Abstract
Human breath investigation offers a non-invasive and quick strategy for recognizing different volatile organic compounds (VOCs) that are markers for various diseases. Scientists have shown that breath acetone is a successful biomarker of Type 2 diabetes which is the most common type of diabetes. The generation of acetone is a consequence of the body processing fats as an alternative of glucose to produce energy. Thus, detection of breath acetone can be a rapid, viable, and patient compliant alternative to the conventional methods of blood glucose determination. To achieve this goal, metal oxide nanostructures with various shapes through different synthesis routes in the nanometer scale, can be used. Owing to its properties such as high surface-to-volume ratios and subsequently large number of surface sites exposed to acetone gas, metal oxide nanostructures facilitate a well-built gas-sensing layer interaction and consequently compared to conventional materials, present a higher sensitivity. This work, presents the progress in metal oxides nanostructures (semiconductor nanomaterials) as gas sensing materials for the exhaled acetone detection, which offers the possibility to help people living with diabetes to screen their disease. The different types of metal oxides materials used in Breath acetone sensors, their limitations and future perspectives have been highlighted.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Human breath contains volatile organic compounds (VOCs), some of which are known as biomarkers on the grounds that their concentration can be legitimately connected to a specific sickness or medical problem and can give basic data to the analysis or the diagnosis of particular diseases.1,2 Nevertheless, the earliest reference point of breath investigation previously began in antiquated occasions, when Hippocrates showed his students how to utilize breath smell so as to recognize patients with liver infection, uncontrolled diabetes, and notwithstanding coming up failing kidneys.3 At the point when a human body cannot discharge energy from glucose, either in light of the fact that glucose is exhausted or on the grounds that the body cannot use it, it can utilize fatty acid as a substitute source of energy. In this procedure, the liver separates fatty acid and creates ketone bodies including acetoacetic acid and, as this immediately degrades, acetone (CH3)2CO) is brought into the blood. At the point when acetone- rich blood reaches the lungs some acetone (CH3)2CO) is discharged and breathed out with the breath. There are a couple of extra significant crossroads in-breath history that convey us to the present. In 1798, John Gallo described the smell of exhaled breath from rotten apples and later in 1857 after 59 years, this smell was recognized as acetone,3 which was utilized as an absolute first biomarker of diabetic unconsciousness. The underestimation of exhaled acetone throughout the years, for the most part on the grounds that there were no appropriate devices to identify it in exhaled breath and correspond it with explicit sicknesses, for example, diabetes. In mid-70 s everything changed, when Linus Pauling (1971) published an original article showing an investigative strategy used to distinguish around 250 compounds in breath.4
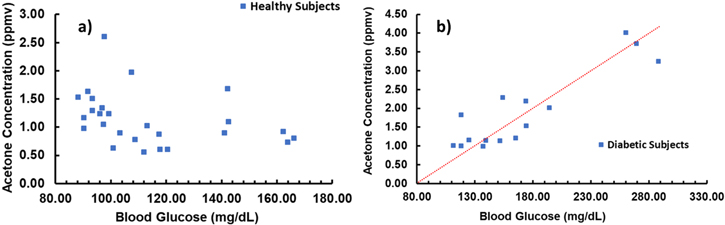
The breath acetone concentration (BAC) can differ significantly on a daily basis, principally influenced by three elements, which are: diet,2,5 exercise.6,7 and medical condition.8,9 The breath acetone concentration (BAC) is unequivocally affected by daily nature of food eaten specifically the quantity of carbohydrates and fats. A decrease in carbohydrates intake results in less availability of glucose for consumption and the organism shifts to fatty acids to work, leading to increase acetone in breath. After normal overnight fasting or long periods without food intake, similar effect is gotten, with the body consuming fats after depleting the willingly accessible resources of glucose. In the same way, during long period and/or hard physical exercise, the body may exhaust its glucose supply and, in this manner, consume fat as an energy source resulting in higher breath acetone concentration. A higher level of acetone concentration in breath may likewise be an indicator of some metabolic conditions, i.e. diabetes. In the breath of pregnant women and infants, a higher concentration of acetone can likewise be found, albeit still questionable, breath acetone concentration may depend additionally on age and Body Mass Index (BMI).2 The well established and known relationships between physiological state and acetone in-breath (Fig. 1) made the evaluation of breath acetone concentration to be a potentially powerful, non-invasive, pain-free, cost-effective and easily repeatable instrument for way of life and medical monitoring.10
Figure 1. Correlation between breath acetone concentration and blood glucose level in (a) healthy patients and (b) diabetes patients. Figure edited with permission from Ref. 11.
Download figure:
Standard image High-resolution imageAccording to the World Health Organization (WHO),12 the number of diabetes patients is increasing yearly. Based on the actual data published by World Health Organization (WHO), 422 million adults have diabetes with 1.6 million deaths registered each year. Hyperglycemia (high level of blood sugar in the body) is known to provoke serious damages in the body, including cardiovascular disease, diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, etc.13,14 Furthermore, diabete is considered as potential sources of health problems such as heart attack, stroke, kidney failure, leg amputation, vision loss, and nerve damagel.12,14 The level of exhaled acetone is usually in the range of 0.2–1.8 ppm for fit people and 1.25–2.5 ppm for people living with diabetes.3,15 and it may increases up to 25 ppm for some people leaving with type-1 diabetes.3,16 The growinfce still based on blood samg number of diabetics patients willing to control diabetes by a noninvasive method, has created a market for portable exhaled breath analyzers, while the current practice still based on blood sampling.11 At the moment, the economically accessible gas sensors for acetone identification work in the 50–5000 ppm, which is out of range for exhaled acetone levels.3 There are still difficulties in understanding these results with respect to all breath gas investigations, specifically concerning breath testing methodology and deconvoluting the distinctive origin of acetone. Though the main key prerequisite is an innovative sensor adequately cost-effective and conservative to be incorporated into individual breath analysers and point-of-care instrumentation.2,17 Sample of commercial metal oxide acetone sensor is shown in Fig. 2.
