Abstract
Mesoporous  film was prepared at low temperatures,
film was prepared at low temperatures,  , on a conductive indium-tin oxide (ITO)-coated polyethylene naphthalate (PEN) plastic sheet by doctor-blade coating of a binder-free paste consisting of nanocrystalline
, on a conductive indium-tin oxide (ITO)-coated polyethylene naphthalate (PEN) plastic sheet by doctor-blade coating of a binder-free paste consisting of nanocrystalline  and interparticle binding agent.
and interparticle binding agent.  -loaded ITO-PEN electrodes were dye-sensitized with Ru bipyridyl complexes to construct photoanodes of plastic dye-sensitized solar cells using iodide/triiodide-based organic redox electrolytes. A solar cell with optimized
-loaded ITO-PEN electrodes were dye-sensitized with Ru bipyridyl complexes to construct photoanodes of plastic dye-sensitized solar cells using iodide/triiodide-based organic redox electrolytes. A solar cell with optimized  particle size and electrolyte composition yielded highest conversion efficiencies of 5.8% and 6.4% for an incident solar energy of 100 and
particle size and electrolyte composition yielded highest conversion efficiencies of 5.8% and 6.4% for an incident solar energy of 100 and  , respectively.
, respectively.
Export citation and abstract BibTeX RIS
Dye-coated mesoscopic titanium oxide films in junction with organic redox electrolytes realize high-efficiency photovoltaic cells, widely known as dye-sensitized solar cell (DSSC). DSSC is the sole utility-type organic solar cell manufactured in the ambient atmosphere without vacuum processes. The last decade has seen a large amount of research and development on improvements of DSSC, which are reflected by numerous patent applications following the pioneering invention by the Grätzel's group.1, 2 Record efficiency of DSSC in power generation has exceeded 11%3, 4 using a small test cell made by high-temperature sintering of a nanocrystalline  paste on an F-doped
paste on an F-doped  (FTO)-coated transparent conductive glass electrode. While competing in efficiency, superiority of DSSC over the conventional Si-based solar batteries is evident in its high environmental performance attained by low-cost high-speed manufacture processes. In particular, a dramatic cost reduction on high-volume manufacturing can be realized when
(FTO)-coated transparent conductive glass electrode. While competing in efficiency, superiority of DSSC over the conventional Si-based solar batteries is evident in its high environmental performance attained by low-cost high-speed manufacture processes. In particular, a dramatic cost reduction on high-volume manufacturing can be realized when  coating, electrolyte sealing, and cell fabrication are conducted by continuous roll-to-roll printing processes using a flexible electrode material. Flexible photovoltaic cells manufactured thereby are open for versatile use and meet recent rapidly growing technologies for industrialization of plastic electronics. Lightweight, bendable solar cells can be attached on rounded surfaces and are suitable for mobile ubiquitous powers. In this respect, our research has been focusing on construction of plastic DSSCs by developing low-temperature semiconductor coating processes. Previously, several methods have been proposed for construction of plastic electrodes for DSSCs. Among those which have attained high photovoltaic performance are mechanical compression of nanocrystalline semiconductor powder5, 6 and hydrothermal synthesis of metal oxide nanocrystals.7 We have demonstrated electrophoretic deposition of nanocrystals as a rapid and efficient means for low-temperature coating of mesoporous
coating, electrolyte sealing, and cell fabrication are conducted by continuous roll-to-roll printing processes using a flexible electrode material. Flexible photovoltaic cells manufactured thereby are open for versatile use and meet recent rapidly growing technologies for industrialization of plastic electronics. Lightweight, bendable solar cells can be attached on rounded surfaces and are suitable for mobile ubiquitous powers. In this respect, our research has been focusing on construction of plastic DSSCs by developing low-temperature semiconductor coating processes. Previously, several methods have been proposed for construction of plastic electrodes for DSSCs. Among those which have attained high photovoltaic performance are mechanical compression of nanocrystalline semiconductor powder5, 6 and hydrothermal synthesis of metal oxide nanocrystals.7 We have demonstrated electrophoretic deposition of nanocrystals as a rapid and efficient means for low-temperature coating of mesoporous  layer.8–10 Combined with chemical treatments of as-deposited
layer.8–10 Combined with chemical treatments of as-deposited  particles for interparticle connection, the electrode sensitized with Ru complex dye reached a maximum conversion efficiency of 4.3% on a plastic substrate.9 These preparation methods required two steps begun with particle deposition and completed with interparticle connection. In this article, we report preparation of a rare binder-free nanocrystalline
particles for interparticle connection, the electrode sensitized with Ru complex dye reached a maximum conversion efficiency of 4.3% on a plastic substrate.9 These preparation methods required two steps begun with particle deposition and completed with interparticle connection. In this article, we report preparation of a rare binder-free nanocrystalline  paste for single-step coating of mesoporous
paste for single-step coating of mesoporous  on plastic electrodes. High conversion efficiency, exceeding 6%, is obtained by optimization for particle size and film thickness, which is easily controlled by the loading of paste using doctor-blade coater or screen printer.
on plastic electrodes. High conversion efficiency, exceeding 6%, is obtained by optimization for particle size and film thickness, which is easily controlled by the loading of paste using doctor-blade coater or screen printer.
Experimental
Highly crystalline  particles of various size ranges were prepared by gas phase pyrolysis from titanium tetrachloride and supplied from Showa Titanium Co. Ltd.
particles of various size ranges were prepared by gas phase pyrolysis from titanium tetrachloride and supplied from Showa Titanium Co. Ltd.  nanocrystalline particles employed are rutile/anatase mixture of various average sizes of
nanocrystalline particles employed are rutile/anatase mixture of various average sizes of  . They were mixed with large
. They were mixed with large  particles of average size
particles of average size  (anatase content 95%) to enhance optical absorption of visible light light-scattering effect.11, 12 Table I shows the size, Brunauer-Emmett-Teller (BET) surface area, and rutile content of the particles examined in this study. An aqueous colloidal sol of titanium oxide was used as an interparticle connection agent, which is an opaque solution containing brookite-type nanocrystalline
(anatase content 95%) to enhance optical absorption of visible light light-scattering effect.11, 12 Table I shows the size, Brunauer-Emmett-Teller (BET) surface area, and rutile content of the particles examined in this study. An aqueous colloidal sol of titanium oxide was used as an interparticle connection agent, which is an opaque solution containing brookite-type nanocrystalline  with a size distribution of
with a size distribution of  at a concentration of
at a concentration of  in a mixture of 25% aqueous hydrochloric acid (pH 4) and 75% ethanol. Indium-tin oxide (ITO)-coated polyethylene naphthalate (PEN) was selected as a transparent conductive plastic electrode (thickness
in a mixture of 25% aqueous hydrochloric acid (pH 4) and 75% ethanol. Indium-tin oxide (ITO)-coated polyethylene naphthalate (PEN) was selected as a transparent conductive plastic electrode (thickness  , sheet resistance
, sheet resistance  , transmittance 80%).
, transmittance 80%).  of PEN,
of PEN,  , practically requires that
, practically requires that  coating processes be made in a temperature range less than
coating processes be made in a temperature range less than  . To prepare a
. To prepare a  nanocrystalline paste that gives a dry mesoporous film works on a plastic substrate without high-temperature sintering process, several key factors should be satisfied. First, sufficiently high viscosity as a coating paste should be obtained without use of polymer binder materials, which remain as an insulating core in the dried
nanocrystalline paste that gives a dry mesoporous film works on a plastic substrate without high-temperature sintering process, several key factors should be satisfied. First, sufficiently high viscosity as a coating paste should be obtained without use of polymer binder materials, which remain as an insulating core in the dried  film and blocks carrier transports between particles. Second, the wet paste should develop high adhesion to the hydrophobic surface of the plastic substrate; ITO surface of PEN shows hydrophobicity without particular treatments. Third, any liquid component of the paste should be finally evaporated at low temperatures
film and blocks carrier transports between particles. Second, the wet paste should develop high adhesion to the hydrophobic surface of the plastic substrate; ITO surface of PEN shows hydrophobicity without particular treatments. Third, any liquid component of the paste should be finally evaporated at low temperatures  . Finally, all components of the paste should be inert so that corrosion of ITO is avoided; acidic precursors, such as
. Finally, all components of the paste should be inert so that corrosion of ITO is avoided; acidic precursors, such as  , useful for interparticle connection deteriorate ITO and cannot be applied. To realize the paste fulfilling the above requirements,
, useful for interparticle connection deteriorate ITO and cannot be applied. To realize the paste fulfilling the above requirements,  nanoparticles were dispersed in a mixed solvent of water and
nanoparticles were dispersed in a mixed solvent of water and  -butanol at volume ration of 1:2.
-butanol at volume ration of 1:2.  -butanol reduces the surface tension of the liquid paste to improve its adhesion to the ITO-PEN surface. Large
-butanol reduces the surface tension of the liquid paste to improve its adhesion to the ITO-PEN surface. Large  particle
particle  of light scattering function was mixed to the above nanoparticle dispersion. An aqueous colloidal
of light scattering function was mixed to the above nanoparticle dispersion. An aqueous colloidal  sol was added to the dispersion as a kind of cement agent to chemically connect nanoparticles.9 The resulting suspension was mixed on a rotation-revolution type mixer at room temperature avoiding the use of ultrasonic treatment that can cause aggregation of nanoparticles. Viscosity was adjusted by the content of water that is mainly brought by the aqueous sol. Viscosity was highly dependent on the ratio of water and
sol was added to the dispersion as a kind of cement agent to chemically connect nanoparticles.9 The resulting suspension was mixed on a rotation-revolution type mixer at room temperature avoiding the use of ultrasonic treatment that can cause aggregation of nanoparticles. Viscosity was adjusted by the content of water that is mainly brought by the aqueous sol. Viscosity was highly dependent on the ratio of water and  -butanol and decreases abruptly by increasing the content of either of water or
-butanol and decreases abruptly by increasing the content of either of water or  -butanol. The paste obtained shows high stability during long-term preservation at room temperature. Viscosity of the optimized binder-free paste reached around
-butanol. The paste obtained shows high stability during long-term preservation at room temperature. Viscosity of the optimized binder-free paste reached around  after adjusting the water content. A similar binder-free paste has been recently prepared by Park et al. , who utilized acid-base interaction to increase viscosity of nano-
after adjusting the water content. A similar binder-free paste has been recently prepared by Park et al. , who utilized acid-base interaction to increase viscosity of nano- colloid.13 Their paste has, however, not been applied to plastic substrates.
colloid.13 Their paste has, however, not been applied to plastic substrates.
