Abstract
Based on the analysis of the precise current–time data and observation of the morphology by scanning electron microscopy, a layer-by-layer growth model of anodic  nanotube (TNT) arrays was presented. Many phenomena appeared during the anodization and can be reasonably explained by this model, such as the first sharp slope of current in initial period, current fluctuation, occurring of ridges in adjacent tubes, and the rings broken off from the tube mouths. Furthermore, key factors which determine the morphology of TNT are discussed and it would be helpful for the design of nanoarchitectures in related material systems.
nanotube (TNT) arrays was presented. Many phenomena appeared during the anodization and can be reasonably explained by this model, such as the first sharp slope of current in initial period, current fluctuation, occurring of ridges in adjacent tubes, and the rings broken off from the tube mouths. Furthermore, key factors which determine the morphology of TNT are discussed and it would be helpful for the design of nanoarchitectures in related material systems.
Export citation and abstract BibTeX RIS
Fabrication of  nanotube (TNT) arrays via anodic oxidation of Ti was first reported in 2001.1 Later studies focused on precise control and extension of the nanotube morphology,2 length and pore size,3 and wall thickness.4 On the basis of the different electrolytes used in the experiments, the process of TNT research can be divided into four generations: the first generation is the nanotube arrays synthesis using aqueous electrolytes; the second is the synthesis using buffered electrolytes; the third is using polar organic electrolytes; and the fourth is using nonfluoride based electrolytes.5 The precisely oriented nature of the TNT makes it an excellent electron percolation pathway for vectorial charge transfer between interfaces and it showed to be suitable for photocatalytic applications,6–10 sensing11–14 photoelectrolysis,15–21 polymer-based bulk heterojunction photovoltaics,22–24 dye-sensitized solar cells,25–31 biofluids filtration, drug delivery, and other biomedical applications.32–35 Many other different morphologies and structures of nanotubes, such as conical-shaped,2 smooth,36 Y-branched37 multiple layers38 and self-standing,39 have also been fabricated successfully.
nanotube (TNT) arrays via anodic oxidation of Ti was first reported in 2001.1 Later studies focused on precise control and extension of the nanotube morphology,2 length and pore size,3 and wall thickness.4 On the basis of the different electrolytes used in the experiments, the process of TNT research can be divided into four generations: the first generation is the nanotube arrays synthesis using aqueous electrolytes; the second is the synthesis using buffered electrolytes; the third is using polar organic electrolytes; and the fourth is using nonfluoride based electrolytes.5 The precisely oriented nature of the TNT makes it an excellent electron percolation pathway for vectorial charge transfer between interfaces and it showed to be suitable for photocatalytic applications,6–10 sensing11–14 photoelectrolysis,15–21 polymer-based bulk heterojunction photovoltaics,22–24 dye-sensitized solar cells,25–31 biofluids filtration, drug delivery, and other biomedical applications.32–35 Many other different morphologies and structures of nanotubes, such as conical-shaped,2 smooth,36 Y-branched37 multiple layers38 and self-standing,39 have also been fabricated successfully.
The promising prospects require a thorough understanding of the nanotube formation mechanism and controllable preparation of the arrays. Three critical aspects are helpful to illustrate the TNT growth, namely, chemical reactions, current–time curve, and detailed morphology. The overall reactions for anodic titania formation and dissolution can be presented as40
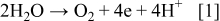
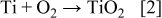
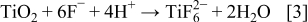
The current–time  curves can both record the anodization behavior and serve as the denotation of anodic process for the experimenters. Generally, a current–time curve consists of three stages described by many reports.12, 18, 22–24, 26, 28, 41 In combination with
curves can both record the anodization behavior and serve as the denotation of anodic process for the experimenters. Generally, a current–time curve consists of three stages described by many reports.12, 18, 22–24, 26, 28, 41 In combination with  curves, a typical TNT growth mechanism named three stage model has been put forward by Ghicov and Schmuki42 The three stage model contains the barrier oxide formation stage (the first stage), pore growing stage (the second stage), and steady-state stage (the third stage). Tao et al. presented a dissolution–breakdown model and made a closer look at the current variation in anodization process.43 In this dissolution–breakdown model, the current can be broken into two parts. One is dissolution current (corresponding to the dissolution stage) and the other is breakdown current (corresponding to the breakdown stage). In the initial stage, a sharp, exponential decrease of current is observed. Then the current increases suddenly, and relatively regular current oscillations that correspond to breakdowns or ridges on the tube wall are observed. When the breakdown is fully developed, the current reaches its maximum. After that the current decreases slowly due to the tube growth, and finally remains steady. Two other models have also been proposed to explain the formation of TNT. The first one is the mechanical splitting model suggested by Yasuda et al.44 It stipulates that high tensile stresses due to the volume expansion associated with the rapidly formed oxide cause the separation seen. An alternate model proposed by Raja et al. suggests that the gaps result from the coalescence of voids that form during the anodization and dissolution processes.45 The model predicts that the voids are generated in the nanotubes as a consequence of the condensation of cation vacancies that result from oxide dissolution. As these vacancies are formed in the inner wall of a tube, they diffuse radially outward where they eventually merge with other vacancies, forming voids, and the coalescence of voids from adjacent tubes creates the separation seen.