Figure 2. Picture of commercial metal oxide VOC gas sensor (Winsen MQ138) .
Download figure:
Standard image High-resolution imageNumerous investigations have been reported on a non-invasive analysis of breath for detection of acetone, such as gas chromatography with mass spectrometry (GC–MS), proton transfer reaction with mass spectrometry (PTR-MS), flame ionization detector (FID), ion mobility spectrometry (IMS) and several other sensitive techniques.18–20 Even though these techniques are sensitive and reliable but they are not portable for daily monitoring. Recent studies are focused on developing gas sensors to cover the exhaled acetone range at room temperature.3
Metal oxides are efficient catalysts of oxidation-reduction reaction.21 Semiconductor metal oxide (SMO) gas sensors are the most explored group of gas sensors.22,23 in the field (Fig. 3). In this review, we are going to summarize metal oxides based chemical sensors for detection of VOCs, more especially for acetone. In the following sections various types of oxygen-containing VOCs in exhaled breath are summarized followed by a review of chemical sensors with different groups. Finally, we described metal oxide nanomaterials gas sensors principle and mechanism, and chemiresistive breath acetone sensors.
Figure 3. Search results of literature review (2017–2019) of the exhaled acetone detection: (a) different methods: Selected‐ion flow‐tube mass spectrometry; Gas Chromatograph—Ion Mobility Spectrometer; Proton‐transfer‐reaction mass spectrometry; Gas chromatograph—mass spectrometry (b) different materials used as receptors in sensor applications presented in papers.
Download figure:
Standard image High-resolution imageVolatile Organic Compounds in Exhaled-Breath
Volatile organic compounds (VOCs) are vaporous molecules that can be sampled rapidly and non-invasively from breath. They are generated either from inside the body so-called endogenous VOCs or from external sources such as food ingestion, metabolization of drugs and environmental exposure known as exogenous VOCs. The exhaled human breath comprises nearly 3500 diverse VOCs. The presence of biomarkers in the exhaled breath are suggestives for a number of medical conditions, such as lung cancer,3,24 asthma,25 chronic obstructive pulmonary disease (COPD) 26, breast cancer,26 and diabetes.27,28 Deplorably, the accurate number of diseases that can be identified or constrained by exhaled breath analysis is so far unidentified. Due to the nature of this work, in this review, only oxygen-containing compounds are highlighted in this review and presented in the section below.
Oxygen-containing compounds
Ketone bodies is a term used to represent three molecules, acetoacetate (AcAc), 3-hydroxybutyrate (3HB) also referred to as β-hydroxybutyrate and acetone that are produced in the liver and generally found in the breath, blood, and urine.29 (Fig. 4). Out of the three ketone bodies, acetoacetate (AcAc) and 3-hydroxybutyrate (3HB) are used to convey energy from the liver to other body tissues due to their energy-rich properties. Acetone is the most abundant component in human breath,30,31 and a minor product in the ketone bodies.29 Acetone is generated by spontaneous decarboxylation or enzymatic conversion of acetoacetate (AcAc).20,29 and the dehydrogenation of isopropanol.8 Acetone is responsible for the sweet odor on the breath of individuals with ketoacidosis. The conversion of acetoacetate process is done through elimination of CO2:
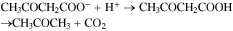
Figure 4. Schematic representation of the working principle of a chemical (biochemical) sensor.
Download figure:
Standard image High-resolution imageChemical Sensors
Principle and composition
A chemical gas sensor can be defined as a device, that can change one or more of its physical properties (mass, electrical conductivity, or dielectric properties) once exposure to a gaseous species or molecules, so that the change can be measured and quantified.32 These changes convey an electrical signal, with a magnitude that is relative to the concentration of the gas under test, which is estimated as an amount of the gas to which the measuring sensor is exposed.
Generally, chemical sensors contain two basic functional units: a receptor element and a transducer element, as shown in Fig. 4. The receptor is part of a sensor where the chemical information is changed into a form of energy so that it can be measured by the transducer. A transducer converts the energy carrying the chemical information about the sample into a useful analytical signal.
In a chemical sensor, the analyte interacts first selectively (more or less) with the recognition (or sensing) element, which should have affinity for the analyte. In addition, the recognition and transduction function are integrated in the same device. An analytical device without recognition function included is called a concentration transducer but not a chemical sensor.33
Classification of chemical sensors
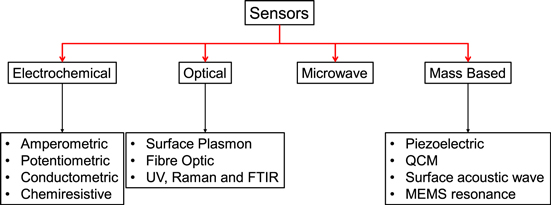
Classification of chemical sensors is accomplished in different ways. In general, there are different ways to categorize sensors in literature. According IUPAC chemical sensors may be classified according to the operating principle of the transducer.34 as listed in Fig. 5. Therefore, based on their transduction methods and modes of measurement, they can be classified into three main classes: (i) electrical and electrochemical properties, (ii) changes in the physical properties, and (iii) optical absorption of the chemical analytes to be measured.35
Figure 5. Transduction base classification of chemical sensors.