Table I. Crystalline  particles of different average sizes used in this study.
particles of different average sizes used in this study.
| Average size (nm) | Nano-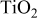 particles particles | Large 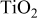
| |||
|---|---|---|---|---|---|
| 30 | 60 | 90 | 150 | 250 | |
BET area (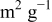 ) ) | 35–60 | 25–35 | 15–25 | 9–14 | 4.5–7.5 |
| Rutile content (%) |
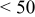
|
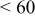
|
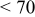
|
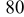
|
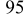
|
The binder-free  paste was coated on an ITO-PEN sheet by a doctor-blade coater or by a screen printer under adjustment of the wet film thickness. The resulted wet coating was allowed for drying at room temperature and, to eliminate water, the film was dried again at
paste was coated on an ITO-PEN sheet by a doctor-blade coater or by a screen printer under adjustment of the wet film thickness. The resulted wet coating was allowed for drying at room temperature and, to eliminate water, the film was dried again at  . A mesoporous
. A mesoporous  film of uniform thickness was formed, which tightly attached to the ITO surface. The amount of
film of uniform thickness was formed, which tightly attached to the ITO surface. The amount of 
 loaded on ITO-PEN could be controlled by adjusting the wet layer thickness based on good linearity established between the
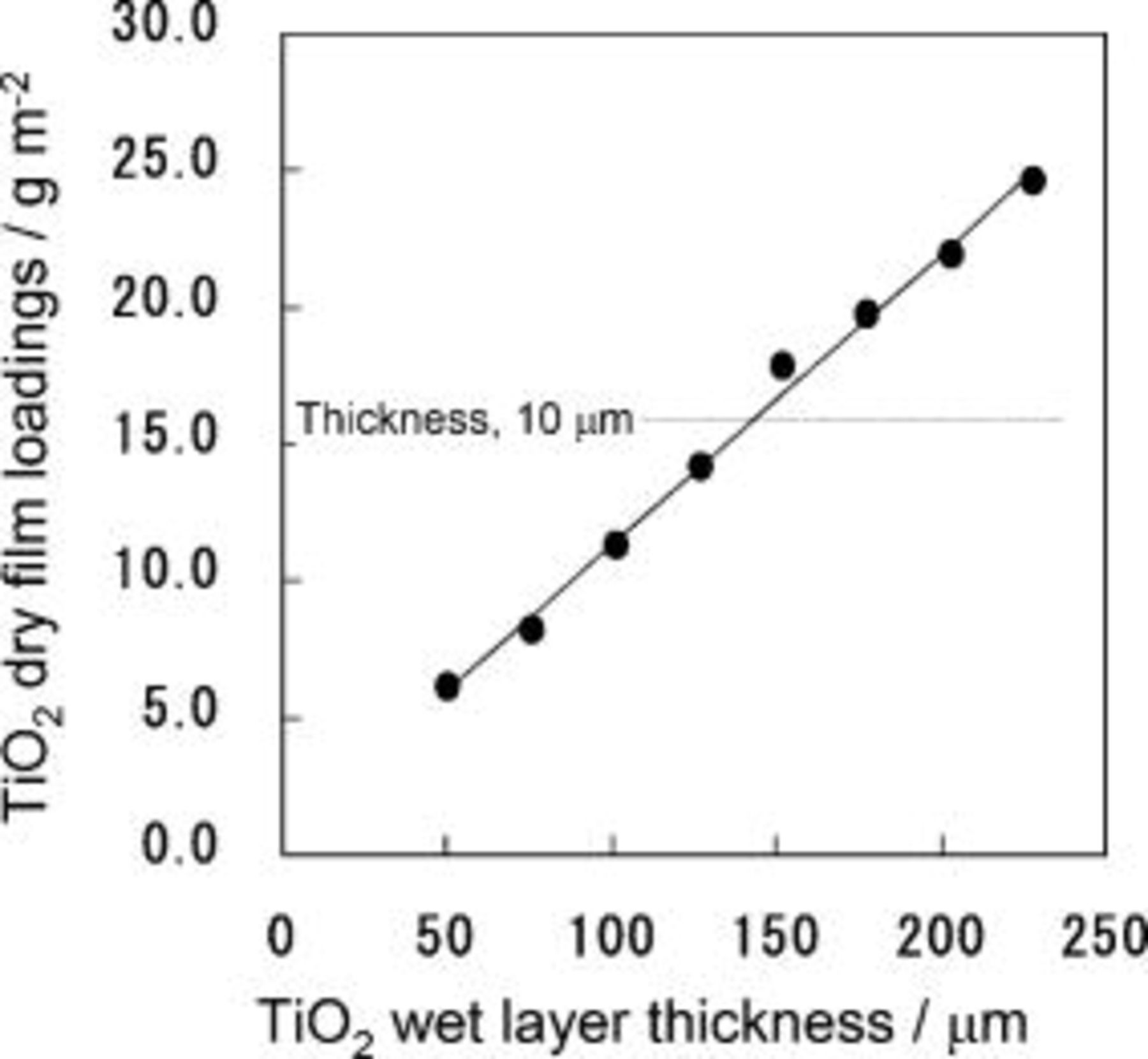
loaded on ITO-PEN could be controlled by adjusting the wet layer thickness based on good linearity established between the  loading and thickness of the wet layer as exhibited in Fig. 1. Thickness of dry
loading and thickness of the wet layer as exhibited in Fig. 1. Thickness of dry  film was proportional to the amount of
film was proportional to the amount of  loading:
loading:  thickness corresponds to
thickness corresponds to 

 . The
. The  film loaded on ITO-PEN sheet showed high anti-exfoliation against bending, giving anti-scratching pencil hardness of more than H. Figure 2 exhibits a
film loaded on ITO-PEN sheet showed high anti-exfoliation against bending, giving anti-scratching pencil hardness of more than H. Figure 2 exhibits a  -coated ITO-PEN sheet under bending, with its surface drawn by pencil and ballpoint pen. Exfoliation was prevented even when the
-coated ITO-PEN sheet under bending, with its surface drawn by pencil and ballpoint pen. Exfoliation was prevented even when the  -coated sheet is bent to a curvature up to
-coated sheet is bent to a curvature up to  (
( diam).
diam).
Figure 1. Linear relationship between the weight loading of dry mesoporous  film and the wet-layer thickness of
film and the wet-layer thickness of  paste coated on the substrate. Inserted line indicates the level of loading giving a film thickness of
paste coated on the substrate. Inserted line indicates the level of loading giving a film thickness of  .
.
Figure 2. (Color online)  -coated ITO-PEN sheet bearing a tightly adhered mesoporous
-coated ITO-PEN sheet bearing a tightly adhered mesoporous  film (before dye sensitization) of pencil hardness H on which writing was made by pencil and ballpoint pen.
film (before dye sensitization) of pencil hardness H on which writing was made by pencil and ballpoint pen.
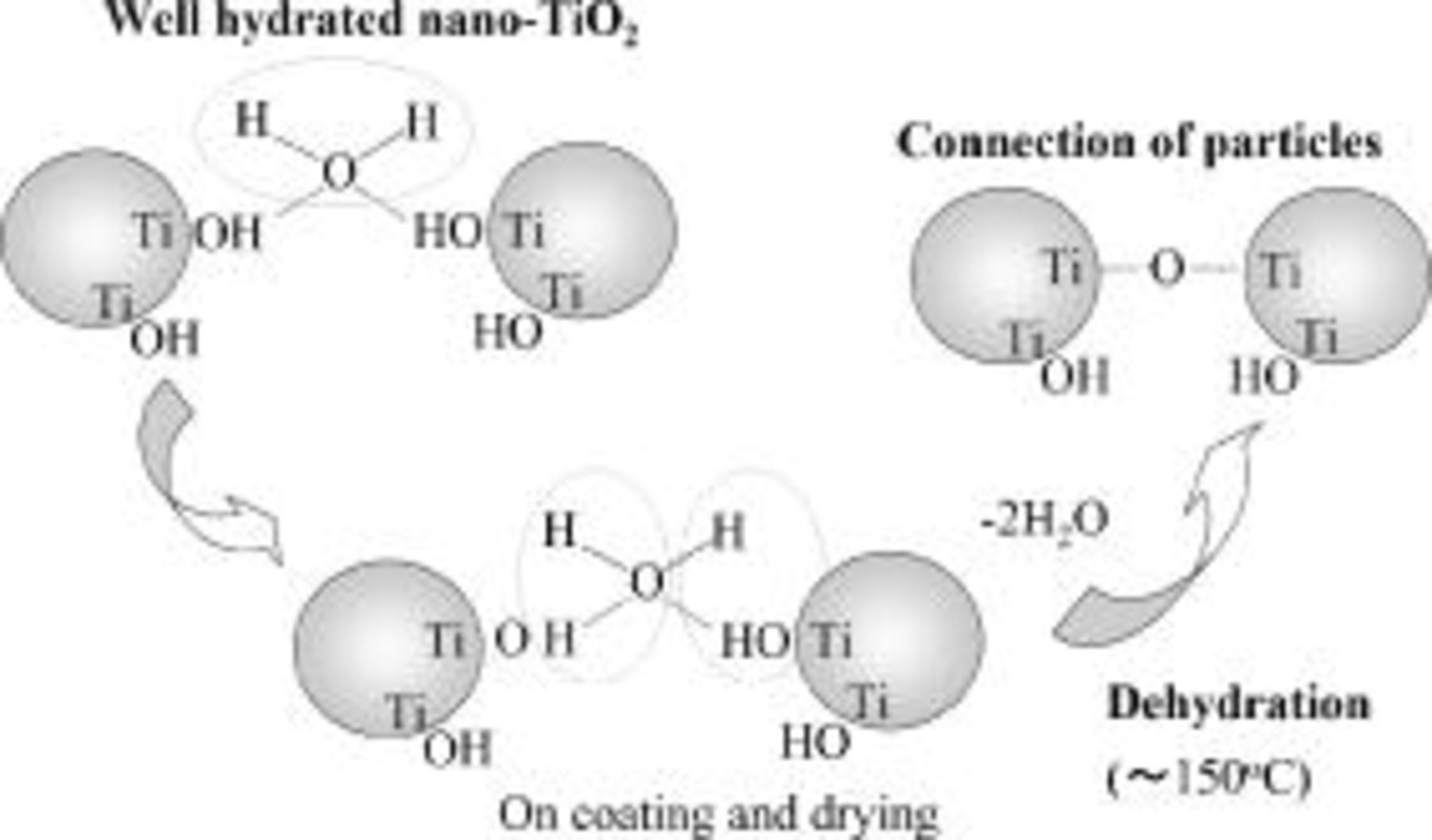
On drying of the paste, interparticle connection is assumed to proceed by dehydration of hydrogen-bonded network of  nanoparticles. This hydrogen-bonded mesoscopic network is effectively introduced by the addition of the well-dispersed aqueous sol of
nanoparticles. This hydrogen-bonded mesoscopic network is effectively introduced by the addition of the well-dispersed aqueous sol of  as cement, in which
as cement, in which  surfaces are covered with hydroxyl groups without undergoing aggregation of particles. Figure 3 illustrates the scheme of interparticle connection in the course of drying the binder-free paste. It is assumed that the hydrogen-bonding connection of main particles (mostly aggregated particles) is enhanced by the use of aqueous dispersion of nonaggregated
surfaces are covered with hydroxyl groups without undergoing aggregation of particles. Figure 3 illustrates the scheme of interparticle connection in the course of drying the binder-free paste. It is assumed that the hydrogen-bonding connection of main particles (mostly aggregated particles) is enhanced by the use of aqueous dispersion of nonaggregated  nanoparticles which produce a large amount of hydroxy groups to react with the surface of main particles.
nanoparticles which produce a large amount of hydroxy groups to react with the surface of main particles.