curves, a typical TNT growth mechanism named three stage model has been put forward by Ghicov and Schmuki42 The three stage model contains the barrier oxide formation stage (the first stage), pore growing stage (the second stage), and steady-state stage (the third stage). Tao et al. presented a dissolution–breakdown model and made a closer look at the current variation in anodization process.43 In this dissolution–breakdown model, the current can be broken into two parts. One is dissolution current (corresponding to the dissolution stage) and the other is breakdown current (corresponding to the breakdown stage). In the initial stage, a sharp, exponential decrease of current is observed. Then the current increases suddenly, and relatively regular current oscillations that correspond to breakdowns or ridges on the tube wall are observed. When the breakdown is fully developed, the current reaches its maximum. After that the current decreases slowly due to the tube growth, and finally remains steady. Two other models have also been proposed to explain the formation of TNT. The first one is the mechanical splitting model suggested by Yasuda et al.44 It stipulates that high tensile stresses due to the volume expansion associated with the rapidly formed oxide cause the separation seen. An alternate model proposed by Raja et al. suggests that the gaps result from the coalescence of voids that form during the anodization and dissolution processes.45 The model predicts that the voids are generated in the nanotubes as a consequence of the condensation of cation vacancies that result from oxide dissolution. As these vacancies are formed in the inner wall of a tube, they diffuse radially outward where they eventually merge with other vacancies, forming voids, and the coalescence of voids from adjacent tubes creates the separation seen.
From the models mentioned above, we found that two critical factors are neglected: one is the explicit explanation of the presence of the ridges; the other is the high accuracy of current data which can detailedly exploit the growth stage of TNT. In this work, based on the experimental details and theoretical reasoning, a layer-by-layer model of the anodic TNT arrays was put forward. The new model can reasonably explain why many phenomena appeared during anodization. Some key factors which determine the TNT morphology were discussed and it would be helpful for the design of nanoarchitectures in related material systems.
Theoretical
Figure 1 is an integrative diagram of the layer-by-layer model. The left side is the typical current–time curve. The right side is the typical cross-sectional image of the nanotube arrays, and the middle is the schematic diagram of the model. Corresponding to the first stage of current curve which shows the sharp slope (oxidation current), the compact initial oxide layer is formed. The reaction is described by Eq. 2. This rough layer endures high potential due to its high resistance; thus, some randomly distributed breakdowns take place. The current (breakdown current) rises for the first time. The enrichment of  ions at the breakdown points leads to the faster chemical dissolution of
ions at the breakdown points leads to the faster chemical dissolution of  at these locations, as described by Eq. 3. The expansion of the breakdown points results in the pits formation which act as pore forming centers. As the size of the pits increase, the electrolyte has the chance to infiltrate into the interface of oxide layer/Ti substrate and the second oxide layer is formed. The current decreases for the second time and the reduction amplitude is much less than that of the first one. Only little amount of electrolyte can penetrate into the Ti substrate, so it is reasonable to believe that the thickness of the second layer is much thinner than that of the first one and the oxidation uniformity is less than that of the first layer either, i.e., some Ti in the second geometrical layer has not been oxidized initially. Similar to the first layer, the second oxide layer is also broken down by the electric field and the current increases quickly but slightly. It is important that the breakdown sites in the subsequent layer are almost in the same places where the breakdown of the former layer happened. The increase of the carrier mobility accelerates the chemical dissolution at the breakdown points, leading to the pits formation in the second layer. Thus, the subsequent oxide would be formed layer by layer and the current shows fluctuation. With the increase of the layer number, the pits in the earlier layers become bigger and then the pores are formed. The remnant Ti has also been oxidized completely. With the increase of the pore size, the internal surface area increases and the surface tension, which causes the shrinking of the pore, increases too. If the adjacent pores become close adequately, the oxide among them would be pulled apart by the surface tension, which eventually leads to the pore-separation and tube formation. Because the pore size increasing in the later layer lags that in the former layer, the pore-separation in subsequent layer occurs later. Therefore, some oxide between adjacent layers would be remained and the ridges are formed, as shown in the places 1–4 in Fig. 1. After the pore-separation, the chemical dissolution takes place both in and out of the tubes.