Download figure:
Standard image High-resolution imageSolid state gas sensors
Solid state sensors principle is to measure the changes in the physical property of the devices relative to chemical on the surface of a sensing element.36 A very important characteristic of solid state gas sensor is the reversible interaction of the gas with the surface of a solid-state material. In addition to the conductivity change of gas-sensing material, the detection of this reaction is also done through the measurement in the change of capacitance, work function, mass, optical characteristics or reaction energy released by the gas/solid interaction.37,38 Solid state gas sensors present the best possibility for the growth of commercial gas sensors for various applications. Recently, scientists have grown interest on solid-state sensors for detecting VOCs.39 for non-invasive diabetes management. There are several types of solid-state gas sensors with different detection principle i.e. Chemiresistive, chemical field effect transistors, calorimetric, potentiometric and amperometric.
Chemiresistors are the simplest and most available gas sensing platform. They have advantages over other platforms such as, simple fabrication, low cost, and simple measurement electronics.32 These sensors work based on the change in electrical resistance of the sensing material in presence of target analytes. Chemiresistive sensor translates chemical information through the changing of electrical resistance.40 The basic device architecture of chemiresistive sensors is generally made by coating an interdigitated electrode (gold and chromium) with a thin film or by using a thin film or other sensing material to bridge the single gap between two electrodes. In both architectures, the chemiresistive sensing material controls the conductance between the two electrodes with each device architecture having its own advantages and disadvantages. The performance of the sensor can be evaluated by evaluating parameters such as, response, selectivity, sensitivity, response time, recovery time, operating temperature, and limit of detection.40 Various materials e.g. metal-oxides, conductive polymers, graphene, carbon nanotubes have Chemiresistive properties. In this review more focus has been given to metal oxides based chemiresistive sensors for VOCs detection.
Metal Oxide Nanomaterials Gas Sensors
Semiconducting metal oxide materials (SMOMs)
Sensing behaviour is one of the most significant property of metal-oxide materials. Beside their sensitivity to light (photon) energy and external pressure, metal oxides display high sensitivity to their chemical environment. They are also able to work in harsh environment; surpass other chemical sensors in their sensitivity, reliability, and durability.32 Therefore, gas sensor industry heavily relies on metal oxide materials as sensing element. Based on the conduction type, the metal oxide semiconductors can be divided into n- and p-type,41 which exhibits different sensing behaviours to the same detecting gas. N-type semiconducting metal oxide materials such as ZnO, SnO2, In2O3, WO3, TiO2, Fe2O3, MoO3, VO2 and CeO2 are the most reported sensing materials for room temperature (RT) resistive gas sensors. They are synthesized at different forms including nanoparticles, nanorods, nanowires, nanoflowers, nanosheets, nanofilms, nanotubes, porous structures and hierarchical nanostructures. These n-type metal oxide materials have been most utilised for the detection of hydrogen sulphide (H2S), nitrogen dioxide (NO2), hydrogen (H2), ammonia (NH3), acetone (CH3COCH3), ethanol (CH3CH2OH), formaldehyde (HCHO), liquefied petroleum gas (LPG), carbon monoxide (CO), etc.42,43 P-type semiconducting metal oxide are also showing promise in gas sensing applications.44 So far, p-type semiconducting metal oxide materials such as CuO, Co3O4, NiO, have been used in RT gas sensing, and the main target gases include ammonia NH3, H2S and NO2.43 Summary of recent various types of semiconducting metal oxide materials used in the detection of oxygen-containing VOCs at RT is listed in Table I (from 2010 up today).
Table I. Summary of semiconducting metal oxide materials and their target VOCs at RT.