Figure 3. A mechanism for interparticle connection (necking) of nanocrystalline  particles dispersed in an aqueous medium via formation of hydrogen-bonded network of particles with surfaces covered with hydroxy groups. Mesoporous film is formed by drying the
particles dispersed in an aqueous medium via formation of hydrogen-bonded network of particles with surfaces covered with hydroxy groups. Mesoporous film is formed by drying the  paste at low temperature
paste at low temperature  that eliminates water.
that eliminates water.
The  -coated ITO-PEN was dye-sensitized by Ru bipyridyl complex dyes, N719 or N712, by dipping in a
-coated ITO-PEN was dye-sensitized by Ru bipyridyl complex dyes, N719 or N712, by dipping in a  dye solution with a mixture of
dye solution with a mixture of  -butanol:acetonitrile:ethanol (2:1:1) at
-butanol:acetonitrile:ethanol (2:1:1) at  for
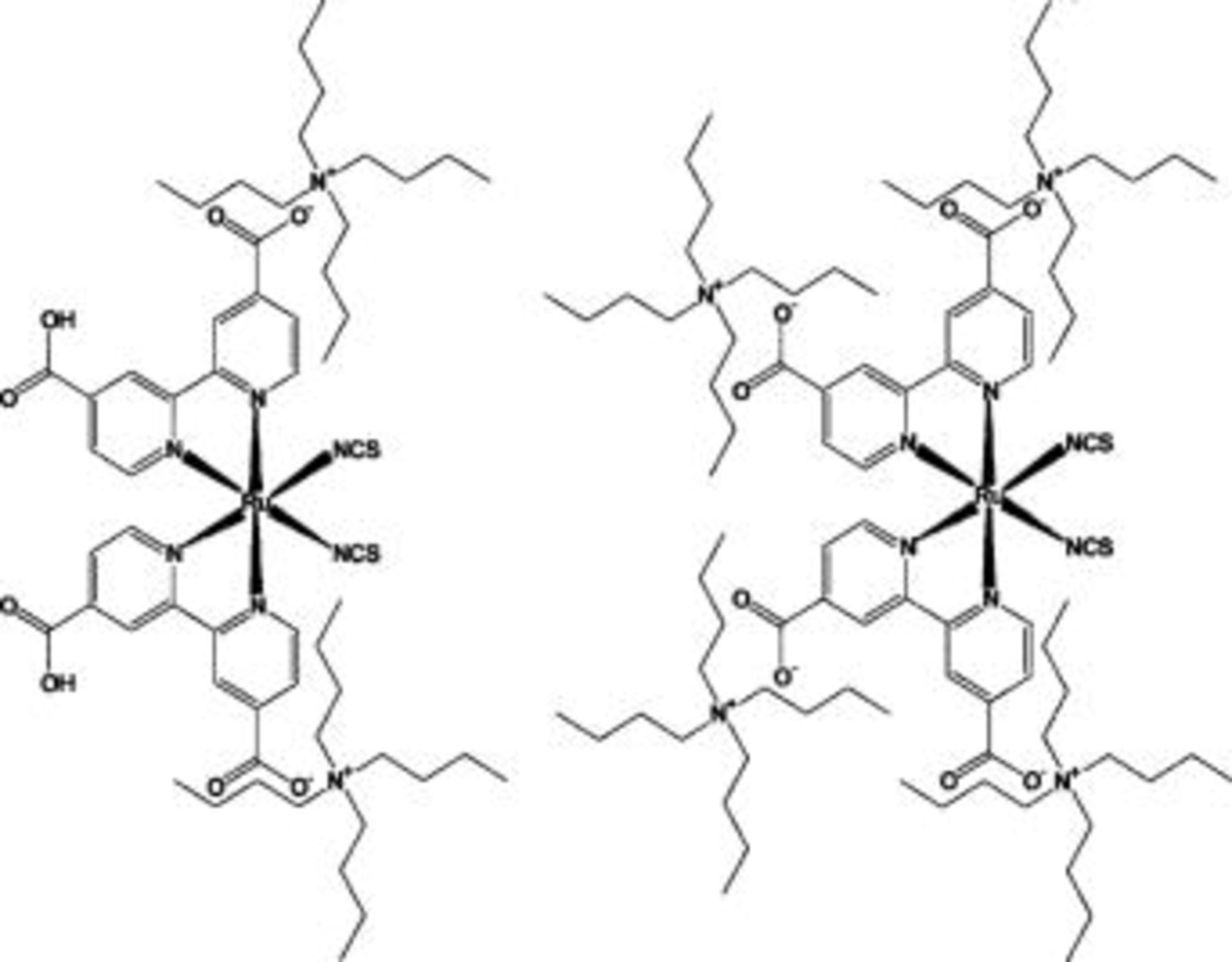
for  under a slowly stirring condition. Figure 4 shows the structure of N719 and N712. N719, cis-bis(isothiocyanato)bis(
under a slowly stirring condition. Figure 4 shows the structure of N719 and N712. N719, cis-bis(isothiocyanato)bis( -bipyridyl-
-bipyridyl- -dicarboxylato)ruthenium(II)bis-tetrabutylammonium, bears two tetrabutylammonium (TBA) groups as the counter cation of carboxy group that anchors on the
-dicarboxylato)ruthenium(II)bis-tetrabutylammonium, bears two tetrabutylammonium (TBA) groups as the counter cation of carboxy group that anchors on the  surface. N712, on the other hand, bears four TBA groups that substitute protons of all carboxy groups. The effect of these two dyes was investigated on the concept that TBA substitution in the Ru complex dye affects the photovoltage of dye-sensitized electrode.14 The photoelectrode was combined with a platinum-coated FTO glass counter electrode by insertion of a
surface. N712, on the other hand, bears four TBA groups that substitute protons of all carboxy groups. The effect of these two dyes was investigated on the concept that TBA substitution in the Ru complex dye affects the photovoltage of dye-sensitized electrode.14 The photoelectrode was combined with a platinum-coated FTO glass counter electrode by insertion of a  thick organic electrolyte layer using a hot-melt type gasket film as a spacer and sealer (Himilan, DuPont-Mitsui Polychemicals, Japan). For enhancement of cell performance, optimization was also done for liquid electrolyte. Four different compositions of electrolyte were examined, which employed a common iodide/triiodide redox components comprising
thick organic electrolyte layer using a hot-melt type gasket film as a spacer and sealer (Himilan, DuPont-Mitsui Polychemicals, Japan). For enhancement of cell performance, optimization was also done for liquid electrolyte. Four different compositions of electrolyte were examined, which employed a common iodide/triiodide redox components comprising  LiI,
LiI,  tetrabutylammonium iodide (TBAI), and
tetrabutylammonium iodide (TBAI), and 
 . With these redox components, different additives and solvents were employed. 4-tert-butylpyridine (TBP) is a conventional additive known to significantly improve photovoltage by suppressing charge recombination (back electron transfer) at the
. With these redox components, different additives and solvents were employed. 4-tert-butylpyridine (TBP) is a conventional additive known to significantly improve photovoltage by suppressing charge recombination (back electron transfer) at the  -electrolyte interface.15 Electrolyte A contained
-electrolyte interface.15 Electrolyte A contained  TBP in 3-methoxypropionitrile (MPN). Electrolyte B contained
TBP in 3-methoxypropionitrile (MPN). Electrolyte B contained  N-methylbenzimidazole (NMB) in MPN as an alternative to TBP. Electrolyte C used the same components as electrolyte B in acetonitrile (AN). Electrolyte D used the same components as electrolyte B dissolved in a mixture solvent AN/MPN (vol ratio 1:1). The photocell assembled was attached an optical shielding mask to confine an effective irradiation area as
N-methylbenzimidazole (NMB) in MPN as an alternative to TBP. Electrolyte C used the same components as electrolyte B in acetonitrile (AN). Electrolyte D used the same components as electrolyte B dissolved in a mixture solvent AN/MPN (vol ratio 1:1). The photocell assembled was attached an optical shielding mask to confine an effective irradiation area as  .
.
Figure 4. Structures of Ru bipyridyl complexes as sensitizers of the mesoporous  electrode. N719 (left) and N712 (right) bear two and four tetrabutyl ammonium (TBA) groups, respectively, as the countercation of carboxy group that anchors on the
electrode. N719 (left) and N712 (right) bear two and four tetrabutyl ammonium (TBA) groups, respectively, as the countercation of carboxy group that anchors on the  surface. These dyes have different effects on the Fermi level of
surface. These dyes have different effects on the Fermi level of  (see text).
(see text).
Photocurrent density-voltage (I-V) characteristics was measured by exposing the cell to air mass (AM) 1.5 simulated sunlight of a solar simulator (Peccell Technologies, L11) at room temperature in combination with Keithley 2400 source meter and I-V curve analyzer (Peccell, PECK2400). I-V curves were recorded by averaging currents generated by the forward and back scanning of voltage with data accumulation at a step of  . Incident intensity was determined by use of a silicon photocell with its photocurrent amplitude correlated for the intensity of standard solar irradiation and was set as
. Incident intensity was determined by use of a silicon photocell with its photocurrent amplitude correlated for the intensity of standard solar irradiation and was set as  (1 sun). Performance with weak intensity light was also examined at
(1 sun). Performance with weak intensity light was also examined at  (
( sun) by using a neutral density filter (S73, Suruga Seiki Ltd.). Incident photon-to-current conversion quantum efficiency (IPCE) was measured as an action spectrum on a Peccell, PEC-S10, which uses an optical fiber (
sun) by using a neutral density filter (S73, Suruga Seiki Ltd.). Incident photon-to-current conversion quantum efficiency (IPCE) was measured as an action spectrum on a Peccell, PEC-S10, which uses an optical fiber ( ) for monochromatic irradiation (incident power,
) for monochromatic irradiation (incident power,  at
at  ). Monochromatic photocurrent was monitored by continuous irradiation (dc measurement) method. All photovoltaic measurements were done at room temperature.
). Monochromatic photocurrent was monitored by continuous irradiation (dc measurement) method. All photovoltaic measurements were done at room temperature.