at these locations, as described by Eq. 3. The expansion of the breakdown points results in the pits formation which act as pore forming centers. As the size of the pits increase, the electrolyte has the chance to infiltrate into the interface of oxide layer/Ti substrate and the second oxide layer is formed. The current decreases for the second time and the reduction amplitude is much less than that of the first one. Only little amount of electrolyte can penetrate into the Ti substrate, so it is reasonable to believe that the thickness of the second layer is much thinner than that of the first one and the oxidation uniformity is less than that of the first layer either, i.e., some Ti in the second geometrical layer has not been oxidized initially. Similar to the first layer, the second oxide layer is also broken down by the electric field and the current increases quickly but slightly. It is important that the breakdown sites in the subsequent layer are almost in the same places where the breakdown of the former layer happened. The increase of the carrier mobility accelerates the chemical dissolution at the breakdown points, leading to the pits formation in the second layer. Thus, the subsequent oxide would be formed layer by layer and the current shows fluctuation. With the increase of the layer number, the pits in the earlier layers become bigger and then the pores are formed. The remnant Ti has also been oxidized completely. With the increase of the pore size, the internal surface area increases and the surface tension, which causes the shrinking of the pore, increases too. If the adjacent pores become close adequately, the oxide among them would be pulled apart by the surface tension, which eventually leads to the pore-separation and tube formation. Because the pore size increasing in the later layer lags that in the former layer, the pore-separation in subsequent layer occurs later. Therefore, some oxide between adjacent layers would be remained and the ridges are formed, as shown in the places 1–4 in Fig. 1. After the pore-separation, the chemical dissolution takes place both in and out of the tubes.
Figure 1. Schematic diagram of the layer-by-layer model.
Based on this model, the following could be assumed: the first layer is much thicker than the subsequent layers and it would remain in the initial period of the tubes formation; from top to bottom of a nanotube, the inner diameter of the tube becomes smaller but the tube wall thicker; the number of the ridges should be equal to the number of the current fluctuation for the nanotube arrays in which the first layer still remains; the uniformity of current fluctuation period relies on the formation and breakdown time of each layer, which is influenced by the Ti substrate structure, electrolyte composition, and other anodic conditions.
Experimental
The Ti foils ( in thickness, 99.6% purity) were degreased prior to anodization by sonicating in acetone, rinsed with deionized water (DI), and dried in a nitrogen stream. The electrochemical setup consists of a high-voltage potentiostat Jaissle IMP 88 and a classical two-electrode cell with
in thickness, 99.6% purity) were degreased prior to anodization by sonicating in acetone, rinsed with deionized water (DI), and dried in a nitrogen stream. The electrochemical setup consists of a high-voltage potentiostat Jaissle IMP 88 and a classical two-electrode cell with  between the two electrodes and
between the two electrodes and  Ti surface open to the electrolyte. The sample numbers and anodization conditions are shown in Table I. The current data were collected and delivered to a computer. After the experiments, the samples were rinsed with DI water and dried in a nitrogen stream. A scanning electron microscope Hitachi field emission scanning electron microscope (FE-SEM, S4800) was employed for the structural and morphological characterizations of the
Ti surface open to the electrolyte. The sample numbers and anodization conditions are shown in Table I. The current data were collected and delivered to a computer. After the experiments, the samples were rinsed with DI water and dried in a nitrogen stream. A scanning electron microscope Hitachi field emission scanning electron microscope (FE-SEM, S4800) was employed for the structural and morphological characterizations of the  nanotubular layers. From scanning electron microscope (SEM) cross-sectional images of mechanically bent samples, the thicknesses of the layers were directly detected.
