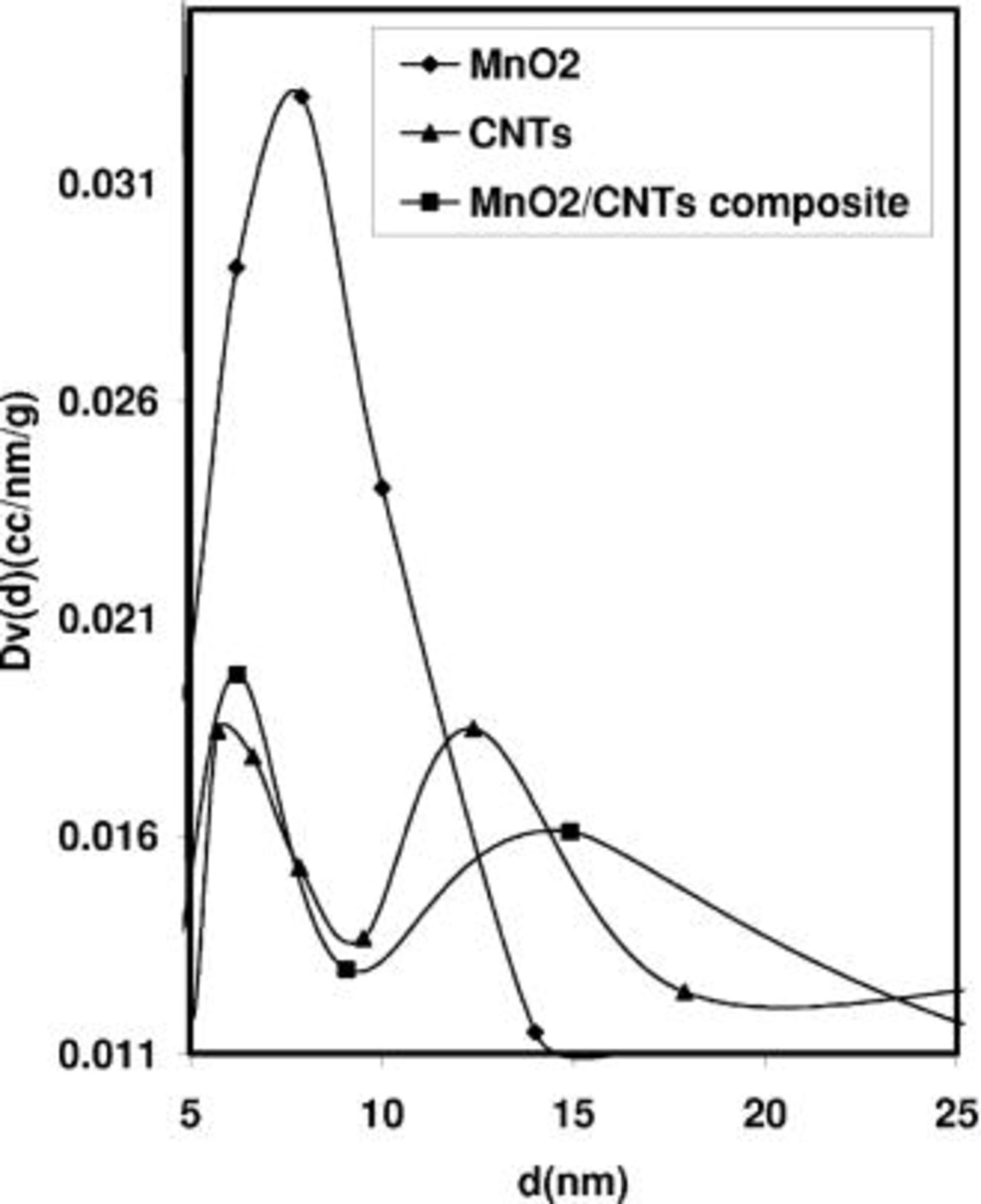
Figure 4 shows the pore size distribution obtained from the  isotherms presented in Fig. 1 by applying the Barret-Joyner-Halendo (BJH) method. This analysis confirms that the mesopore size distribution of the
isotherms presented in Fig. 1 by applying the Barret-Joyner-Halendo (BJH) method. This analysis confirms that the mesopore size distribution of the  /CNTs composite and CNTs are comparable. Moreover, compared with
/CNTs composite and CNTs are comparable. Moreover, compared with  the
the  /CNTs composite presents an additional mesoporosity. We can postulate that, due to this open network, the ions accessibility from the electrolyte to the active mass of the
/CNTs composite presents an additional mesoporosity. We can postulate that, due to this open network, the ions accessibility from the electrolyte to the active mass of the  /CNTs composite should be favored, while keeping the advantage of the excellent electrical conductivity provided by the nanotubes. Moreover, the flexibility and entanglement of the nanotubes should ensure good mechanical properties of the
/CNTs composite should be favored, while keeping the advantage of the excellent electrical conductivity provided by the nanotubes. Moreover, the flexibility and entanglement of the nanotubes should ensure good mechanical properties of the  /CNTs composite electrodes. In the following part of this manuscript, we show that all these assumptions are proved to a large extent.
/CNTs composite electrodes. In the following part of this manuscript, we show that all these assumptions are proved to a large extent.
Figure 4. Pore size distributions calculated by the BJH method from the nitrogen adsorption isotherms at 77 K of  pristine nanotubes (CNTs) and the
pristine nanotubes (CNTs) and the  /CNTs composite with 15 wt % of nanotubes
/CNTs composite with 15 wt % of nanotubes  /CNTs composite).
/CNTs composite).