Results and Discussion
Optimization for film thickness and average particle size
The dry mesoporous  film formed by the binder-free paste coating without sintering gave porosity of about 60%, which is high enough to compare with those of sintered
film formed by the binder-free paste coating without sintering gave porosity of about 60%, which is high enough to compare with those of sintered  films (50–70%) with pores created by firing of binder polymers.16, 17 The construction of the film was optimized to elicit high photovoltaic performance. Effect of the loading of
films (50–70%) with pores created by firing of binder polymers.16, 17 The construction of the film was optimized to elicit high photovoltaic performance. Effect of the loading of  (thickness of
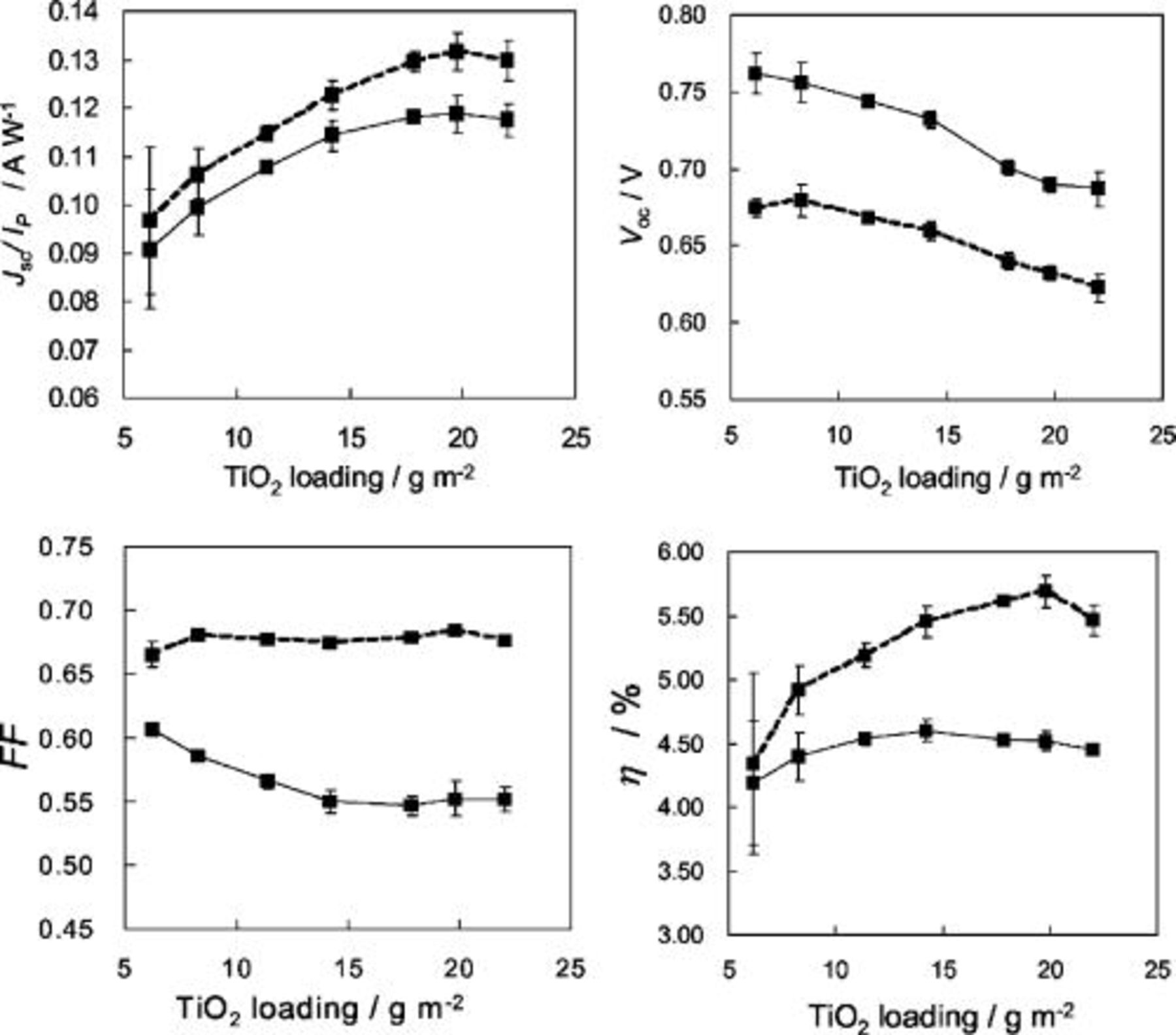
(thickness of  film) on the photovoltaic performance was first investigated with the sensitizer N719 in combination of electrolyte A. Figure 5 shows dependences of short circuit photocurrent density
film) on the photovoltaic performance was first investigated with the sensitizer N719 in combination of electrolyte A. Figure 5 shows dependences of short circuit photocurrent density  , open-circuit voltage
, open-circuit voltage  , fill factor
, fill factor  , and power conversion efficiency
, and power conversion efficiency  on the loading of
on the loading of  per unit area
per unit area  . The results are compared between conditions of high intensity (1 sun) and low intensity (
. The results are compared between conditions of high intensity (1 sun) and low intensity ( sun) irradiances of simulated AM1.5 sunlight, where the amplitude of
sun) irradiances of simulated AM1.5 sunlight, where the amplitude of  is normalized for constant incident intensity per unit area in terms of
is normalized for constant incident intensity per unit area in terms of  . Amplitude of
. Amplitude of  , which largely influences the efficiency, increased with increasing
, which largely influences the efficiency, increased with increasing  loading and showed saturation at around
loading and showed saturation at around  , which corresponds to film thicknesses of
, which corresponds to film thicknesses of  . The saturation in photocurrent indicates that the diffusion length of photoexcited electrons traveling the
. The saturation in photocurrent indicates that the diffusion length of photoexcited electrons traveling the  particle network is limited to less than around
particle network is limited to less than around  . The existence of a constant gap in photocurrent density between high and low irradiances is a sign of an electron transport loss occurring by enhanced electrons generation at high intensity. The above phenomena reflect that there exists still high electric resistance between interconnected
. The existence of a constant gap in photocurrent density between high and low irradiances is a sign of an electron transport loss occurring by enhanced electrons generation at high intensity. The above phenomena reflect that there exists still high electric resistance between interconnected  particles against the electron diffusion as a result of low-temperature preparation of the
particles against the electron diffusion as a result of low-temperature preparation of the  network. This matter directly affects
network. This matter directly affects  , which, in the high irradiance causing high IR drop (ohmic loss), showed a marked decrease with increased
, which, in the high irradiance causing high IR drop (ohmic loss), showed a marked decrease with increased  loading and thickness.
loading and thickness.  showed a simple decrease with increased thickness, apparently due to the resistance of thick
showed a simple decrease with increased thickness, apparently due to the resistance of thick  film. A similar phenomenon has been observed in our previous work with electrophoretically deposited
film. A similar phenomenon has been observed in our previous work with electrophoretically deposited  layers.9 As a result of the above influences, overall energy conversion efficiency
layers.9 As a result of the above influences, overall energy conversion efficiency  did not simply follow the photocurrent increase but was affected by the layer thickness as well as by light intensity. It gave a maximum at
did not simply follow the photocurrent increase but was affected by the layer thickness as well as by light intensity. It gave a maximum at 
 (thickness of
(thickness of  ) for low irradiance (
) for low irradiance ( sun) and at
sun) and at 
 (thickness of
(thickness of  ) for high irradiance (1 sun).
) for high irradiance (1 sun).
Figure 5. Dependences of photocurrent density  , open-circuit voltage
, open-circuit voltage  , fill factor
, fill factor  , and energy conversion efficiency
, and energy conversion efficiency  on the amount of
on the amount of  loading on the ITO-PEN plastic electrode. Photocurrent density is normalized for constant incident power
loading on the ITO-PEN plastic electrode. Photocurrent density is normalized for constant incident power  in terms of
in terms of  . Solid and dashed lines show data measured for high intensity light (1 sun) and low intensity light (
. Solid and dashed lines show data measured for high intensity light (1 sun) and low intensity light ( sun), respectively.
sun), respectively.  films for these measurements contained average
films for these measurements contained average  size nanopartilces mixed with
size nanopartilces mixed with  size large particles.
size large particles.  loading of
loading of  corresponds to a thickness of
corresponds to a thickness of  . Electrolyte A was employed.
. Electrolyte A was employed.
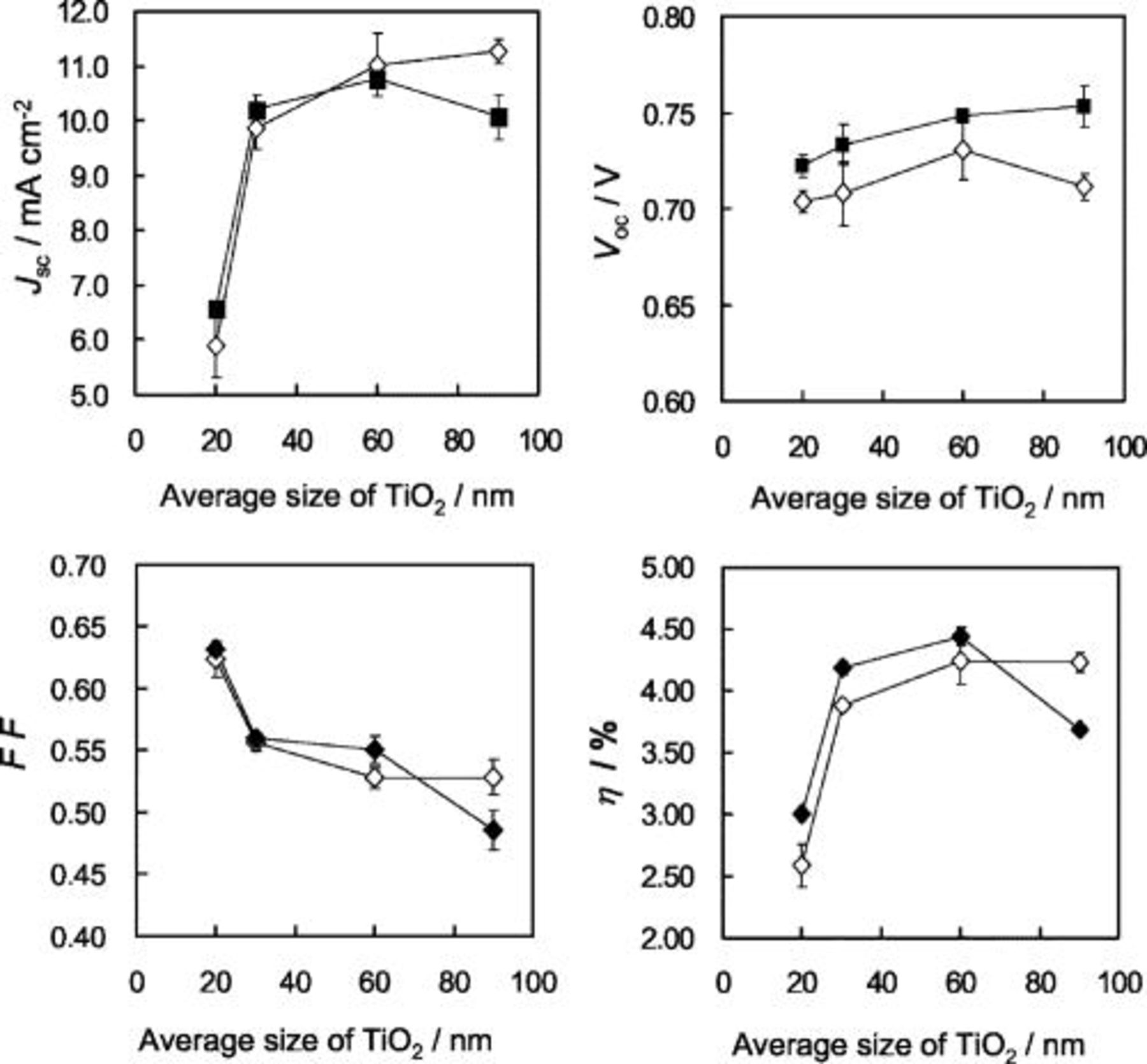
The size of  nanocrystals also showed a dominant effect on the photovoltaic performance. Because high-temperature sintering
nanocrystals also showed a dominant effect on the photovoltaic performance. Because high-temperature sintering  that causes firing of polymer binders and melting of
that causes firing of polymer binders and melting of  nanoparticles is not applied in our preparation, average particle size in the coating paste is assumed to determine the pore size distribution, which influences the diffusion rate of electrolyte into the mesoporous
nanoparticles is not applied in our preparation, average particle size in the coating paste is assumed to determine the pore size distribution, which influences the diffusion rate of electrolyte into the mesoporous  film. Thus, using a relatively thin film with
film. Thus, using a relatively thin film with  loadings of
loadings of  (thickness of
(thickness of  ), average size of nanoparticle was changed for improvement of photovoltaic performance. Figure 6 exhibits the photovoltaic performance (
), average size of nanoparticle was changed for improvement of photovoltaic performance. Figure 6 exhibits the photovoltaic performance ( ,
,  ,
,  , and η) of N719-sensitized electrode as a function of average size of
, and η) of N719-sensitized electrode as a function of average size of  nanoparticles. Size-dependent characteristics are compared between
nanoparticles. Size-dependent characteristics are compared between  films with and without the mixing of the large
films with and without the mixing of the large  particle (content,
particle (content,  ). Both systems with and without the large particle showed similar size-dependent profiles in the photovoltaic performance. The photocurrent density increased with the average size and gave a maximum at a size range more than
). Both systems with and without the large particle showed similar size-dependent profiles in the photovoltaic performance. The photocurrent density increased with the average size and gave a maximum at a size range more than  . The result may indicate that small pore size formed from particle size around
. The result may indicate that small pore size formed from particle size around  is inefficient to yield sufficient density of photocurrent due to suppressed diffusion of electrolyte in the mesopore. In the presence of the large particle, photocurrent tends to decrease in the size range more than
is inefficient to yield sufficient density of photocurrent due to suppressed diffusion of electrolyte in the mesopore. In the presence of the large particle, photocurrent tends to decrease in the size range more than  , which must be influenced by a large decrease in the amount of dye molecules adsorbed on the
, which must be influenced by a large decrease in the amount of dye molecules adsorbed on the  film.