nanotubular layers. From scanning electron microscope (SEM) cross-sectional images of mechanically bent samples, the thicknesses of the layers were directly detected.
Table I. Anodization conditions of all samples.
| Samples | Electrolyte composition | Anodization time (h) | Voltage (V) |
|---|---|---|---|
| A1 |
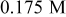 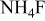 /glycerol/water (Vol. 30:1) /glycerol/water (Vol. 30:1) | 3 | 30 |
| A2 | 6 | ||
| A3 | 12 | ||
| B |
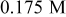 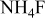 /glycerol/water (Vol. 1:1) /glycerol/water (Vol. 1:1) | 2 | 20 |
| C | 3 | 15 | |
| D |
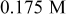 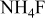 /glycerol /glycerol | 3 | 30 |
Results and Discussion
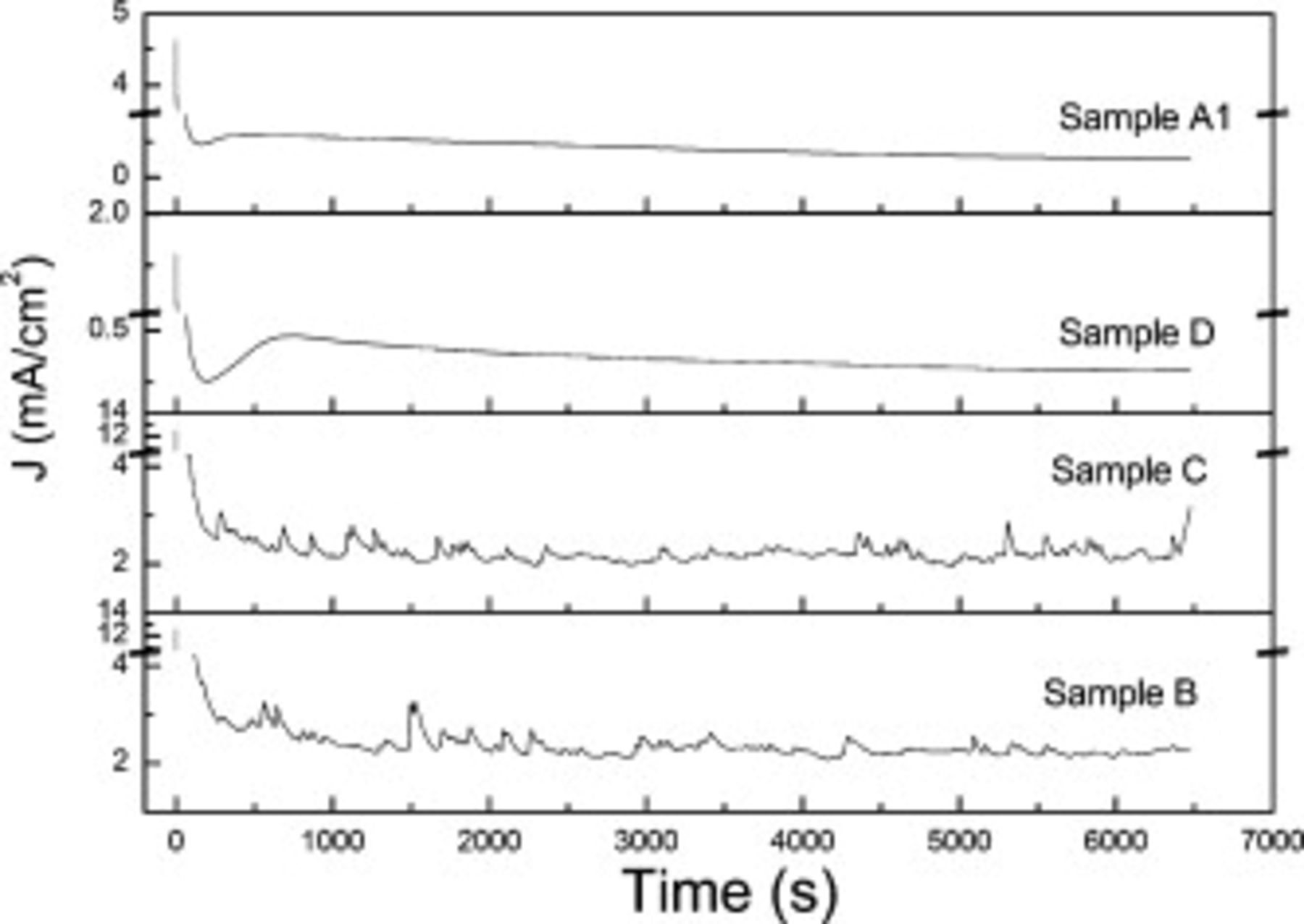
Figure 2 shows the  patterns of A1, B, C, and D samples in the beginning
patterns of A1, B, C, and D samples in the beginning  of anodization. Current curves of B and C exhibit obvious fluctuation, indicating the periodical growth of nanotube arrays. Current curves of A1 and D show the typical three stages and it seems that there is no fluctuation in the third stage. But the current curve shown in Fig. 3, taken out from A1 current data (current curves of A2, A3, and D are quite similar to these of A1), indicates the periodical fluctuation even in the electrolyte containing little or no water. It is interesting that the fluctuation is unremitting in the whole anodic duration indicating no steady-state of current like that mentioned in Ref. 43.
of anodization. Current curves of B and C exhibit obvious fluctuation, indicating the periodical growth of nanotube arrays. Current curves of A1 and D show the typical three stages and it seems that there is no fluctuation in the third stage. But the current curve shown in Fig. 3, taken out from A1 current data (current curves of A2, A3, and D are quite similar to these of A1), indicates the periodical fluctuation even in the electrolyte containing little or no water. It is interesting that the fluctuation is unremitting in the whole anodic duration indicating no steady-state of current like that mentioned in Ref. 43.
Figure 2. Current curves of the samples A1, D, C, and B.
Figure 3. A current curve section for the sample A1.