| Material | Shape | Synthesis Process | Type | Target gas | Agent | LOD (ppm) | References |
|---|---|---|---|---|---|---|---|
| CuO | Nanowires | Thermal oxidation | p | C2H5OH | Ox | 10 | 45 |
| CuO | Nanoribbons | Wet chemical | p | C2H5OH | Ox | 20 | 46 |
| In2O3 | Cubic crystals | Hydrothermal | n | C2H5OH | Ox | 10 | 47 |
| Au/In2O3 | Nanofibres | Electrospinning | — | C2H5OH | Ox | 20 | 48 |
| β-MnO2 | Thin films | Spray pyrolysis | p | CH3COH | Ox | 10 | 49 |
| V2O5 | Nanoneedles | Vapour deposition | n | CH3COCH3 | Ox | 0.941 | 50 |
| SnO2 | Nanoporous | Hydrothermal | n | CH3COCH3 | Ox | 10 | 51 |
| SnO2-Sb | Nanowires | CVD | — | C2H5OH | Ox | 40 | 52 |
| SnO2/graphene | Nanohybrids | Electrochemical deposition | — | HCHO | Red | 0.02 | 53 |
| SnO2/rGO | Hybrid films | Hydrothermal | — | CH3COCH3 | Ox | 10 | 54 |
| TiO2 | Nanotubes arrays | Electrochemical anodization | n | HCHO | Red | 0.04 | 55 |
| TiO2 | Nanotubes | Electrochemical | n | CH3COCH3 | Ox | 10 | 56 |
| Ag/TiO2 | Nanoparticles | Sol-gel | — | C2H5OH | Ox | 0.15 | 57 |
| Ag/TiO2 | Nanorods | Wet chemical | — | C2H5OH | Ox | 58 | |
| Co/TiO2 | Nanoparticles | Sol-gel | — | C2H5OH | Ox | — | 59 |
| TiO2/rGO | Nanosheets | Spray | — | HCHO | Red | 0.1 | 60 |
| ZnO | Thin films | Thermal evaporation | n | C2H5OH | Ox | — | 61 |
| ZnO | Tetrapod network | Thermal oxidation | n | C2H5OH | Ox | 10 | 62 |
| ZnO | Nanorods | Laser ablation | n | C2H5OH | Ox | 1 | 63 |
| ZnO | Nanorods | Electrospinning | n | C2H5OH | Ox | 1 | 64 |
| ZnO | Nanowires | Electrospinning | n | C2H5OH | Ox | 1 | 65 |
| Au/ZnO | Core-shells | Sol-gel | — | HCHO | Red | 0.5 | 66 |
| Al/ZnO | Nanowires | Electrodeposition | — | C2H5OH | Ox | — | 67 |
| Al/ZnO | Hexagonal facets | Sol-gel | — | C2H5OH | Ox | — | 68 |
| Ni/ZnO | Nanorods | Electrodeposition | — | CH3COCH3 | Ox | — | 69 |
| Na/ZnO | Nanoflowers | Solution route | — | CH3COCH3 | Ox | 0.2 | 70 |
| VO2/ZnO | Heteronanostructures | Heteroepitaxial | het | CH3COCH3 | Ox | 10 | 71 |
| α-Fe2O3/ZnO | Nanowires | Piezo-surface coupling | het | C2H5OH | Ox | 100 | 72 |
| ZnO/MWCNT | Nanorods | Hydrothermal | — | C2H5OH | Ox | 5 | 73 |
| WO3−x | Quantum dots | Solvothermal | n | HCHO | Red | 1.5 | 74 |
| WO3 | Nano-films | Thermal evaporation | n | C2H5OH | Ox | 10 | 75 |
| PANI/Cellulose/WO3 | Granules | Chemical solution | p-n | CH3COCH3 | Ox | 10 | 76 |
Note: Ox = oxidizing agent; Red = reducing agent; LOD = limit of detection; het = heterojunction (n-p; p-n; n-n; p-p).
Principle and mechanism
The sensing properties of chemiresistive gas sensors are controlled by three factors, i.e. the receptor function, transducer function, and utility factor, as shown in Fig. 6. The main concern of receptor, is how every constituent reacts to the surrounding atmosphere containing oxygen and target gases. The quantity of oxygen adsorbed in this procedure decides the sensing properties, which is directly dependent on the specific surface area of the sensing materials.77
Figure 6. Diagram description of the gas-sensing mechanism and the conduction model based on n-type and p-type Semiconducting metal oxides72 [Reprinted with permission from Royal Society of chemistry].
Download figure:
Standard image High-resolution imageThe working temperature of gas sensors plays an important role in the determination of the types of chemisorbed oxygen species (ions).43,78 The oxygen species are formed depending on working temperature as summarised in following equations.43
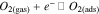
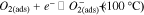
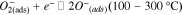
The formation of oxygen species results in the capture of electrons from conduction band of the surface layer, leading to an alteration in conductivity of the semiconducting metal oxide. Furthermore, that alteration in conductivity results from a change in the charge carrier concentration.78 The increase or decrease in conductivity of the semiconducting metal oxide material depends on the type of majority carriers in the semiconducting metal oxide material (n-type: electron and p-type: hole) and the nature of gas molecules (either oxidizing or reducing) at room temperature.
Gas sensing mechanism of N and P-type SMOMs
Generally for n-type semiconducting metal oxide materials, electrons are removed from the conduction band of the surface layer by the absorbed oxygen molecules resulting in the formation of negatively charged chemisorbed oxygen ions, and at RT the oxygen ions on the surface are basically O2-ions.43 The decrease in the electron density brings the formation of an electron depletion layer on the surface of SMOMs and formation of a potential energy barrier. (see Fig. 6). As a result, the resistance of the SMOMs increases,42 consequently the conductivity of the SMOMs decreases. Since the target plays also important role in the performance of the gas sensor. In the case of reducing gases (donors), such as H2S, H2, NH3, HCHO, etc, the chemical reaction releases electrons, which are reinjected back to the electron depletion layer. Then, the reduction of the potential barrier energy occurs due to the decrease in the electron depletion layer. Therefore, the surface resistance of semiconducting metal oxide materials is decreased and conductivity increased. Conversely, when the target gases are oxidizing gases (acceptors), such as NO, NO2, CH3COCH3, Cl2 and O3, the reaction with the chemisorbed oxygen ions will capture the electrons, which will expand the electron depletion layer, resulting in an increase of the potential barrier energy. Consequently, the surface resistance of the semiconducting metal oxide materials is increased and the conductivity decreased. In conclusion, in the presence of the oxidizing gases, n-type SMOM based sensors resistance increases and decreases in the presence of the reducing gases.