film.  was improved by increasing the average size and by using the
was improved by increasing the average size and by using the  film containing the large particle. This tendency is interpreted as reflecting the decrease of
film containing the large particle. This tendency is interpreted as reflecting the decrease of  surface area, which leads to suppression of the back electron transfer, i.e., dark current, at the
surface area, which leads to suppression of the back electron transfer, i.e., dark current, at the  -electrolyte (iodine) interface.
-electrolyte (iodine) interface.  of the cell, on the other hand, showed a simple decrease with increasing size. This indicates that the size increase is causing an increase in the resistance of the
of the cell, on the other hand, showed a simple decrease with increasing size. This indicates that the size increase is causing an increase in the resistance of the  film. The latter may arise from an increase in the film thickness brought about by the size increase. As a result of the above phenomena, highest power conversion efficiency was yielded at sufficiently large particle size of
film. The latter may arise from an increase in the film thickness brought about by the size increase. As a result of the above phenomena, highest power conversion efficiency was yielded at sufficiently large particle size of  in the presence of the large light-scattering particle. The
in the presence of the large light-scattering particle. The  class particle optimized in the present nonsintering coating method is much larger than
class particle optimized in the present nonsintering coating method is much larger than  normally used for sintering method16 where pore distribution are created by burning of polymer binder materials. This size is also larger than the
normally used for sintering method16 where pore distribution are created by burning of polymer binder materials. This size is also larger than the 
 which we have previously used in the study of binder-free electrophoretic deposition method in which size effect was not optimized.9 Difference in the optimized
which we have previously used in the study of binder-free electrophoretic deposition method in which size effect was not optimized.9 Difference in the optimized  size of the paste as a starting material is apparently related to the use of binders. In sintering, firing of polymer binders stimulates connection of small particles forming new large particles and pores. In the nonsintering binder-free process, pore size distribution is substantially determined by the initial particle size. It is assumed that the mixture of average
size of the paste as a starting material is apparently related to the use of binders. In sintering, firing of polymer binders stimulates connection of small particles forming new large particles and pores. In the nonsintering binder-free process, pore size distribution is substantially determined by the initial particle size. It is assumed that the mixture of average  nanoparticle and
nanoparticle and  particle gives a desirable pore distribution which may resemble the sintered
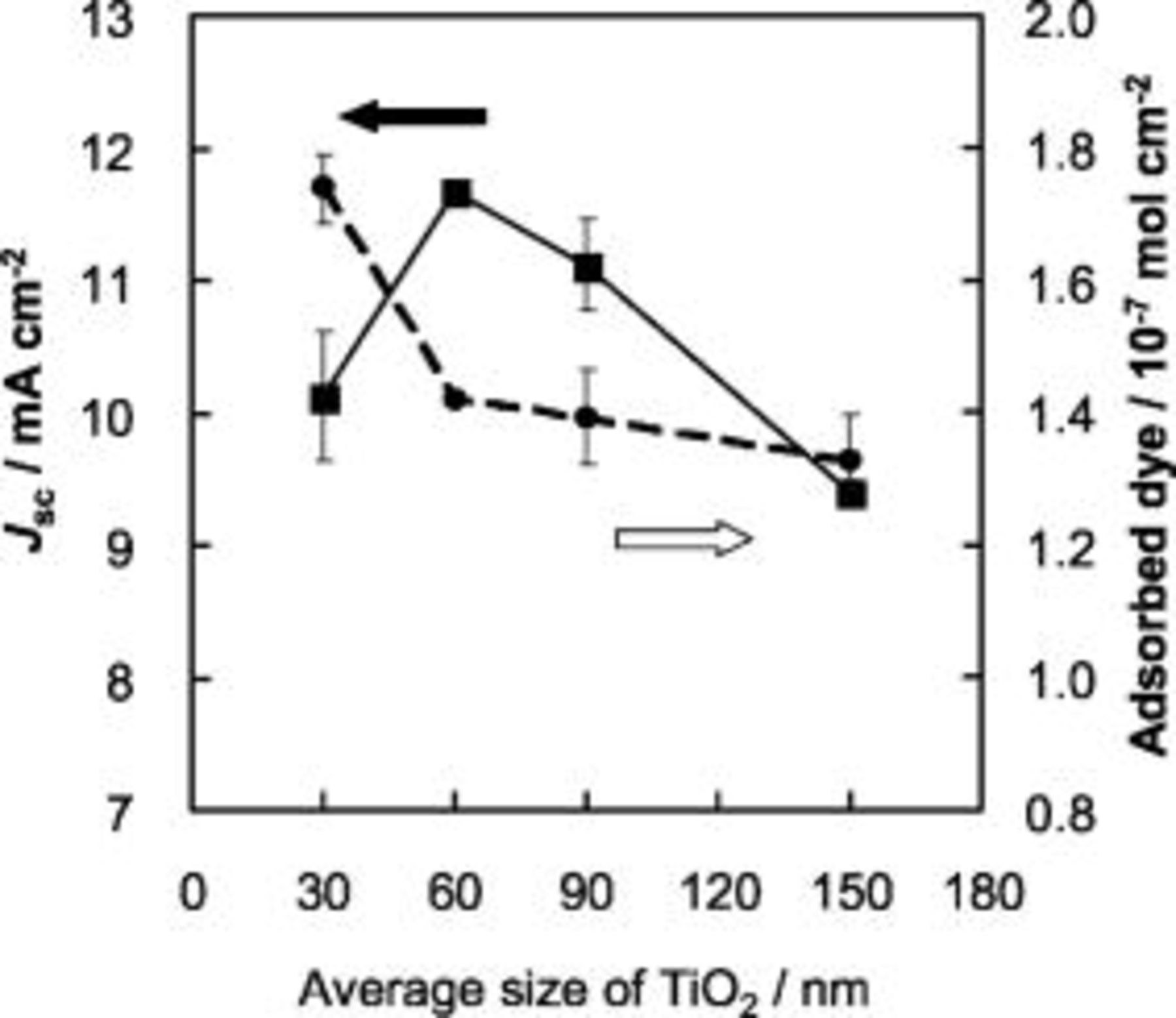
particle gives a desirable pore distribution which may resemble the sintered  network. In Fig. 7, the size-dependent change of photocurrent is exhibited for an extended size range of nanoparticles from
network. In Fig. 7, the size-dependent change of photocurrent is exhibited for an extended size range of nanoparticles from  and compared with the change of the amount of adsorbed dye molecules. The adsorbed dye amount was determined by optical absorption of the dye dissolved in a
and compared with the change of the amount of adsorbed dye molecules. The adsorbed dye amount was determined by optical absorption of the dye dissolved in a  aqueous KOH solution. In this measurement, a high
aqueous KOH solution. In this measurement, a high  loading of
loading of  was employed to enhance the photocurrent density in combination of electrolyte D consisting of
was employed to enhance the photocurrent density in combination of electrolyte D consisting of  LiI,
LiI,  tetrabutylammonium iodide (TBAI),
tetrabutylammonium iodide (TBAI), 
 ,
,  NMB in a mixture of AN and MPN (vol. ratio 1:1). The optimum size for photocurrent of
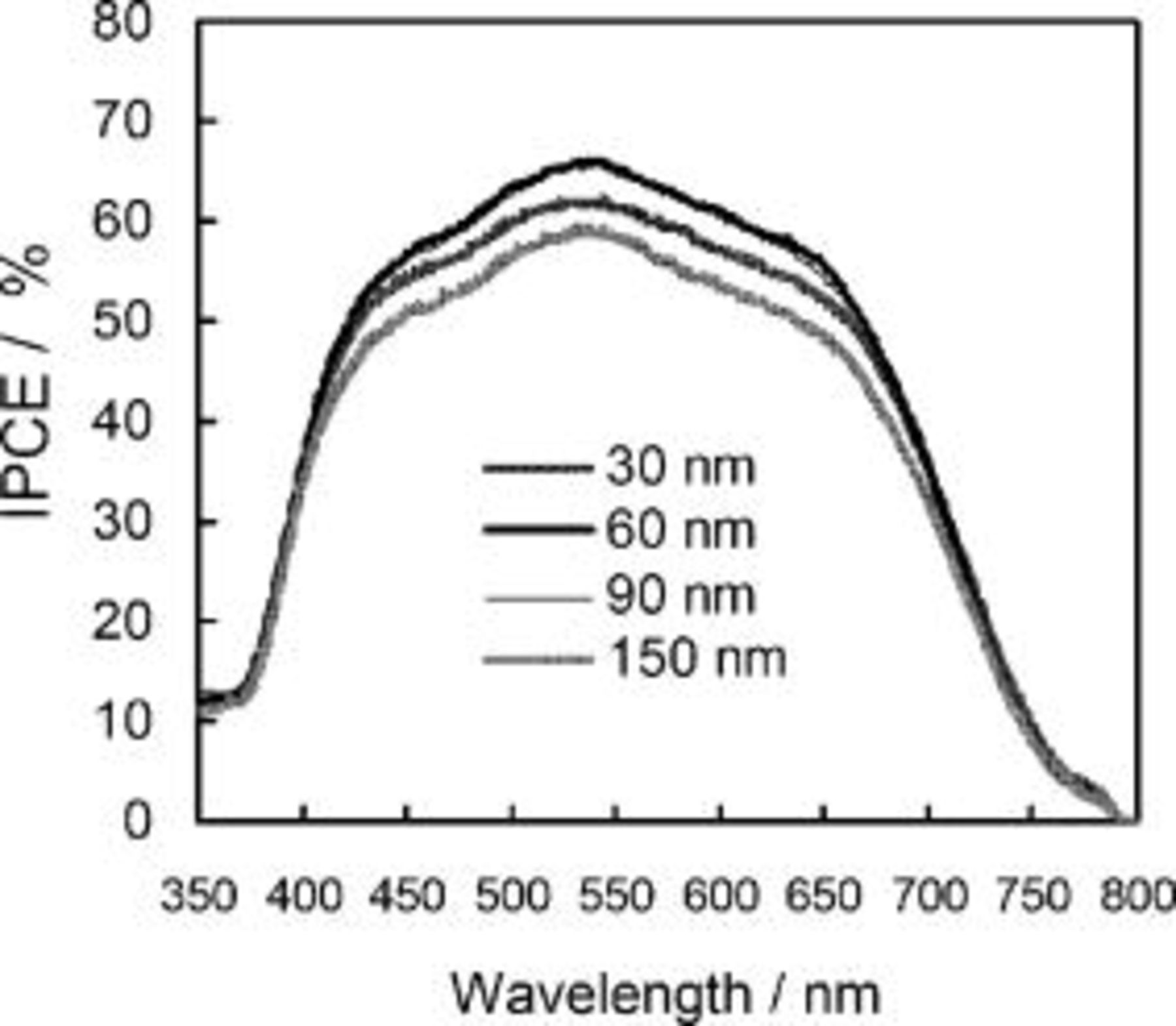
NMB in a mixture of AN and MPN (vol. ratio 1:1). The optimum size for photocurrent of  was reproduced in this system while the surface dye amount is simply decreased with increasing the size. IPCE of the cell was measured using this system. Figure 8 shows the IPCE action spectra for plastic photoelectrodes bearing different size
was reproduced in this system while the surface dye amount is simply decreased with increasing the size. IPCE of the cell was measured using this system. Figure 8 shows the IPCE action spectra for plastic photoelectrodes bearing different size  films. Using the
films. Using the  particle cell, IPCE value reached at maximum of 66% around wavelengths of
particle cell, IPCE value reached at maximum of 66% around wavelengths of  where the N719 dye adsorbed has a peak optical absorption. The spectra also show sufficiently high response at long wavelengths over
where the N719 dye adsorbed has a peak optical absorption. The spectra also show sufficiently high response at long wavelengths over  as light scattering effect by the mixing of large particle. The particle size of
as light scattering effect by the mixing of large particle. The particle size of  is theoretically effective for scattering at wavelengths up to
is theoretically effective for scattering at wavelengths up to  . The effect obtained in longer wavelength region must reflect that larger particles containing in the size distribution and aggregated particles are also involved in the light scattering. At wavelengths shorter than