Electrochemical characterization.—
Figure 5 shows the cyclic voltammograms (CVs), with a scan rate of potential of 2 mV/s, for pure  (pressing a mixture of 90% active
(pressing a mixture of 90% active  PVDF) and for the CNTs (pressing a mixture of 90% active
PVDF) and for the CNTs (pressing a mixture of 90% active  PVDF) in 1 mol L−1
PVDF) in 1 mol L−1  electrolyte. It can be observed that
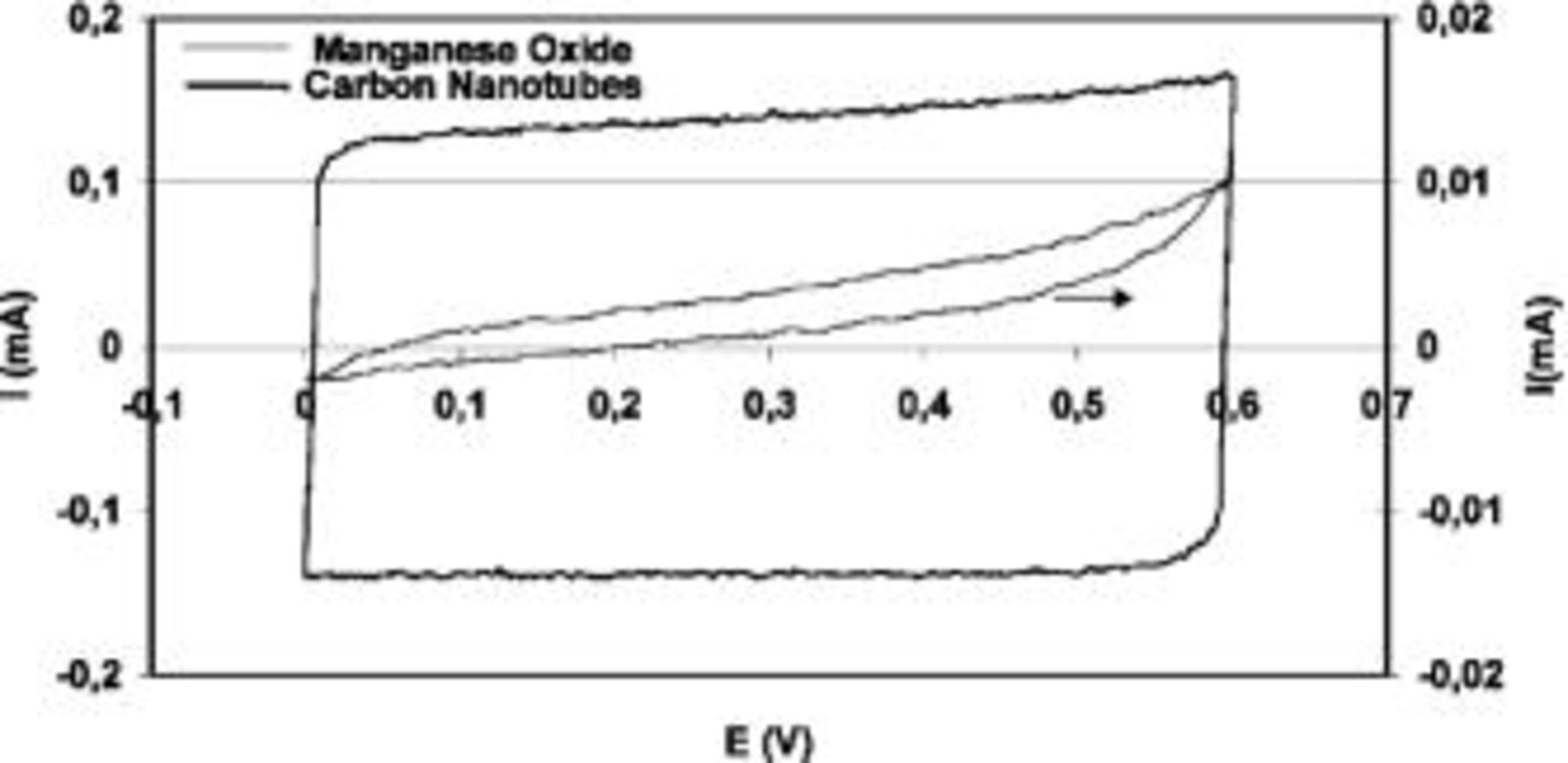
electrolyte. It can be observed that  presents a very high resistance and the specific capacitance obtained is only 0.1 F/g, because the electrolyte probably does not penetrate in the bulk of the material. On the contrary, carbon nanotubes present the characteristic box-like shape of an ideal capacitor, but the specific capacitance obtained is not higher than 20 F/g. Therefore, carbon nanotubes cannot be considered as an electrochemical active mass giving a significant additional capacitance in the composites.
presents a very high resistance and the specific capacitance obtained is only 0.1 F/g, because the electrolyte probably does not penetrate in the bulk of the material. On the contrary, carbon nanotubes present the characteristic box-like shape of an ideal capacitor, but the specific capacitance obtained is not higher than 20 F/g. Therefore, carbon nanotubes cannot be considered as an electrochemical active mass giving a significant additional capacitance in the composites.
Figure 5. Cyclic voltammograms at a scan rate of 2 mV/s in 1 mol L−1  for two-electrode cells made of the pristine carbon nanotubes and
for two-electrode cells made of the pristine carbon nanotubes and  respectively.
respectively.
Table I displays the specific capacitance of composites prepared with different nanotube loadings during the synthesis of  and the resistance estimated from the galvanostatic charge/discharge cycling experiments. It is clearly seen that the addition of carbon nanotubes improves the behavior of
and the resistance estimated from the galvanostatic charge/discharge cycling experiments. It is clearly seen that the addition of carbon nanotubes improves the behavior of  as a capacitor electrode. The specific capacitance values referred to the oxide
as a capacitor electrode. The specific capacitance values referred to the oxide  increase with the amount of CNTs, indicating that more energy can be extracted from manganese oxide, in other words that the active mass of
increase with the amount of CNTs, indicating that more energy can be extracted from manganese oxide, in other words that the active mass of  is more accessible with a large enough amount of CNTs. In addition, the resistance decreases when increasing the amount of CNTs, proving their efficiency as a conductivity agent. However, when the specific capacitance is referred to the total mass of electrode material, to be realistic, it can be noticed that carbon nanotubes loadings higher than 10-15 wt % are not improving the performance of the electrodes because they are not electrochemically active enough by themselves. Therefore, 10-15 wt % of CNTs as additive are sufficient to increase the capacitance of
is more accessible with a large enough amount of CNTs. In addition, the resistance decreases when increasing the amount of CNTs, proving their efficiency as a conductivity agent. However, when the specific capacitance is referred to the total mass of electrode material, to be realistic, it can be noticed that carbon nanotubes loadings higher than 10-15 wt % are not improving the performance of the electrodes because they are not electrochemically active enough by themselves. Therefore, 10-15 wt % of CNTs as additive are sufficient to increase the capacitance of  from 0.1 to 140 F/g. Hence, carbon nanotubes are found to be useful as additives for manganese oxide, but it is necessary to test if they present any advantage over another conventional additive, as carbon black.
from 0.1 to 140 F/g. Hence, carbon nanotubes are found to be useful as additives for manganese oxide, but it is necessary to test if they present any advantage over another conventional additive, as carbon black.
Table I.