Figure 4a, surface image of A1, shows the porouslike structure suggesting that the initial oxide layer has not been dissolved completely. From Figs. 4b–4e, one can observe that the tube mouth diameters of the samples A2, A3, B, and C are  ,
,  ,
,  , and
, and  , respectively. From Figs. 4f–4j cross-sectional images of A1, A2, A3, B, and C,
, respectively. From Figs. 4f–4j cross-sectional images of A1, A2, A3, B, and C,  ,
,  ,
,  ,
,  , and
, and  long nanotubes, can be observed, respectively. Protected by initial oxide layer, the sample A1 shows the high tube growth rate of
long nanotubes, can be observed, respectively. Protected by initial oxide layer, the sample A1 shows the high tube growth rate of  . The tube growth rates of A2 and A3 are less than these of A1. By calculating the current data of A1, the current fluctuation period is about
. The tube growth rates of A2 and A3 are less than these of A1. By calculating the current data of A1, the current fluctuation period is about  counting from the multiplied cross-sectional image of A1 whose initial oxide layer still remains, the number of the ridges is about 100 along the sidewall. The mean ridge number along the sidewall of the sample A1 is in agreement with the number of current oscillation period. But for A2, A3, B, and C samples, the mean ridge number is less than the current oscillation period number and this may be due to the chemical dissolution of the ridges by the electrolyte which easily flowed into the interspaces among the tubes after the disappearance of the initial oxide layer. In Fig. 4h, the multiplied image (inset) of the area identified by the arrow shows the approximate size of nanotube wall thickness with the inner diameter in the middle of a tube. By contrast with that at tube mouth, it confirms the aforementioned assumption that the inner diameter decreases while tube wall thickness increases from top to bottom of a tube. This reduction in pore size could also be due to the accumulation of anodization debris. In the SEM side views of Fig. 4, the tubes of the samples B and C show less straight-line than those of A1, A2, and A3. Even Y-branched tubes can be observed in B and C. In more water contained electrolyte, due to the higher carrier mobility, the oxide layer can be easily broken down and the continuity of the breakdown points along the growth direction would be less than that in less water containing electrolyte. This leads to the slightly less straight and branched tubes.