. The effect obtained in longer wavelength region must reflect that larger particles containing in the size distribution and aggregated particles are also involved in the light scattering. At wavelengths shorter than  , there occurs an abrupt drop in photocurrent, which is due to the sharp cut off of ultraviolet light by the PEN film. Although this UV-filtering function brings about a loss in photocurrent, it can keep the dye-sensitized photoelectrode from deterioration of dye by the photocatalytic reaction on excitation of
, there occurs an abrupt drop in photocurrent, which is due to the sharp cut off of ultraviolet light by the PEN film. Although this UV-filtering function brings about a loss in photocurrent, it can keep the dye-sensitized photoelectrode from deterioration of dye by the photocatalytic reaction on excitation of 
 .
.
Figure 6. Dependences of photocurrent density  , open-circuit voltage
, open-circuit voltage  , fill factor
, fill factor  , and energy conversion efficiency
, and energy conversion efficiency  on average size of
on average size of  nanocrystalline particles loaded on the ITO-PEN plastic electrode. Closed points and open points indicate data with and without the 250 nm size particles.
nanocrystalline particles loaded on the ITO-PEN plastic electrode. Closed points and open points indicate data with and without the 250 nm size particles.  loadings of films are constant at
loadings of films are constant at  . Electrolyte A was employed.
. Electrolyte A was employed.
Figure 7. Effects of the average size of  nanocrystalline particles on photocurrent density
nanocrystalline particles on photocurrent density  (solid line) and the amount of adsorbed dye molecules at
(solid line) and the amount of adsorbed dye molecules at  films (dashed line) on the ITO-PEN plastic electrode. For all particle sizes,
films (dashed line) on the ITO-PEN plastic electrode. For all particle sizes,  films contained
films contained  large particles and had a relatively high
large particles and had a relatively high  loading of
loading of  . Electrolyte D was employed.
. Electrolyte D was employed.
Figure 8. Effects of the average size of  nanocrystalline particles on IPCE action spectra of N719-sensitized
nanocrystalline particles on IPCE action spectra of N719-sensitized  films coated on the ITO-PEN plastic electrodes. Experimental conditions are the same as in Fig. 7.
films coated on the ITO-PEN plastic electrodes. Experimental conditions are the same as in Fig. 7.
Comparison of low-temperature coating with high-temperature sintering
Photovoltaic performance obtained by the present low-temperature coating technique was assessed in comparison with the conventional high-temperature sintering method. For this examination, an F-doped tin oxide (FTO) transparent conductive glass (Nippon Sheet Glass, Co., sheet resistance < ) was employed as a heat-resistant electrode substrate. The binder-free
) was employed as a heat-resistant electrode substrate. The binder-free  paste (average
paste (average  nanoparticles mixed with
nanoparticles mixed with  particles) was doctor-blade coated on FTO glasses and the
particles) was doctor-blade coated on FTO glasses and the  layers were subjected to heating and sintering in an oven at
layers were subjected to heating and sintering in an oven at  and
and  , respectively, to form mesoporous layer of
, respectively, to form mesoporous layer of  thickness. In both heating processes, no polymer binders were used in the coating pastes. This condition differs from the ordinary sintering method in which polyethylene glycol is normally added as a viscous binder and is fired to form porous surfaces. In our comparative experiments, high-temperature effect was examined which causes surface melting of nano-
thickness. In both heating processes, no polymer binders were used in the coating pastes. This condition differs from the ordinary sintering method in which polyethylene glycol is normally added as a viscous binder and is fired to form porous surfaces. In our comparative experiments, high-temperature effect was examined which causes surface melting of nano- particles
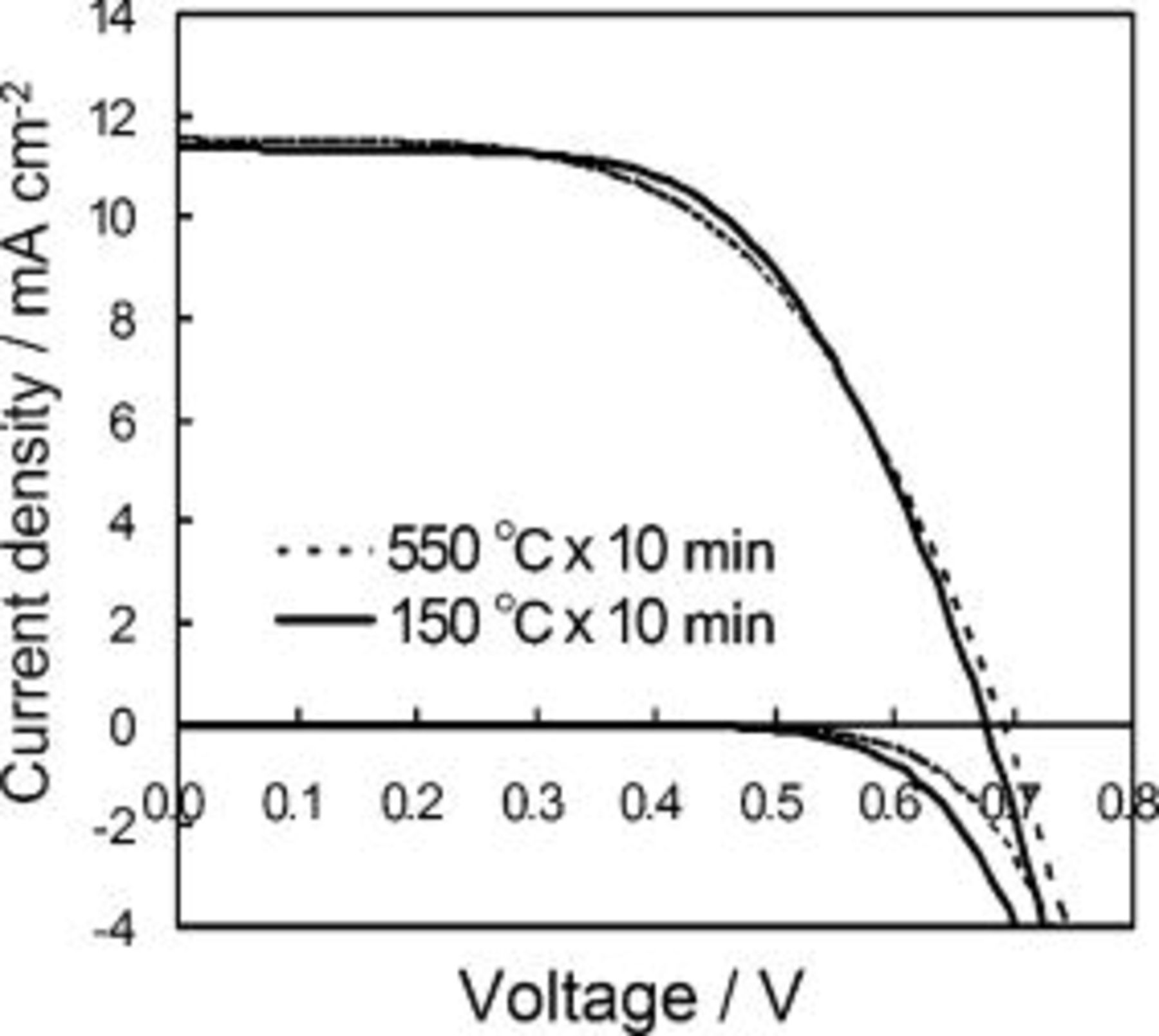
particles  leading to interparticle connection. Figure 9 demonstrates the result of I-V performances for
leading to interparticle connection. Figure 9 demonstrates the result of I-V performances for  sintering of the binder-free film in comparison with that of
sintering of the binder-free film in comparison with that of  heating. Note that the
heating. Note that the  sintering can only yield a slight change in I-V characteristic accompanied by voltage improvement. This fact indicates that our binder-free low-temperature coating method is capable of giving a level of interparticle connection which can be introduced by
sintering can only yield a slight change in I-V characteristic accompanied by voltage improvement. This fact indicates that our binder-free low-temperature coating method is capable of giving a level of interparticle connection which can be introduced by  heating. This conclusion, however, does not exclude the merit of electrode preparation by sintering of a binder-containing paste. In the latter, burning of polymer binders becomes a key step in pore formation17 and, most probably, reinforcement of particle interconnection assisted by carbonization of the polymer, which leads to realize a high level of photocurrent density.
heating. This conclusion, however, does not exclude the merit of electrode preparation by sintering of a binder-containing paste. In the latter, burning of polymer binders becomes a key step in pore formation17 and, most probably, reinforcement of particle interconnection assisted by carbonization of the polymer, which leads to realize a high level of photocurrent density.