Specific capacitance in 1 mol L−1 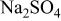 medium for medium for 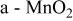 loaded with different weight percentages of carbon nanotubes and capacitor resistance. loaded with different weight percentages of carbon nanotubes and capacitor resistance. | |||
|---|---|---|---|
| Carbon nanotubesloading(% wt) | Specificcapacitance
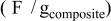 | Specificcapacitance
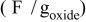 | Resistance(Ω cm2) |
| 0 | 0.1 | 0.1 | 2000 |
| 5 | 19 | 20 | 625 |
| 10 | 137 | 154 | 5.2 |
| 15 | 137 | 161 | 4.0 |
| 20 | 139 | 174 | 3.8 |
| 25 | 141 | 188 | 3.5 |
| 30 | 138 | 197 | 3.0 |
| 40 | 140 | 234 | 2.7 |
| 50 | 138 | 277 | 2.5 |
| 100 | 20 | ⋯ | 2.0 |
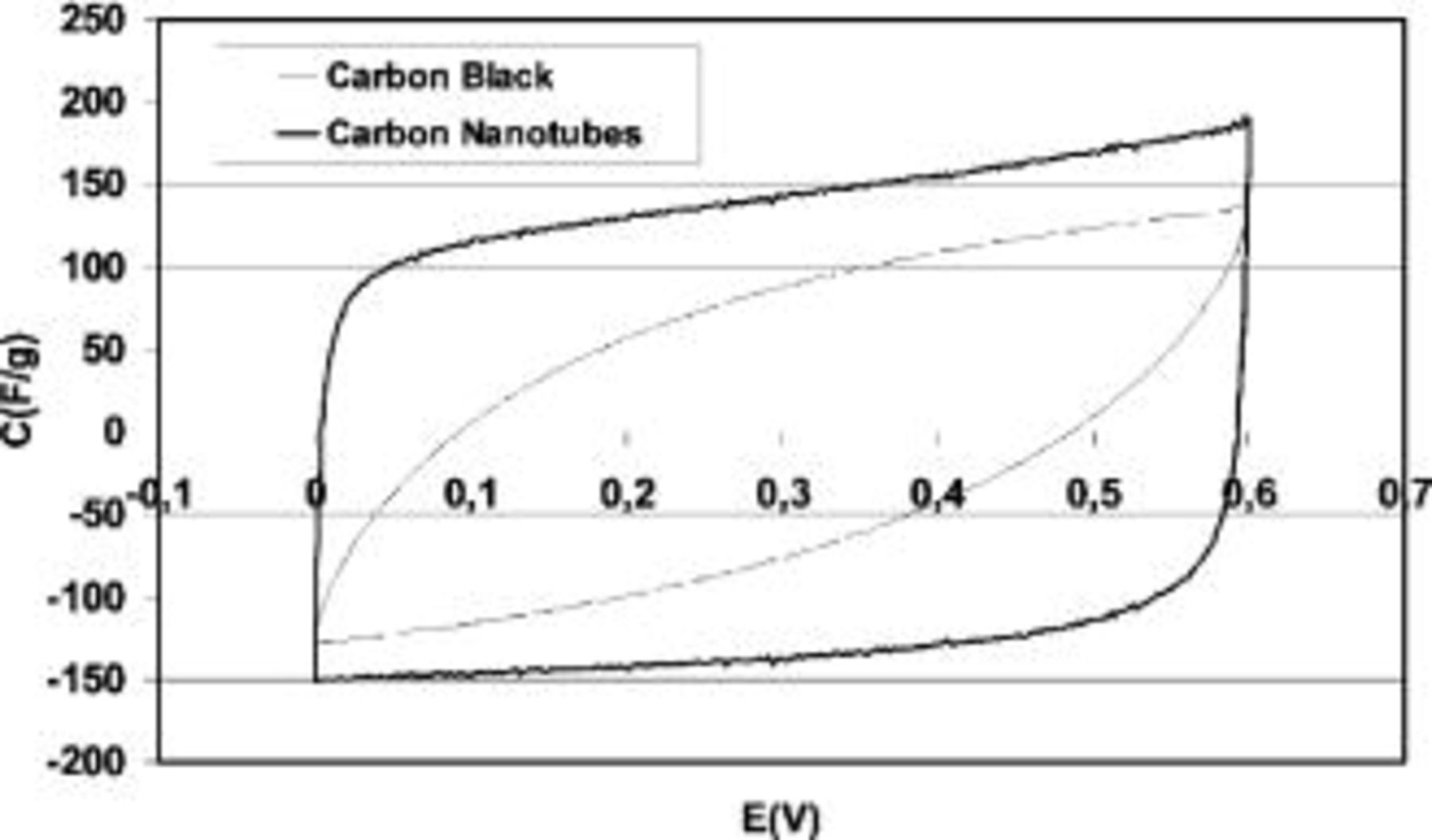
The comparative voltamperometric analyses presented in Fig. 6 for  electrodes loaded either with 15 wt % of CNTs or with 15 wt % of carbon black demonstrate that the electrochemical behavior of both composites is completely different. The capacitor built from the material prepared with carbon black is highly resistive, presenting a CV curve very far from the ideal box-like shape. The obtained capacitance with this composite material is not higher than ∼70 F/g. On the contrary, when carbon nanotubes are used as an additive to
electrodes loaded either with 15 wt % of CNTs or with 15 wt % of carbon black demonstrate that the electrochemical behavior of both composites is completely different. The capacitor built from the material prepared with carbon black is highly resistive, presenting a CV curve very far from the ideal box-like shape. The obtained capacitance with this composite material is not higher than ∼70 F/g. On the contrary, when carbon nanotubes are used as an additive to  the resistance of the electrodes is much lower than in the case of using the conventional carbon black, finding a performance closer to an ideal capacitor. Hence, the conductivity of
the resistance of the electrodes is much lower than in the case of using the conventional carbon black, finding a performance closer to an ideal capacitor. Hence, the conductivity of  was very effectively enhanced by the presence of carbon nanotubes. Additionally, another highly valuable effect of the use of CNTs is the possibility of extracting more energy from the
was very effectively enhanced by the presence of carbon nanotubes. Additionally, another highly valuable effect of the use of CNTs is the possibility of extracting more energy from the  electrodes, obtaining specific capacitance values per gram of electrode material of ∼140 F/g.
electrodes, obtaining specific capacitance values per gram of electrode material of ∼140 F/g.
Figure 6. Comparative voltamperograms of two-electrode cells built with  /carbon black and
/carbon black and  /CNTs electrodes, respectively. Carbon black or CNTs loading: 15 wt %. Electrolyte, 1 mol L−1.
/CNTs electrodes, respectively. Carbon black or CNTs loading: 15 wt %. Electrolyte, 1 mol L−1.  scan rate 2 mV/s.
scan rate 2 mV/s.