counting from the multiplied cross-sectional image of A1 whose initial oxide layer still remains, the number of the ridges is about 100 along the sidewall. The mean ridge number along the sidewall of the sample A1 is in agreement with the number of current oscillation period. But for A2, A3, B, and C samples, the mean ridge number is less than the current oscillation period number and this may be due to the chemical dissolution of the ridges by the electrolyte which easily flowed into the interspaces among the tubes after the disappearance of the initial oxide layer. In Fig. 4h, the multiplied image (inset) of the area identified by the arrow shows the approximate size of nanotube wall thickness with the inner diameter in the middle of a tube. By contrast with that at tube mouth, it confirms the aforementioned assumption that the inner diameter decreases while tube wall thickness increases from top to bottom of a tube. This reduction in pore size could also be due to the accumulation of anodization debris. In the SEM side views of Fig. 4, the tubes of the samples B and C show less straight-line than those of A1, A2, and A3. Even Y-branched tubes can be observed in B and C. In more water contained electrolyte, due to the higher carrier mobility, the oxide layer can be easily broken down and the continuity of the breakdown points along the growth direction would be less than that in less water containing electrolyte. This leads to the slightly less straight and branched tubes.
Figure 4. (a)–(e) SEM top views and (f)–(j) SEM side views for the samples (A1), (A2), (A3), (B), and (C), respectively. The inset of (h) is the multiplied image identified by the arrow.
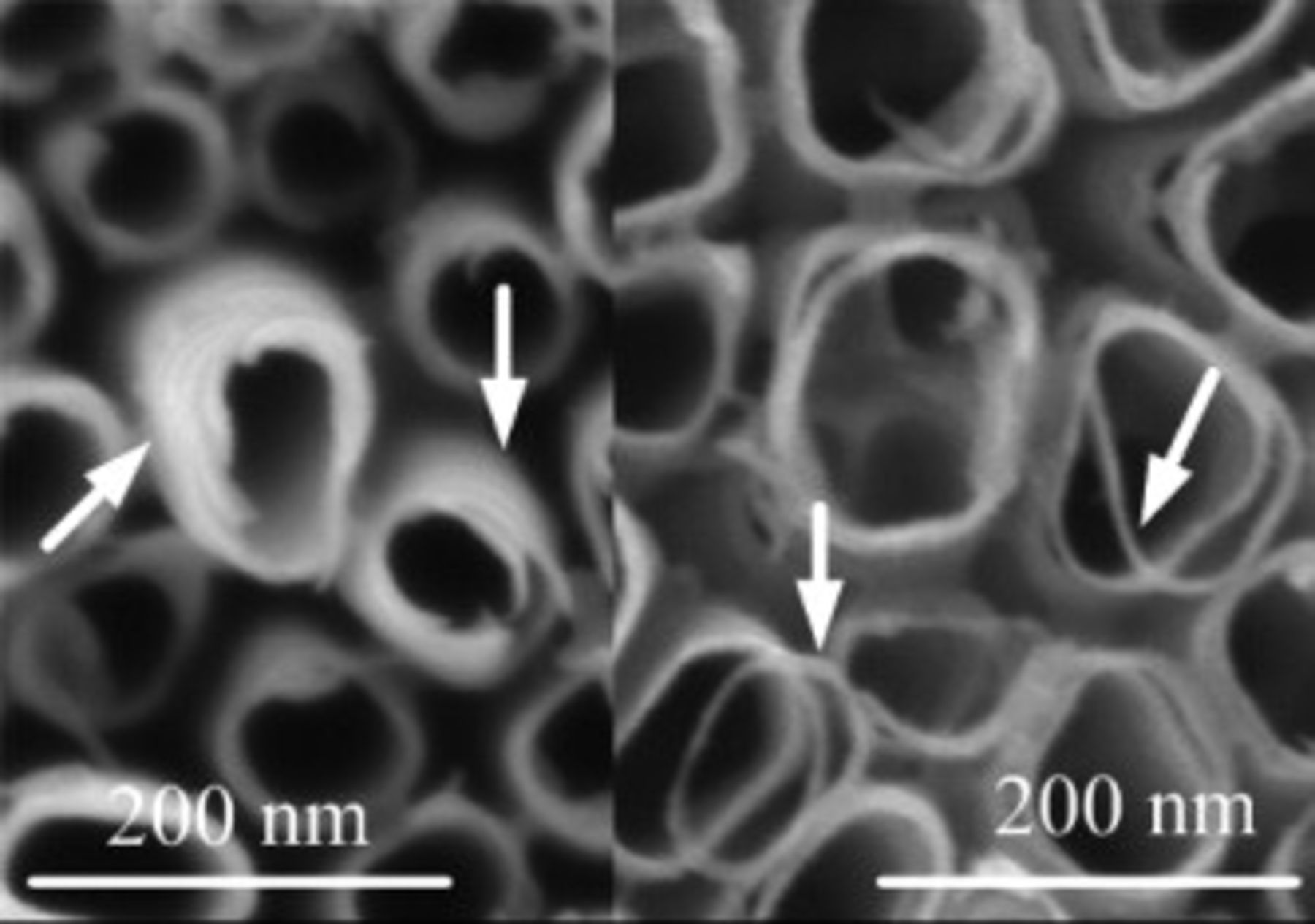
Further evidential SEM images which approve the layered-growth model are shown in Fig. 5. In the left column of Fig. 5, the arrows point at the salient nanotube mouths, from which the layered-structure can be observed obviously. In the right column of Fig. 5, the rings, broken off from nanotube mouths by chemical dissolution, also indicate the layered-structure of the nanotube. The rings can be found in many samples except for A1 and D (not shown) in which the initial layers have not been dissolved.
Figure 5. SEM top views of typical samples. The arrows identify the layered-structure of the tubes (left column) and the rings broken from the tube mouths (right column).
Conclusions