Figure 9. Effects of the sintering temperature on the photoelectric performance of N719-sensitized  electrode. I-V characteristics of the binder-free
electrode. I-V characteristics of the binder-free  films coated on FTO glasses are compared, in which films were heated to
films coated on FTO glasses are compared, in which films were heated to  according to the method of this study (solid line) and sintered to
according to the method of this study (solid line) and sintered to  (dashed line) for
(dashed line) for  . Comparison of photocurrent densities (upper set of curves) was made under incident intensity of 1 sun using electrolyte D for both cells. Comparison of corresponding dark currents (lower set of curves) is also given.
. Comparison of photocurrent densities (upper set of curves) was made under incident intensity of 1 sun using electrolyte D for both cells. Comparison of corresponding dark currents (lower set of curves) is also given.
Optimization of electrolyte composition with the Ru complex dyes N719 and N712
Based on the above optimized recipe of binder-free  film preparation, we have next examined the different combinations of dye sensitizer and electrolyte for maximizing the photovoltaic performance.18 N719 and N71214 were examined which have two and four TBA cations, respectively, replacing proton at the anchoring carboxy group. Adsorption of N712, which bears no protonated carboxy groups, is assumed to negatively shift the Fermi level of
film preparation, we have next examined the different combinations of dye sensitizer and electrolyte for maximizing the photovoltaic performance.18 N719 and N71214 were examined which have two and four TBA cations, respectively, replacing proton at the anchoring carboxy group. Adsorption of N712, which bears no protonated carboxy groups, is assumed to negatively shift the Fermi level of  with respect to the level occurring with N719 that has one protonated carboxy group.14 As a result of a expanded gap between the Fermi level and the redox couple iodide/teriiodide, use of N712 is expected to cause an increase in photovoltage. A similar photovoltage improvement can be caused by the presence of nonredox additive tert-butylpyridine (TBP) in the electrolyte,15 which contributes to back electron transfer suppression at the
with respect to the level occurring with N719 that has one protonated carboxy group.14 As a result of a expanded gap between the Fermi level and the redox couple iodide/teriiodide, use of N712 is expected to cause an increase in photovoltage. A similar photovoltage improvement can be caused by the presence of nonredox additive tert-butylpyridine (TBP) in the electrolyte,15 which contributes to back electron transfer suppression at the  -electrolyte interface and negatively shifts the potential where dark cathodic current starts. Four electrolyte compositions, electrolytes A-D, were compared in this viewpoint. All electrolytes contained a common redox system comprising LiI
-electrolyte interface and negatively shifts the potential where dark cathodic current starts. Four electrolyte compositions, electrolytes A-D, were compared in this viewpoint. All electrolytes contained a common redox system comprising LiI  ,
, 
 , and TBAI
, and TBAI  . Electrolyte A contained
. Electrolyte A contained  TBP while electrolyte B contained
TBP while electrolyte B contained  NMB as additive in MPN. Effect of solvents was examined in electrolyte C and electrolyte D, which contained NMB in AN and in a mixture solvent AN/MPN (volume ratio 1:1), respectively.
NMB as additive in MPN. Effect of solvents was examined in electrolyte C and electrolyte D, which contained NMB in AN and in a mixture solvent AN/MPN (volume ratio 1:1), respectively.
Table II summarizes the results of I-V characteristics by N719 sensitization obtained under 1 sun and  sun intensities with different electrolyte compositions. For each condition of light intensity and electrolyte, average was taken from I-V data of three and more independent cells, in which fabrication of electrode employed an amply high
sun intensities with different electrolyte compositions. For each condition of light intensity and electrolyte, average was taken from I-V data of three and more independent cells, in which fabrication of electrode employed an amply high  loading of
loading of  comprising average
comprising average  nanoparticles mixed with
nanoparticles mixed with  particles.
particles.  -coated ITO-PEN electrodes were heated at
-coated ITO-PEN electrodes were heated at  for
for  before dye sensitization. With this high
before dye sensitization. With this high  loading, photocurrent density exceeding
loading, photocurrent density exceeding  and
and  close to
close to  were obtained under 1 sun irradiation. Use of AN as a low viscosity solvent effectively improved
were obtained under 1 sun irradiation. Use of AN as a low viscosity solvent effectively improved  in electrolytes C and D, accompanied by a slight increase in
in electrolytes C and D, accompanied by a slight increase in  . Maximum conversion efficiency reaches 5.5% with AN-based electrolytes C and D. For weak
. Maximum conversion efficiency reaches 5.5% with AN-based electrolytes C and D. For weak  sun irradiation, efficiency is further improved, closely achieving 6%. The top efficiency, however, was obtained by combination with an electrolyte using MPN as solvent (electrolyte B). With low irradiance yielding low photocurrent density, there is less influence of the viscosity of electrolyte on ionic diffusion that limits photocurrent. Improvement of efficiency by the MPN-based electrolyte arose from an increase in
sun irradiation, efficiency is further improved, closely achieving 6%. The top efficiency, however, was obtained by combination with an electrolyte using MPN as solvent (electrolyte B). With low irradiance yielding low photocurrent density, there is less influence of the viscosity of electrolyte on ionic diffusion that limits photocurrent. Improvement of efficiency by the MPN-based electrolyte arose from an increase in  , which may be associated with suppression of dark current (reduction of triiodide) at the
, which may be associated with suppression of dark current (reduction of triiodide) at the  -electrolyte interface. From these results we chose electrolyte D as the electrolyte composition useful for high irradiance performance and electrolyte B for low irradiance performance.
-electrolyte interface. From these results we chose electrolyte D as the electrolyte composition useful for high irradiance performance and electrolyte B for low irradiance performance.
Table II. Photoelectric I-V characteristics of N719-sensitized ITO-PEN plastic electrodes under high (1 sun) and low ( sun) irradiances for different electrolyte compositions. Each experiment shows average data taken for three or more cells.
sun) irradiances for different electrolyte compositions. Each experiment shows average data taken for three or more cells.
With irradiance of 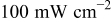 (1 sun) (1 sun) | |||||
|---|---|---|---|---|---|
| Electrolyte |
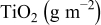
|
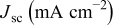
|
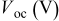
|
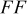
|
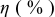
|
| A | 19 |
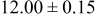
|
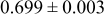
|
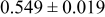
|
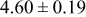
|
| B | 19 |
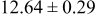
|
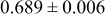
|
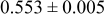
|
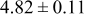
|
| C | 18 |
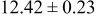
|
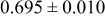
|
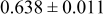
|
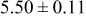
|
| D | 18 |
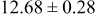
|
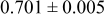
|
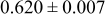
|
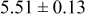
|
With irradiance of 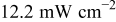 ( ( sun) sun) | |||||
| Electrolyte |
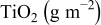
|
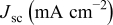
|
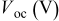
|
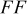
|
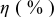
|
| A | 19 |
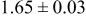
|
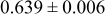
|
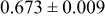
|
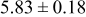
|
| B | 19 |
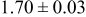
|
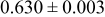
|
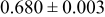
|
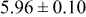
|
| C | 18 |
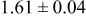
|
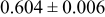
|
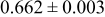
|
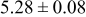
|
| D | 18 |
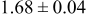
|
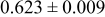
|
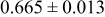
|
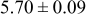
|
A: LiI  , TBAI
, TBAI  ,
, 
 , TBP
, TBP  in MPN.B: LiI
in MPN.B: LiI  , TBAI
, TBAI  ,
, 
 , NMB
, NMB  in MPN.C: LiI
in MPN.C: LiI  , TBAI
, TBAI  ,
, 
 , NMB
, NMB  in AN.D: LiI
in AN.D: LiI  , TBAI
, TBAI  ,
, 
 , NMB
, NMB  in AN/MPN (vol. ratio 1:1).
in AN/MPN (vol. ratio 1:1).
Table III summarizes I-V characteristics for N712 sensitization with the electrolyte compositions B and D for high (1 sun) and low ( sun) irradiances.
sun) irradiances.  loading,
loading,  , is slightly lower than that used in the N719-sensitized cells (Table II). In this system, best conversion efficiencies obtained were 5.81% and 6.43% for 1 sun irradiance with use of electrolyte D and
, is slightly lower than that used in the N719-sensitized cells (Table II). In this system, best conversion efficiencies obtained were 5.81% and 6.43% for 1 sun irradiance with use of electrolyte D and  irradiance with electrolyte B, respectively.18 These efficiencies are both higher than the corresponding efficiencies in the N719-sensitized cells (Table II). Although photocurrent amplitudes became slightly low compared with the N719 series, probably influenced by
irradiance with electrolyte B, respectively.18 These efficiencies are both higher than the corresponding efficiencies in the N719-sensitized cells (Table II). Although photocurrent amplitudes became slightly low compared with the N719 series, probably influenced by  loading, photovoltage,
loading, photovoltage,  , was improved by around
, was improved by around  over the N719-sensitized cells. This voltage increase is assumed to be due to the Fermi level shift by the four TBA substitution in N712.14 The highest efficiency in global power conversion
over the N719-sensitized cells. This voltage increase is assumed to be due to the Fermi level shift by the four TBA substitution in N712.14 The highest efficiency in global power conversion  , 6.4%, was achieved with N712 in combination of MPN-based iodide/triiodide electrolytes in our low-temperature manufactured plastic electrode.
, 6.4%, was achieved with N712 in combination of MPN-based iodide/triiodide electrolytes in our low-temperature manufactured plastic electrode.
Table III. Phtoelectric I-V characteristics of N712-sensitized ITO-PEN plastic electrodes under high (1 sun) and low ( sun) irradiances. Electrolytes B and D were employed for comparison. Each experiment shows average data taken for three or more cells.
sun) irradiances. Electrolytes B and D were employed for comparison. Each experiment shows average data taken for three or more cells.