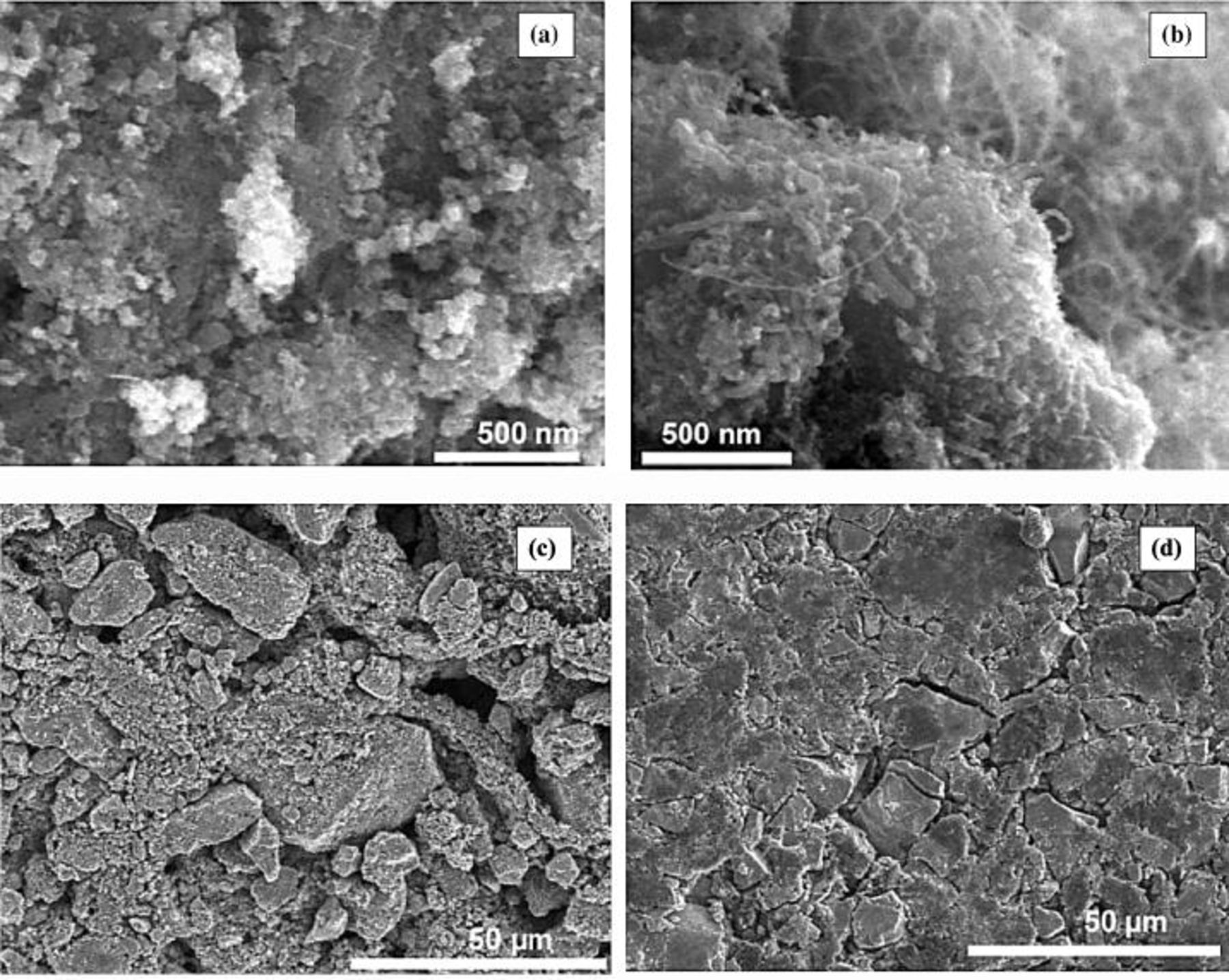
The scanning electron microscopy (SEM) analyses helped to better understand the different behaviors of both composite materials. The SEM pictures of the  /carbon black and
/carbon black and  /CNTs composites, both with 15 wt % of carbon additive, are shown in Fig. 7. Figure 7a shows that the carbon black and manganese oxide particles are homogeneously mixed. In the case of CNTs (Fig. 7b), the manganese oxide particles are integrated and surrounded in the entangled network of nanotubes in agreement with the
/CNTs composites, both with 15 wt % of carbon additive, are shown in Fig. 7. Figure 7a shows that the carbon black and manganese oxide particles are homogeneously mixed. In the case of CNTs (Fig. 7b), the manganese oxide particles are integrated and surrounded in the entangled network of nanotubes in agreement with the  adsorption results presented in Fig. 4. In both cases the dispersion of the carbon additive is very good. However, remarkable differences between both materials are observed on the surface of the film electrodes prepared with 10 wt % of binder. Figure 7c shows that when using carbon black as an additive, the surface of the film electrode is not very homogeneous and the connectivity of manganese oxide particles is rather poor. On the contrary, a film electrode prepared in similar conditions with the same proportion of carbon nanotubes presents a more homogeneous surface (Fig. 7d). This better homogeneity is attributed to the entangled network of CNTs which improves the connectivity of the
adsorption results presented in Fig. 4. In both cases the dispersion of the carbon additive is very good. However, remarkable differences between both materials are observed on the surface of the film electrodes prepared with 10 wt % of binder. Figure 7c shows that when using carbon black as an additive, the surface of the film electrode is not very homogeneous and the connectivity of manganese oxide particles is rather poor. On the contrary, a film electrode prepared in similar conditions with the same proportion of carbon nanotubes presents a more homogeneous surface (Fig. 7d). This better homogeneity is attributed to the entangled network of CNTs which improves the connectivity of the  particles. As a consequence of this good connectivity between the
particles. As a consequence of this good connectivity between the  particles, the conductivity is improved, as observed in the voltammograms of Fig. 6, by comparison with a traditional additive as carbon black. Additionally, the carbon nanotubes generate an open mesoporous network which facilitates the contact between the electrolyte and the active mass of the nanocomposite, allowing more energy to be extracted from manganese oxide.
particles, the conductivity is improved, as observed in the voltammograms of Fig. 6, by comparison with a traditional additive as carbon black. Additionally, the carbon nanotubes generate an open mesoporous network which facilitates the contact between the electrolyte and the active mass of the nanocomposite, allowing more energy to be extracted from manganese oxide.
Figure 7. SEM observations of the  /carbon nanocomposites obtained by mixing
/carbon nanocomposites obtained by mixing  with 15 wt % of carbon (a)
with 15 wt % of carbon (a)  /carbon black; (b)
/carbon black; (b)  /CNTs; (c)
/CNTs; (c)  /carbon
/carbon  wt % Teflon; (d)
wt % Teflon; (d)  wt % Teflon.
wt % Teflon.