In contrast, for p-type SMOMs the sensing mechanism depends on the changes of surface resistance resulting in the changes in the concentrations of hole carriers due to the oxidation-reduction reaction between SMOMs surface and the target gases. In the presence of air at RT, two phenomena occurred on the surface of p-type SMOMs, the formation of the oxygen ions of O2- due to the oxygen molecules that have been adsorbed and electrons capture from the conduction band. Thus, an increase of density of hole carriers and a decrease in the surface layer's Fermi level, are observed. In the end, the sensors conductivity increases and their resistance decreases due to an accumulation of hole layer formed on the surface of p-type SMOMs, as shown in Fig. 6. In case the reducing gas molecules are adsorbed on the surface of SMOMs, electrons are released, which will combine with the holes, consequential, Fermi level increases and the hole accumulation layer decreases due to the reaction between the reducing gas molecules and O2− ions. Therefore, the reduction of the conductivity of the SMOMs layer is observed. Conversely, for the oxidizing gases, the surface of the p-type SMOMs allows extra free electrons to be captured. The molecules adsorbed on the sensor surface will capture electrons from the p-type SMOMs to form a negatively charged chemisorbed oxygen ions. Therefore, the concentrations of hole carriers will significantly increase, consequently the conductivity of the p-type SMOMs-based gas sensors increases. In brief, reducing gases increase the resistance of p-type SMOM based sensors and it decreases with the oxidizing gases. The mechanism for p-type SMOM based sensors is opposite to the mechanism for n-type SMOM based sensors discussed earlier.
Sensitizations of SMOMs
The sensing performance of the SMOMs gas sensors is prejudiced by its poor selectivity and addition of a suitable quantity of additives such as Pd, Pt, Au, RuO2,etc can enhance gas sensitivity and rate of response under certain conditions by modifying its surface.37,44 The mechanism of sensitization or response enhancement by additives depends on the type of metal oxide materials and can be achieved through two different types of sensitization mechanisms: chemical and electronic. In chemical sensitization, chemical reactions between target gas and metal oxide surface are assisted by the promoters that exist on the metal oxide surface through a phenomenon called spill- over. For example noble metals have been added on the metal oxides surfaces and remarkably enhanced the sensing properties of SMOMs sensors, including Pt/ZnO,79 Pd/ZnO,80 Pt/SnO2,81 Pd/SnO2,82 Pd/TiO2,83 Pt/TiO2,84 etc. In electronic sensitization, there is possibility of modification in the electrical property of the metal oxide, which is done via change in redox state of the promoter, by acceptor or donor charges from gas molecules, for example Ag/SnO2,85 Sb/SnO2,86 Na/ZnO,70 Ni/ZnO,69 Zn/NiO,87 Al/NiO,88 Sb/WO3.89
Effect of grain size on the gas sensing performance of SMOMs
The morphology of the semiconducting metal oxide materials also influences the performance of the gas sensor. Parameters such as grain size, number of activated adsorption sites and gas diffusion ability are responsible for remarkable low performance of gas-sensing. In this review only grain size is discussed since it affects the sensitivity of gas sensors. A reduction of grain size to the nanoscale level is one of the most efficient strategies for enhancing the gas-sensing properties.78,90
There are three cases which are related to the relationships between the grain size (D) and the thickness of the depletion layer (L). These mechanisms are described in terms of the boundary control, neck control and grain control.
In case of large grains with a small surface-to-volume ratio, L is significantly smaller than the single crystallite size (D ≫ 2L). Most of the grains are unaffected by the surface interactions with the gas phase. The electron conducting channels through necks are too wide to be influenced by the surface effect. Fundamentally, the conductivity of the gas sensors depends on the grain boundary barriers. Thus, the gas-sensing mechanism is controlled by the grain boundary and the sensitivity of the metal oxide materials is independent of the grain size. In case of higher surface-to-volume ratio, specifically smaller grains but still larger than twice the depletion layer (D ≥ 2L), that region extends into the grains forming necks. As a consequence, the conductivity is a product of collaboration between the cross-section area of these necks and the grain boundary barriers, resulting in sensitivity enhancement (neck controlled). Furthermore, the sensitivity of metal oxide materials depends on the grain size and increases when the grain size reduces. When D < 2L, the depletion layer extends throughout the whole grain and the crystallites are almost completely depleted. Under this situation, grains share a major part of the resistance and control the gas sensitivity. Thus, the conductivity considerably decreases due to absence of the conduction channels between the grains. The energy bands are almost flat all through the entire structure of the interconnected grains and lack of important barriers for intercrystallite charge transport, thus mostly the grains size controls the sensitivity (grain controlled). In theory and empirically the highest gas sensitivity is gotten only if (D < 2L). Inspired by the model proposed by Xu et al.,91 an excellent monodisperse α-Fe2O3 nanoparticles based acetone sensor was developed by Liang and co authors.92 The almost fully depleted grains induced changes to the overall conductivity, leading to improvement of the sensitivity.
Chemiresistive breath acetone sensors
Noninvasive diabetes detection is the future of point of care diabetes management. Hence, the number of researches in this field is increasing significantly. The recent chemiresistive breath acetone sensors for diabetes detection are presented in Table II (2017–2019).