In this paper, a new growth layer-by-layer model of titania nanotube arrays is presented in which the key processes can be described as (1) establishment and breakdown of each oxide layer subsequently, (2) pores formation due to the longitudinal prolongation and the lateral expansion of the pits, (3) tubes formation after pores separation and the ridges formation between adjacent layers. Based on the model and experimental results, several conclusions can be made:
- (1)A tube growth starts from a breakdown point in the initial oxide layer. So it is reasonable to believe that the distribution of the breakdown points in the initial layer determines the tube diameter. The tube with bigger diameter can be obtained from the anodic conditions under which the initial layer is formed with fewer breakdown points in unit area.
- (2)The continuity of the breakdown points layer by layer determines if the tube is straight or not. The slower the formation and breakdown of the layers, the straighter the tubes obtained. However, the Y-branched and other shapes of tubes can also be made by successfully controlling the formation and breakdown of oxide layers.
- (3)The ridges hinder the charge transport but can reinforce the nanotube arrays' structure. So an appropriate sum of ridges is necessary for the structural stabilization of TNT.
- (4)A fluctuation (containing an oxidation current and a breakdown current) corresponds to the formation and breakdown stages of an oxide layer. By quantitative comparison of the first sharp slope and the raise in the current–time curve under different anodic conditions, detailed information of the initial layer can be obtained and the nanotube arrays' morphology can be predicted by and large. After calculating the relationship between the period time and the tube growth rate, the tube length can be controlled successfully.
Acknowledgments
Project supported by the National Natural Science Foundation of China (50872001), the Research Fund for the Doctoral Program of Higher Education of China (20060357003), the Higher Educational Natural Science Foundation of Anhui Province, China (KJ2008B015, KJ2010A123), the Open Foundation of Anhui Key Laboratory of Information Materials and Devices, China, Key Project of Anhui Province, China (05021028), the Talent Foundation of Anhui Province, China (2004Z029), and the Talent Development Foundation of Anhui University, Anhui Province, China.