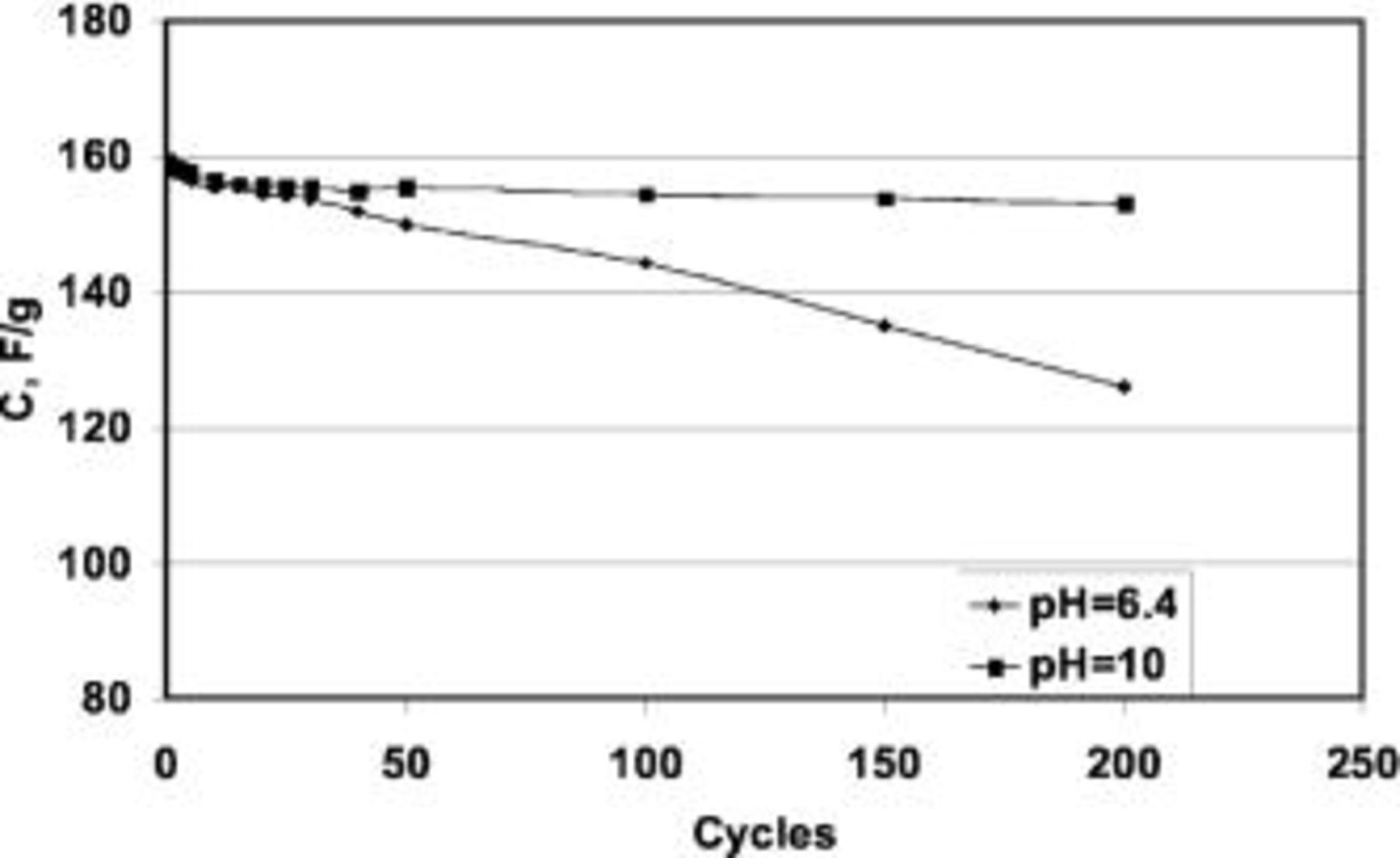
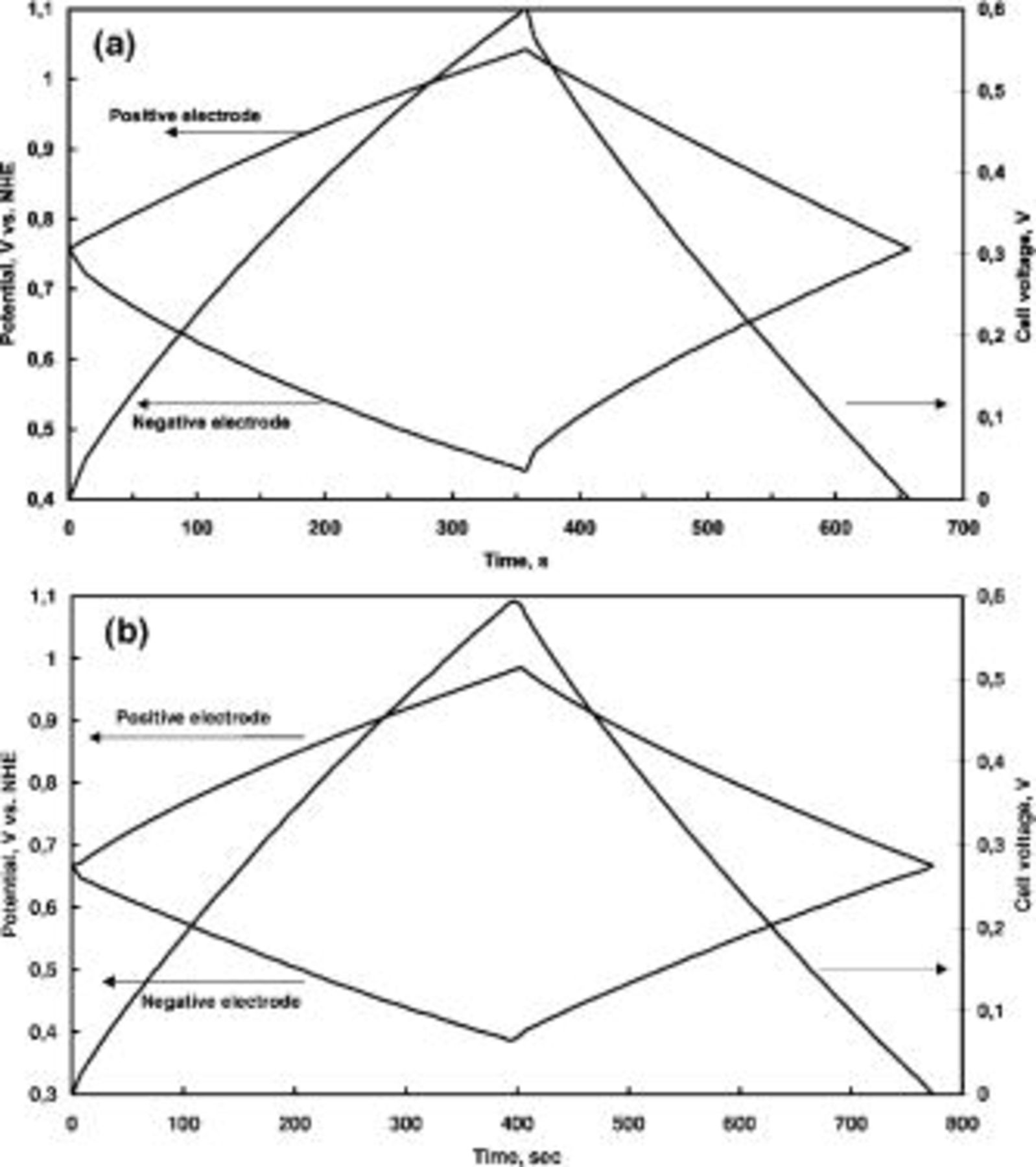
Another important aspect for a material to be used as a capacitor electrode is the electrochemical stability through repeated charge/discharge cycling. Figure 8 presents the specific discharge capacitance vs. the cycle number for  (with 15 wt % of CNTs) in 1 mol L−1
(with 15 wt % of CNTs) in 1 mol L−1  at two different pHs. At pH 6.4, the specific capacitance loss reaches 20% after 200 cycles. However, if the pH of the electrolyte is fixed at 10 by adding NaOH, the stability is remarkably increased, with a loss of only 4% of the initial specific capacitance after 200 cycles.
at two different pHs. At pH 6.4, the specific capacitance loss reaches 20% after 200 cycles. However, if the pH of the electrolyte is fixed at 10 by adding NaOH, the stability is remarkably increased, with a loss of only 4% of the initial specific capacitance after 200 cycles.