With irradiance of 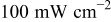 (1 sun) (1 sun) | |||||
|---|---|---|---|---|---|
| Electrolyte |
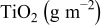
|
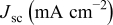
|
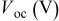
|
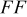
|
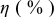
|
| B | 17 |
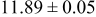
|
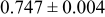
|
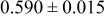
|
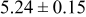
|
| D | 17 |
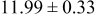
|
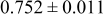
|
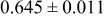
|
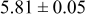
|
With irradiance of 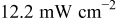 ( ( sun) sun) | |||||
| Electrolyte |
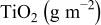
|
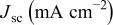
|
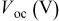
|
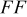
|
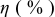
|
| B | 17 |
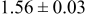
|
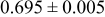
|
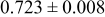
|
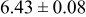
|
| D | 17 |
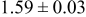
|
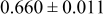
|
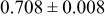
|
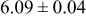
|
B: LiI  , TBAI
, TBAI  ,
, 
 , NMB
, NMB  in MPN.D: LiI
in MPN.D: LiI  , TBAI
, TBAI  ,
, 
 , NMB
, NMB  in AN/MPN (1:1).
in AN/MPN (1:1).
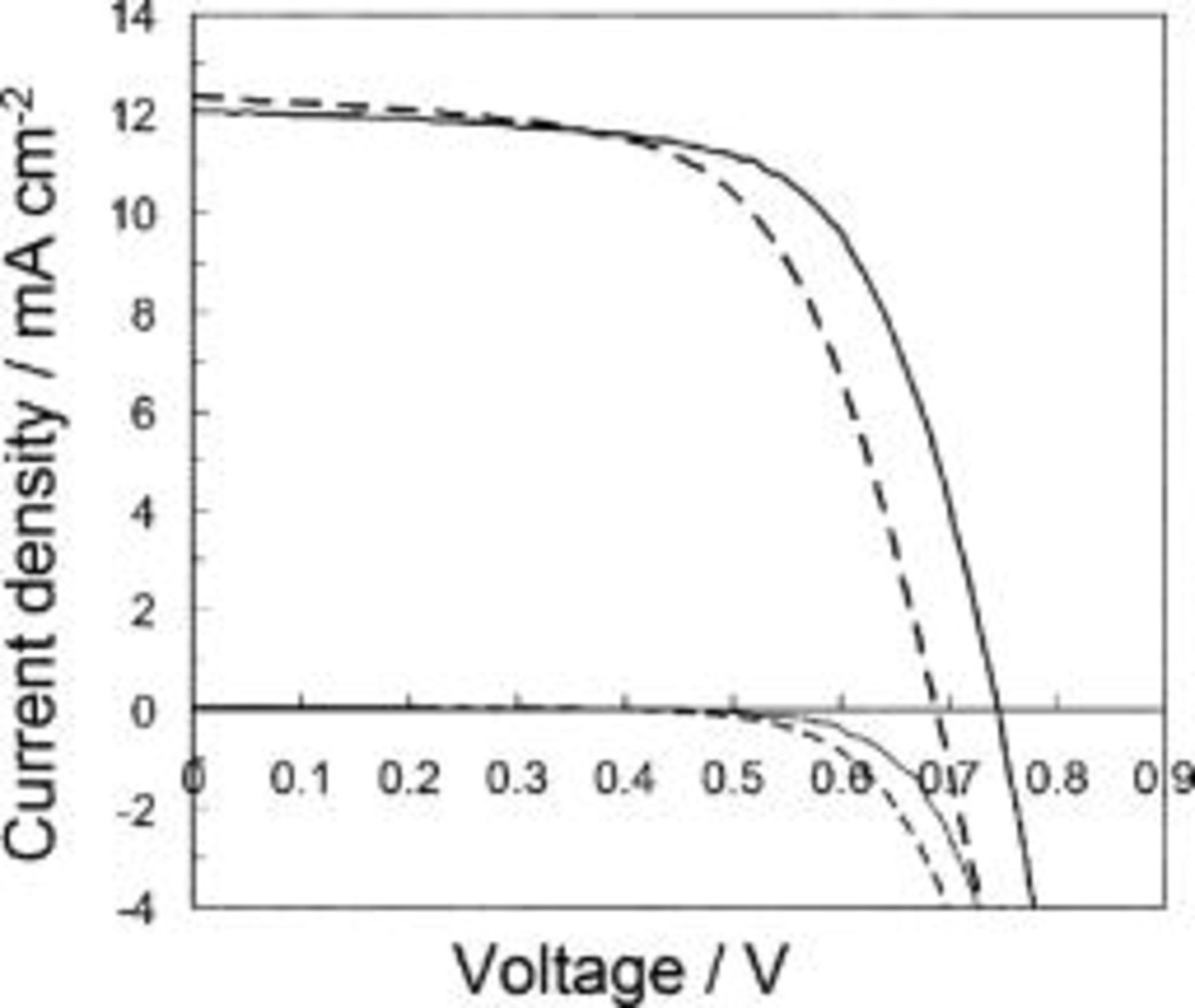
Figure 10 compares the typical I-V performances obtained by the N719 and N712-sensitized  -ITO-PEN plastic electrodes under 1 sun irradiance. A major difference is seen as a voltage increase for N712 both in photocurrent and dark current characteristics. The N712-sensitized electrodes give
-ITO-PEN plastic electrodes under 1 sun irradiance. A major difference is seen as a voltage increase for N712 both in photocurrent and dark current characteristics. The N712-sensitized electrodes give  of around
of around  , which is more than
, which is more than  higher than the level obtained by N719 (see Tables II and III). With sintered
higher than the level obtained by N719 (see Tables II and III). With sintered  films on glass electrodes, same effect has been observed yielding global conversion efficiency over 8%. The efficiency obtained by the present low-temperature manufactured plastic photoanode, 6.4%, is therefore around 70–80% those obtained by glass-based cells with sintered
films on glass electrodes, same effect has been observed yielding global conversion efficiency over 8%. The efficiency obtained by the present low-temperature manufactured plastic photoanode, 6.4%, is therefore around 70–80% those obtained by glass-based cells with sintered  films. This relation is also indicated by IPCE values (Fig. 8) in which the peak IPCE 66% is 25% lower than the top IPCE achieved by the Ru complex dyes.16 As mentioned previously on the comparison of low-temperature and sintering methods using the binder-free paste (Fig. 9), no significant difference in performance occurs unless binder-containing paste is employed. However, the cell performance of the present method is difficult to compete with the top efficiency of DSSC based on the sintering of polymer binder-mixed paste. The efficiency loss in the plastic DSSC is not only due to the low-temperature preparation of
films. This relation is also indicated by IPCE values (Fig. 8) in which the peak IPCE 66% is 25% lower than the top IPCE achieved by the Ru complex dyes.16 As mentioned previously on the comparison of low-temperature and sintering methods using the binder-free paste (Fig. 9), no significant difference in performance occurs unless binder-containing paste is employed. However, the cell performance of the present method is difficult to compete with the top efficiency of DSSC based on the sintering of polymer binder-mixed paste. The efficiency loss in the plastic DSSC is not only due to the low-temperature preparation of  network but also apparently affected by the high sheet resistance
network but also apparently affected by the high sheet resistance  of the ITO-PEN plastic electrode relative to FTO glass (7–9 Ω∕◻), which inevitably reduces
of the ITO-PEN plastic electrode relative to FTO glass (7–9 Ω∕◻), which inevitably reduces  in the I-V characteristics. In this respect, fabrication of a transparent plastic substrate bearing a highly electroconductive network of current-collecting materials is the key factor to ensuring high photovoltaic performance.
in the I-V characteristics. In this respect, fabrication of a transparent plastic substrate bearing a highly electroconductive network of current-collecting materials is the key factor to ensuring high photovoltaic performance.
Figure 10.
I-V characteristics for N719-sensitized (dashed line) and N712-sensitized (solid line)  -coated ITO-PEN plastic electrodes based on low-temperature preparation using the binder-free
-coated ITO-PEN plastic electrodes based on low-temperature preparation using the binder-free  paste.
paste.  loadings were
loadings were  according to the condition of Table II and III.
according to the condition of Table II and III.  -coated ITO-PEN electrodes were heated at
-coated ITO-PEN electrodes were heated at  for
for  before dye sensitization. Photocurrent densities (upper set of curves) were measured under incident intensity of 1 sun using electrolyte D. Comparison of corresponding dark currents (lower set of curves) is also given.
before dye sensitization. Photocurrent densities (upper set of curves) were measured under incident intensity of 1 sun using electrolyte D. Comparison of corresponding dark currents (lower set of curves) is also given.
Conclusion
Binder-free viscous nano- paste was prepared by mixing colloidal
paste was prepared by mixing colloidal  sol. as cement to interconnect the particles. Doctor-blade coating of the paste and heating
sol. as cement to interconnect the particles. Doctor-blade coating of the paste and heating  provides a mesoscopic
provides a mesoscopic  film formed on ITO-PEN conductive plastic sheet with high adhesion strength. Sensitized with Ru complex dyes, plastic electrode yielded energy conversion efficiency of 5.8–6.4% under simulated sunlight (AM 1.5) of intensities
film formed on ITO-PEN conductive plastic sheet with high adhesion strength. Sensitized with Ru complex dyes, plastic electrode yielded energy conversion efficiency of 5.8–6.4% under simulated sunlight (AM 1.5) of intensities  . Significantly high efficiency was obtained through optimization of
. Significantly high efficiency was obtained through optimization of  particle size and film thickness and with selected kind of organic electrolyte. High performance of the flexible plastic electrode is backed by well-connected network of
particle size and film thickness and with selected kind of organic electrolyte. High performance of the flexible plastic electrode is backed by well-connected network of  particles whose porosity is as high as 60%. Our fabrication method does not apply mechanical compression of the
particles whose porosity is as high as 60%. Our fabrication method does not apply mechanical compression of the  film for adhesion reinforcement to avoid possible destruction of the pore structure. For fabrication of flexible DSSCs, recently, stainless steel has also been attempted as a substrate alternative to the plastic electrode.19 Corrosion of the substrate with the redox electrolyte, however, always matters in utilization of metallic substrates. In this respect, use of flexible plastics is highly sought after for industrialization of low-cost DSSCs manufacturing by roll-to-roll processes. Based on the present technology, we have fabricated a large-area full-plastic DSSC module with a series connection of unit cells, in which counter electrodes were also made on PEN sheet. The lifetime of the module when preserved in the ambient atmosphere at room temperature is less than
film for adhesion reinforcement to avoid possible destruction of the pore structure. For fabrication of flexible DSSCs, recently, stainless steel has also been attempted as a substrate alternative to the plastic electrode.19 Corrosion of the substrate with the redox electrolyte, however, always matters in utilization of metallic substrates. In this respect, use of flexible plastics is highly sought after for industrialization of low-cost DSSCs manufacturing by roll-to-roll processes. Based on the present technology, we have fabricated a large-area full-plastic DSSC module with a series connection of unit cells, in which counter electrodes were also made on PEN sheet. The lifetime of the module when preserved in the ambient atmosphere at room temperature is less than  . To ensure high durability, our study is in progress by developing a new conductive layer (CL) on PEN and stabilizing the interfacial structures of plastic-CL and CL-
. To ensure high durability, our study is in progress by developing a new conductive layer (CL) on PEN and stabilizing the interfacial structures of plastic-CL and CL- .
.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (417) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by New Energy and Industrial Technology Development Organization (NEDO), Japan
Toin University of Yokohama assisted in meeting the publication costs of this article.