Table II. Electrochemical based Metal Oxides breath acetone sensors (2017–2019).
| Sensing Materials | LOD (ppm) | Temperature (°C) | Response (Ra/Rg) | References |
|---|---|---|---|---|
| TiO2 functionalized In2O3 | 0.8 | 250 | 33.34 | 93 |
| NiO/ZnO hollow spheres | 100 | 275 | 94 | |
| ZnO decorated with Pt and Nb | 50 | 450/400 | 5.2/45.8 | 95 |
| TiO2 nanoporous | 500 | 370 | 25.97 | 96 |
| Au modified α-Fe2O3 | 50 | 150 | 42.0 | 97 |
| SnO2/MWCNT | 1 | 250 | — | 98 |
| PPy-WO3 Hybrid | 37 | 90 | — | 99 |
| Pt-loaded α-Fe2O3 | 0.8 | 220 | 27.2 | 100 |
| Ca-doped YbFeO3 | 0.1 | 250 | 1.72 | 101 |
| C-doped WO3 | 0.9 | 350 | 5.1 | 102 |
| CdMoO4 | 0.5 | 625 | — | 103 |
| ZnO:Ni | 116 | 340 | 68 | 104 |
| Pd-ZnO/ZnCo2O4 | 0.4 | 250 | — | 105 |
| C3N4-SnO2 | 0.067 | 380 | 29 | 106 |
| In loaded WO3/SnO2 | 50 | 200 | 66.5 | 107 |
| SnO2/SiO2 | 1 | 170 | 9.4 | 108 |
| CdNb2O6 | 0.2 | 600 | — | 109 |
| Mesoporous α-Fe2O3 nanospheres | 0.1 | 170 | 16.0 | 110 |
| Cuboid WO3 | 0.5 | 300 | 49.1 | 111 |
| ZnO-Fe3O4 | 0.1 | 485 | 47 | 112 |
| ZnO/TiO2 nanofibers | 5 | 350 | 3.08 | 113 |
| NiO/SnO2 | 0.01 | 300 | 6 | 114 |
| PANI/Cellulose/WO3 | 10 | RT (25) | — | 76 |
| NiFe2O4 | 0.52 | 160 | 1.9 | 115 |
| Ru-loaded WO3 | 0.5 | 300 | — | 116 |
| Co1−xZnxFe2O4 | 0.3 | 650 | — | 117 |
| Fe and C codoped WO3 | 0.9 | 300 | 7.3 | 118 |
| Pt functionalized SnO2 | 0.0036 | 300 | 7.0 | 119 |
| MgFe2O4/g-C3N4 | 500 | 320 | — | 120 |
| Apo-Pt@HP_WO3 | 1 | 350 | 10.80 | 121 |
| Sm2O3/SnO2 | 0.1 | 250 | 41.14 | 122 |
| WO3/Pt-GNs | 10 | 200 | — | 123 |
| Rh/WO3 | 0.5 | 250 | — | 124 |
| Sb-doped In2O3 | 50 | 240 | 64.3 | 125 |
| Cr-doped CuO | 0.32 | 450 | — | 126 |
| Go-SnO2-TiO2 | 0.25 | 200 | 6.28 | 127 |
| Pt@In2O3 | 0.01 | 320 | 6.23 | 28 |
| g-C3N4/WO3 | 100 | 340 | 35 | 128 |
| N-SnO2 | 0.007 | 300 | 357 | 129 |
Note: LOD = limit of detection; Ra/Rg = electrical resistance under exposure to air and target gas (acetone).
Metal oxide based chemiresistive acetone sensors
It can be seen from Table II that metal oxides such as, WO3, SnO2, FexOy, TiO2, CuO, ZnO and In2O3, etc have been used mostly for the detection and screening of diabetes. A brief review of some of the latest publications (2017–2019) are highlighted in the sections below.
WO3 based acetone chemiresistive sensors
Due to its chemical and physical properties WO3 has been one of the metal oxides that is frequently used for exhaled acetone detection and attracted researchers' interest. Its doping is very important in order to increase its sensing properties. The first work on WO3 based thin film sensor for automotive applications was developed and published by Gouma et al.,130 in 2003. Early this year, wang et al.,128 have developed a new acetone sensors using WO3 nanosheets and g-C3N4/WO3 composite with different amount of g-C3N4 loaded. Comparatively to pristine WO3 nanosheets and g-C3N4 acetone sensors, g-C3N4/WO3 gas sensor presented good response, excellent selectivity, ephemeral response and trace detection ability to acetone vapor. The results obtained with 1 wt% g-C3N4/WO3 at 340 °C (100 ppm acetone) have shown better response (Ra/Rg) of 300% higher than the response value of pure WO3 sensor with a fast response/recovery speed (9 s /3.8 s) and large linear detection range (0.5 ppm−500 ppm). Li et al.,116 have used Ru-loaded WO3 nanoparticles for acetone detection. The response obtained with Ru-loaded WO3 sensors has been times higher than neat WO3 with a low limit of detection (0.5 ppm). However, 1 wt% Ru loaded WO3 gave highest response (Ra/Rg) around 7.3 at 300 °C at 1.5 ppm acetone vapour. Kim et al.,121 have presented WO3 nanofibers (NFs) with hierarchically interconnected porosity (HP_WO3 NFs) acetone sensor. The sensor exhibits response (Ra/Rg) of 10.80 at 1 ppm of acetone with high humidity atmosphere (90% Relative Humidity (RH)). Qiu et al.,124 prepared WO3 nanosheets dispersed with Rh at its surface through a wet impregnation method. The resistance response to acetone obtained with 1wt. and 2wt.% Rh-WO3 , were about three orders of pristine at 250 °C with linear range of 0.5 to 10 ppm. Chen et al.,123 have worked on the acetone sensors using for gravure-printed WO3/Pt-decorated rGO nanosheets composites. The highest response for the sensor at 200 °C was 12.2 to 10 ppm. Shen et al.,118 have used iron and carbon codoped WO3 with hierarchical walnut-like microstructure to develop a selective acetone sensor. The sensor showed better response to 10 ppm of acetone of almost 17 (Ra/Rg) at 300 °C for 0.992 at1 % Fe/WO3.