Figure 8. Specific discharge capacitance of a two-electrode capacitor based on the  /CNTs composite (with 15 wt % of CNTs) vs. the number of cycles at two different values of pH. Cutoff cell voltage 0.6 V; charge/discharge current 100 mA/g.
/CNTs composite (with 15 wt % of CNTs) vs. the number of cycles at two different values of pH. Cutoff cell voltage 0.6 V; charge/discharge current 100 mA/g.
In order to explain this behavior, it has to be considered that the pseudocapacitance of hydrous oxides like  is attributed to reversible redox transitions involving protons and/or cations exchange with the electrolyte following the equation20
is attributed to reversible redox transitions involving protons and/or cations exchange with the electrolyte following the equation20
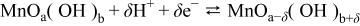
where  and
and  indicate interfacial
indicate interfacial  at high and low oxidation states, respectively. However, due to the solubility of either Mn(II) or Mn(VII) in the electrolytic solution, the irreversible reactions Mn(IV) to Mn(II) and Mn(IV) to Mn(VII) should be avoided during charging and discharging the capacitor. If one of these reactions takes place, it will result in a partial dissolution of the electrode and a subsequent decrease of capacitance. From the Pourbaix potential-pH diagram for manganese,38 it is possible to determine that the reaction Mn(IV) to Mn(II) occurs at potentials of 0.47 and 0.05 V (vs. NHE) for pH 6.4 and 10, respectively. In the case of the transformation of Mn(IV) into Mn(VII) the equilibrium potentials are 1.19 and 0.98 V (vs. NHE) for pH 6.4 and 10, respectively. Therefore, in order to confirm that the behavior found in Fig. 8 is related to some dissolution of
at high and low oxidation states, respectively. However, due to the solubility of either Mn(II) or Mn(VII) in the electrolytic solution, the irreversible reactions Mn(IV) to Mn(II) and Mn(IV) to Mn(VII) should be avoided during charging and discharging the capacitor. If one of these reactions takes place, it will result in a partial dissolution of the electrode and a subsequent decrease of capacitance. From the Pourbaix potential-pH diagram for manganese,38 it is possible to determine that the reaction Mn(IV) to Mn(II) occurs at potentials of 0.47 and 0.05 V (vs. NHE) for pH 6.4 and 10, respectively. In the case of the transformation of Mn(IV) into Mn(VII) the equilibrium potentials are 1.19 and 0.98 V (vs. NHE) for pH 6.4 and 10, respectively. Therefore, in order to confirm that the behavior found in Fig. 8 is related to some dissolution of  we have measured the potential of both electrodes when the cell voltage varies between 0 and 0.6 V during the galvanostatic charge-discharge cycling in the two aqueous solutions of different pH.
we have measured the potential of both electrodes when the cell voltage varies between 0 and 0.6 V during the galvanostatic charge-discharge cycling in the two aqueous solutions of different pH.
For this purpose, we have used a three-electrode Swagelok-type cell consisting of two electrodes from the  /CNTs composite, as the working and the auxiliary ones, and a
/CNTs composite, as the working and the auxiliary ones, and a  reference electrode. Figure 9 presents, for the two electrolytic media, the variation of the cell voltage during charge-discharge cycling at a constant current density of 100 mA/g (taking into account the total mass of the electrode) and the potential profile for both positive and negative electrodes. Figure 9a shows that with the electrolyte of pH 6.4, the initial potential for both electrodes in the discharged state of the capacitor is 0.76 V vs. NHE. After charging the capacitor to 0.6 V, the potential of the positive electrode increases up to 1.04 V, while for the negative one it decreases from 0.76 to 0.44 V vs. NHE. Since the irreversible reduction of Mn(IV) into Mn(II) starts to occur at 0.47 V for a pH 6.4,38 it demonstrates that the negative electrode is actually working below this potential, which results in a partial dissolution of the active material in the electrolyte. Hence, the capacitance decrease observed in Fig. 8 during repeated cycling of a capacitor in this medium is due to the deterioration of the negative electrode. On the contrary, from Fig. 9b it is clear that at pH 10 both electrodes work in the electrochemical stability window where there is not any irreversible reaction producing either Mn(II) or Mn(VII). In this electrolyte, the initial potential for both electrodes of the discharged capacitor is 0.66 V vs. NHE and, after charging the capacitor up to 0.6 V, the potential of the negative electrode decreases to 0.38 V, very far from the value of 0.05 V needed for the destructive reaction Mn(IV) to Mn(II) at pH 10.38 This fact explains the good cycle life observed in Fig. 8 for a capacitor based on
reference electrode. Figure 9 presents, for the two electrolytic media, the variation of the cell voltage during charge-discharge cycling at a constant current density of 100 mA/g (taking into account the total mass of the electrode) and the potential profile for both positive and negative electrodes. Figure 9a shows that with the electrolyte of pH 6.4, the initial potential for both electrodes in the discharged state of the capacitor is 0.76 V vs. NHE. After charging the capacitor to 0.6 V, the potential of the positive electrode increases up to 1.04 V, while for the negative one it decreases from 0.76 to 0.44 V vs. NHE. Since the irreversible reduction of Mn(IV) into Mn(II) starts to occur at 0.47 V for a pH 6.4,38 it demonstrates that the negative electrode is actually working below this potential, which results in a partial dissolution of the active material in the electrolyte. Hence, the capacitance decrease observed in Fig. 8 during repeated cycling of a capacitor in this medium is due to the deterioration of the negative electrode. On the contrary, from Fig. 9b it is clear that at pH 10 both electrodes work in the electrochemical stability window where there is not any irreversible reaction producing either Mn(II) or Mn(VII). In this electrolyte, the initial potential for both electrodes of the discharged capacitor is 0.66 V vs. NHE and, after charging the capacitor up to 0.6 V, the potential of the negative electrode decreases to 0.38 V, very far from the value of 0.05 V needed for the destructive reaction Mn(IV) to Mn(II) at pH 10.38 This fact explains the good cycle life observed in Fig. 8 for a capacitor based on  and an electrolyte of pH 10.
and an electrolyte of pH 10.
Figure 9. Galvanostatic charge/discharge cycle  mA/g) for the capacitor and for the positive and negative electrodes in a three-electrode cell, where the working and auxiliary electrodes are made from the
mA/g) for the capacitor and for the positive and negative electrodes in a three-electrode cell, where the working and auxiliary electrodes are made from the  /CNTs composite with 15 wt % of CNTs. The results are presented vs. NHE at two different values of pH: (a) pH 6.4; (b) pH 10.
/CNTs composite with 15 wt % of CNTs. The results are presented vs. NHE at two different values of pH: (a) pH 6.4; (b) pH 10.
The results presented above demonstrate that irreversible electrode reactions leading to a specific capacitance loss with time can be avoided by controlling the pH of the electrolytic solution. However, Fig. 9b shows that using the electrolyte with pH 10, the positive electrode is just working at the limit of the irreversible transformation of manganese oxide to Mn(VII). As a consequence, the operating voltage of this capacitor cannot be increased to a value higher than 0.6 V.
The value of specific capacitance obtained in a two-electrode cell for amorphous manganese oxide prepared with 15 wt % of carbon nanotubes, ca. 140 F/g (of total electrode mass), seems to be lower than some of the values presented in the literature for amorphous manganese oxide prepared with 25 wt % of acetylene black, ca. 200 F/g.6 21 These promising results were obtained in three-electrode cell devices, as all the other studies on the use of manganese oxide as an electrode material for capacitors.16 17 18 19 20 21 22 23 24 25 The following part demonstrates that the values of specific capacitance are overestimated when they are obtained from a three-electrode configuration.
In a two-electrode cell, each electrode works in a different potential range. Taking into account the experiment presented in Fig. 9a for pH 6.4, it is very easy to observe that after charging the capacitor to 0.6 V, the polarization of the negative electrode  V) is larger than the polarization of the positive electrode
V) is larger than the polarization of the positive electrode  V). The specific capacitance calculated per gram of electrode material is 133 and 144 F/g for the negative and the positive electrode, respectively. The same effect is observed in Fig. 9b for pH 10, where the negative electrode is working in a potential range of 0.28 V giving a specific capacitance of 150 F/g, while the range is 0.32 V for the positive electrode, giving a specific capacitance of 134 F/g. Hence, it is clear that the specific capacitance of each
V). The specific capacitance calculated per gram of electrode material is 133 and 144 F/g for the negative and the positive electrode, respectively. The same effect is observed in Fig. 9b for pH 10, where the negative electrode is working in a potential range of 0.28 V giving a specific capacitance of 150 F/g, while the range is 0.32 V for the positive electrode, giving a specific capacitance of 134 F/g. Hence, it is clear that the specific capacitance of each  electrode depends on its working potential range. In addition, according to the known relationship
electrode depends on its working potential range. In addition, according to the known relationship
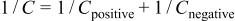
the capacitance measured in a two-electrode cell is mainly imposed by the electrode with the smallest capacitance.
Completely different capacitance values are determined when the  /CNTs composite is tested in a three-electrode cell being used only as a working electrode, with a Pt wire as an auxiliary electrode and
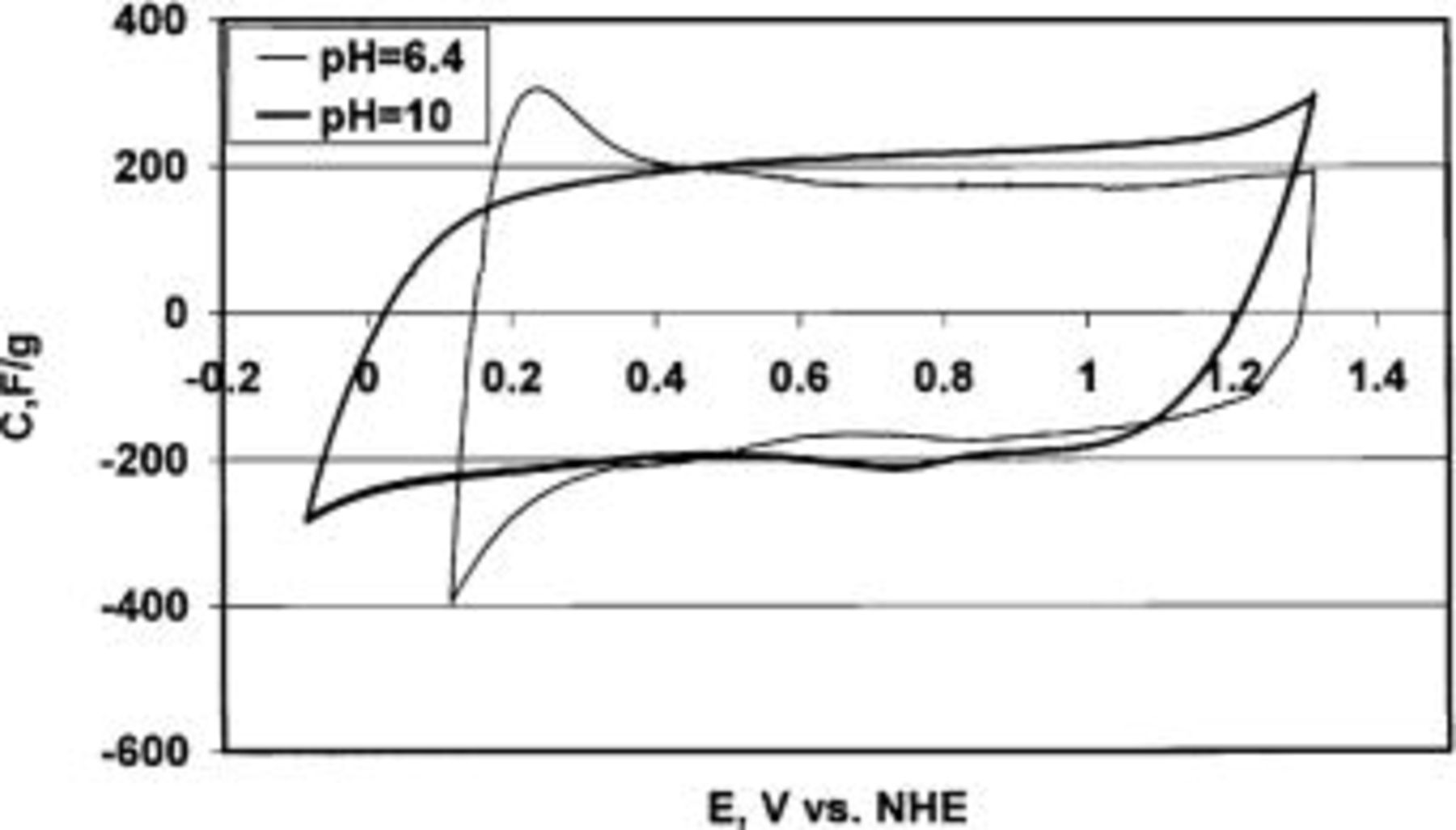
/CNTs composite is tested in a three-electrode cell being used only as a working electrode, with a Pt wire as an auxiliary electrode and  as the reference electrode. Figure 10 presents the cyclic voltammograms of the
as the reference electrode. Figure 10 presents the cyclic voltammograms of the  /CNTs composite obtained at pH 6.4 and 10 with a 2 mV/s scan rate of potential. First, Fig. 10 confirms that the possible working potential of
/CNTs composite obtained at pH 6.4 and 10 with a 2 mV/s scan rate of potential. First, Fig. 10 confirms that the possible working potential of  depends on the pH of electrolyte. At pH 10,
depends on the pH of electrolyte. At pH 10,  can be polarized with smaller potential values than at pH 6.4. In the latter case, some redox process starts to take place at ca. 0.4 V, as shown by the peak appearing from this potential, due to the Mn(IV) to Mn(II) transformation. An opposite behavior is rather found under positive polarization at pH 6.4, for which the electrode can be reversibly polarized at potentials as high as 1.2 V, whereas at pH 10, the potential corresponding to the Mn(IV) to Mn(VII) transformation should not exceed 1.0 V.