FexOy-based acetone chemiresistive sensors
This group of iron oxides involves commonly: FeO, Fe2O3, and Fe3O4, with Fe2O3 being the most used in gas-sensors. The high operating required for Fe2O3-based gas sensors (450 °C–1075 °C), made its use less compare to other metal oxides. Nevertheless, recently Zhang et al.,120 have synthesized magnesium ferrite (MgFe2O4) decorated with g-C3N4 porous microspheres composites to detect acetone. The sensor response of MgFe2O4/g-C3N4 composites with 10 wt% g-C3N4 based sensor showed an enhancement of ∼145 times at lower temperature of 60 °C in comparison to a pristine MgFe2O4. Another group Wang et al.,115 have presented the acetone sensor working at low concentrations ∼1 ppm and lower temperatures ∼160 °C, which is based on NiFe2O4 nanocubes. The maximum response R = Rg/Ra was 30.4 (160 °C/2 00 ppm) and around 12 under 50 ppm of acetone (160 °C).
SnO2-based acetone chemiresistive sensors
SnO2 has high chemical stability and exceptional electrical properties, with broad band-gap energy (3.6 eV).39 SnO2 has been the most broadly used material in gas sensors, including improved acetone detection, especially when doped with other semiconductor metal oxides (e.g. ZnO, CuO and Sm2O3). Recently, Guan et al.,129 have developed Nitrogen-incorporated SnO2 nanostructure for high-performance acetone gas sensing. The sensor showed sensor response of (Ra/Rg − 1 = 357), low limit of detection (7 ppb) as well a wonderful sensitivity for acetone gas at 300 °C. Du et al.,113 developed an acetone vapour sensor using a synthesized hollow SnO2/ZnO heterojunctions nanofibers. The SnO2/ZnO sensor exhibits the highest response to each concentration, compared to the sensors with SnO2 alone and ZnO alone. The SnO2/ZnO, SnO2, and ZnO based sensors exhibited the responses of 3.08, 1.17, and 1.14, to 5 ppm acetone at 375 °C, respectively. This result showed better response for SnO2/ZnO compare to the other two alone, and has response time of 12-s as well recovery time of 27-s. Hu et al.,114 synthesized NiO/SnO2 (p/n) hierarchical structures via hydrothermal method for acetone sensor in the temperature range of 210 °C–390 °C. The maximum response obtained at 300 °C under 50 ppm of acetone was 20.18 (R = Ra/Rg). Kalidoss et al.,127 have investigated acetone in diabetes mellitus patients' breath in the linear range of 0.25 ppm to 30 ppm at 200 °C using of GO-SnO2-TiO2 ternary nanocomposites. The GO-SnO2-TiO2 sensor exhibits the response of 60 (Ra/Rg) to 5 ppm acetone gas. Tomer et al.,107 have synthesized an indium loaded WO3/SnO2 nanohybrid for acetone sensor. The results obtained from In/WO3-SnO2 (2 wt% Indium) sensor were 66.5 (Ra/Rg) as sensor response to 50 ppm of acetone with limit of detection ∼1 ppm at 200 °C, which were the highest compare to the other loaded. Asgari et al.,108 have developed acetone sensors based on SiO2 decorated with different wt% SnO2 in a wide temperatures range (70 °C–420 °C) and concentrations (0.5–5 ppm) as well. The highest response of ∼ 2193.7 with 300 ppm of acetone at 270 °C was obtained with 80 wt% SnO2/SiO2. The response (S = Ra/Rg − 1) of 80 wt% SnO2/SiO2 sensor to 0.5, 1, 2.5, and 5 ppm of the exhaled acetone was 1.4, 9.4, 24.1, and 37.5, respectively.
TiO2-based acetone chemiresistive sensors
Its gas-sensing properties are especially related to its structure; therefore many research groups continue to utilize this material with specific complement on its differing nanostructures. In 2017, Park.93 has done measurements of exhaled acetone using TiO2 nanoparticles functionalized with In2O3 nanowires for exhaled acetone measurements. The results of measurements were completed as a function of 0.1, 0.2, 0.5, 1, 2, 5, and 10 ppm acetone at 250 ◦C with the corresponding responses (Rg/Ra) of 4.07, 4.83, 6.17, 8.8, 12.25, 20.55, and 33.34, respectively. Chen et al.,96 synthesized nanoporous TiO2 by a facile hydrothermal method which does not use surfactant or template. It was discovered an improvement in the gas response of TiO2 to acetone due to a high concentration of Oads in the nanoporous TiO2 that was synthesized. Excellent response/recovery time, good linear dependence, certain selectivity as well as repeatability and long-term stability at 370 °C were also obtained with nanoporous TiO2, which making it as a potential material for acetone sensing.
In2O3-based acetone chemiresistive sensors
Indium oxide (In2O3), especially in its cubic form, has been generally utilized in the microelectronic field, including gas sensors. Its use in gas sensing depends strongly on its synthesis conditions, which will decide the atomic structure formation, phase composition, and electronic states in the sensing material.10 In 2018, Liu et al.,125 have developed a sensor based on Sb-doped In2O3 microstructures for acetone detection. 2 mol% Sb composite sensor presented the maximum response (Ra/Rg) of 64.3 with 50 ppm of acetone at 240 °C as well fast response/recovery time of (8/27 s), and long-term stability.