can be polarized with smaller potential values than at pH 6.4. In the latter case, some redox process starts to take place at ca. 0.4 V, as shown by the peak appearing from this potential, due to the Mn(IV) to Mn(II) transformation. An opposite behavior is rather found under positive polarization at pH 6.4, for which the electrode can be reversibly polarized at potentials as high as 1.2 V, whereas at pH 10, the potential corresponding to the Mn(IV) to Mn(VII) transformation should not exceed 1.0 V.
Figure 10. Cyclic voltammetry in a three-electrode cell system using the  /CNTs composite (with 15 wt % of CNTs) as working electrode, Pt as the auxiliary electrode, and
/CNTs composite (with 15 wt % of CNTs) as working electrode, Pt as the auxiliary electrode, and  as the reference electrode at pH 6.4 and pH 10. Scan rate of potential, 2 mV/s.
as the reference electrode at pH 6.4 and pH 10. Scan rate of potential, 2 mV/s.
When the specific capacitance is calculated from the CV curves presented in Fig. 10 for the three-electrode cell, the values obtained are 230 F/g (total mass of electrode) and 200 F/g for pH 6.4 and 10, respectively, that are similar to the maximum values presented by other authors.16
21 The reasons for such noticeable differences depending on whether using a two- or a three-electrode cell can be interpreted by comparing the data presented in Fig. 9 and 10. In the three-electrode cell, the total potential range of the working electrode is much higher than the operating potential range of each electrode in the two-electrode cell (ca. 0.3 V). In an ideal capacitor, the capacitance should not depend on the potential window. However, when the electrode is from a material with pseudo-capacitance properties such as  the capacitance is the result of the overlapping of several redox reactions, each of them occurring in a given potential range. Thus, taking into account that operating with a higher potential range, additional redox processes can take place, this explains why high values of specific capacitance are only determined in the three-electrode configuration. Hence, when a material with pseudo-capacitance properties, e.g.,
the capacitance is the result of the overlapping of several redox reactions, each of them occurring in a given potential range. Thus, taking into account that operating with a higher potential range, additional redox processes can take place, this explains why high values of specific capacitance are only determined in the three-electrode configuration. Hence, when a material with pseudo-capacitance properties, e.g.,  is used for both electrodes of a symmetric capacitor, the data found with a three-electrode configuration cannot be extrapolated.
is used for both electrodes of a symmetric capacitor, the data found with a three-electrode configuration cannot be extrapolated.
Although our results state clearly that symmetric capacitors based on  can operate reversibly in a voltage of 0.6 V, Fig. 10 shows that at pH 6.4 the
can operate reversibly in a voltage of 0.6 V, Fig. 10 shows that at pH 6.4 the  /CNTs composite remains a very promising material for a positive electrode, allowing the polarization to be extended up to 1.2 V. Taking into account that
/CNTs composite remains a very promising material for a positive electrode, allowing the polarization to be extended up to 1.2 V. Taking into account that  is irreversibly dissolved at low potentials, an interesting alternative to circumvent this problem in a neutral medium would be to use
is irreversibly dissolved at low potentials, an interesting alternative to circumvent this problem in a neutral medium would be to use  only for the positive electrode and another material for the negative electrode. Such so-called asymmetric systems have been already successfully tested with conducting polymers39
40
41 and activated carbon as the positive and negative electrodes, respectively. Recently, it has been confirmed that a relatively high voltage can be reached in an asymmetric system based on manganese oxide as the positive electrode, and activated carbon as the negative one.42
43 We are presently investigating this system in order to better understand the respective roles of both electrodes in the charge-storage mechanism.
only for the positive electrode and another material for the negative electrode. Such so-called asymmetric systems have been already successfully tested with conducting polymers39
40
41 and activated carbon as the positive and negative electrodes, respectively. Recently, it has been confirmed that a relatively high voltage can be reached in an asymmetric system based on manganese oxide as the positive electrode, and activated carbon as the negative one.42
43 We are presently investigating this system in order to better understand the respective roles of both electrodes in the charge-storage mechanism.
Conclusions
For the first time, the performance of manganese oxide has been studied in a real two-electrode system, using  /carbon nanotubes composites as electrode material. As compared to carbon black, carbon nanotubes are effective for increasing the capacitance and improving the electrochemical properties of the
/carbon nanotubes composites as electrode material. As compared to carbon black, carbon nanotubes are effective for increasing the capacitance and improving the electrochemical properties of the  electrodes which show an ideal box-like shape of voltammetry characteristics. Hence, multiwalled carbon nanotubes are a very promising material as a conductive additive for capacitor or battery electrodes, replacing the conventional carbon black.
electrodes which show an ideal box-like shape of voltammetry characteristics. Hence, multiwalled carbon nanotubes are a very promising material as a conductive additive for capacitor or battery electrodes, replacing the conventional carbon black.
The performance of real capacitors based on manganese oxide is limited by the two irreversible reactions Mn(IV) to Mn(II) and Mn(IV) to Mn(VII), which potential depends on the electrolyte pH. In particular, with real capacitors, the conditions usually used in previous studies with three-electrode cells lead to the dissolution of the negative electrode. However, by choosing the right electrolyte and adjusting its pH, it is possible to improve the electrochemical stability of  /CNTs electrodes for being used in real capacitors. Beside the high influence of the electrolyte on the electrochemical performance, the carbon nanotubes help in preserving the electrodes integrity during cycling. Undoubtedly, owing to their excellent mechanical properties, they can adapt to any volume variation during cycling.
/CNTs electrodes for being used in real capacitors. Beside the high influence of the electrolyte on the electrochemical performance, the carbon nanotubes help in preserving the electrodes integrity during cycling. Undoubtedly, owing to their excellent mechanical properties, they can adapt to any volume variation during cycling.
Acknowledgments
This research was supported by a Marie Curie fellowship of the European Community program "Improving Human Research Potential and the Socio-Economic Knowledge Base" under contract no. HPMF-CT-2001-01453.