ZnO-based acetone chemiresistive sensors
Zinc oxide (ZnO) is a n-type material and a promising semiconducting metal oxide for gas-detecting applications. In 2017, Wongrat et al.,95 reported on Pt and Nb decorated ZnO nanostructures. Maximum sensor response values of 188.0 and 240.0 respectively were obtained for sensors based on ZnO:Pt and ZnO:Nb upon exposure toward acetone vapor at 1000 ppm concentration at 400 °C.
CuO-based acetone chemiresistive sensors
Among the copper oxides, due to its p-type semiconducting property, CuO is the most reported material used in gas sensors. Late in 2018, Szkudlarek, A., et al.,126 have investigated the effect of Cr-doping on the electronic structure of CuO and Cr-doped CuO thin films, deposited via DC-pulsed magnetron sputtering at 100 °C. The results showed that the highest response was obtained with 3.2 ppm of acetone at 450 °C and limit of detection ∼0.4 ppm. The developed sensor shows high sensitivity to acetone at lower concentrations with very high operating temperature compared to the latest achievement in this field. Bernascon et al., developed an ultra-low cost, a single-use flexible electrochemical sensor based on plated Ni/Pt and inkjet printed with CuO NPs, with a PET as supporting layer. The results obtained the sensor showed a good reproducibility, high sensitivity (around 1600 μA mM−1 cm−2), a wide linear range and a low detection limit, coupled with a good insensitivity to other sugars present in blood sample.131
Challenges in metal oxides-based breath acetone sensors
Current challenges in metal oxide based chemiresistive gas sensors are the management of improvement of sensitivity, selectivity and stability.132 A better management of these challenges can lead to a good breath sensor with low detection limit, fast response time and stability. Few of the strategies to improve sensor characteristics are presented in the section below.
General strategies for improvement of sensor characteristics
As mentioned above, management of particle size and porosity of the material can improve the sensitivity of the material in gas sensing. For instance, usually nanostructured metal oxide shows better electrocatalytic activities than its corresponding bulk material leading to the surface interactions and curvature properties enhancement.133 Another way to have a high sensitivity is the synthesis of small size metal oxides materials especially in nanometers level.20,134 Doping of metal oxides with low concentration of metals, such as gold, silver, copper, palladium, platinum and fluorine enhance sensitivity of metal oxides gas sensors as well.
The enhancement of selectivity of metal oxides gas sensors is achieved through two different ways. The first way consists on the synthesis of a material that is strictly sensitive to analyte of interest having a low or zero cross-sensitivity to other analytes that are present in VOCs. This is possible by initially optimizing the working temperature, doping elements and their concentrations during synthesis.20,135 The second way is based on the preparation of materials for discrimination between several analytes in a mixture. This can be done by using one sensor signal; which is normally done either by doing sensor temperature modulation.20,136 or by using sensor arrays.20,137
The low stability of metal oxides gas sensor leads to problems such as false alarms, uncertain results and finally the sensor replacement. Synthesis of Metal oxides nanomaterials such as nanorods, nanotubes, nanowires and so on does not solve totally the problems since those nanomaterials can be easily degraded because of their high reactivity. However, the stability of metal oxides gas sensor can be improved by calcination during preparation and annealing of the film as the post-processing treatment and furthermore reducing the working temperature of the sensor element.20 Another way of increasing stability of metal oxides breath sensor is to synthesize a mixed metal oxides or/and dope metal oxides with carbon nanotubes.135 Zhang and coauthors.122 managed to increase the response of pure SnO2 by more than 2.29 times after loading samarium oxide (Sm2O3). The lowest detection limit (LOD) obtained (around 100 ppb) due to the increase in oxygen vacancies created by the substitution of samarium in the SnO2 lattice. Moon et al.,119 functionalized SnO2 hemipill network with a hollow Pt. The result of functionalized SnO2 has shown high quality response compared to non-functionalized one with detection limit of 3.6 ppb obtained with 200 ppb of acetone and high humidity (RH 80%). Shen et al.,102 developed a porous C-doped WO3 hollow spheres biosensor that has managed to get selectivity and selectivity for breath acetone detection. The sensor has shown better response with good selectivity ∼ to 0.1ppm over other VOCs such as ethanol, toluene, methanol, benzene, NH3 and CO. Kim and coauthors.138 worked on an important factor, the catalytic sensitization of the sensing layers to increase sensor selectivity. The functionalization of WO3 nanofibers (NFs) by Rhodium nanoparticles (Rh2O3NPs) has been used to achieve highly sensitive and selective breath acetone detection. A biosensing layer based on maize straw-templated hierarchical porous Ni doped ZnO (STHPS ZnO:Ni) has been synthesized by Zhang et al.,104 The breath acetone measurement result has shown the short response of 6 s, recovery times of 2 s with detection limit of 116 ppb. The stability of breath acetone sensor for more than one year was obtained by Narjinary et al.,98 using nanocomposites of multiwall carbon nanotubes (MWCNT)–SnO2. Because of the MWCNT loaded in pristine SnO2, the sensor response increases from 50% to 80%.
Effects of operational temperature and relative humidity
Operational temperature is another major limitation that affects sensing application. Even though a lot of work has been done to overcome sensitivity and selectivity of the breath acetone sensors, high operational temperature still the main disadvantage. The disadvantage associated are lack of flexibility, high power consumption, safety hazards, reduced device lifetime,139 unfeasible accommodation of inflammable substances and other ecological impacts.10 Many researches have been performed to develop room temperature acetone sensor. Conducting polymers due to their possible high sensitivity, short response time, room temperature operation, etc are useful in overcoming the problem. For example, a conductive polymeric matrix of PANI-WO3 nanomaterial doped with cellulose was applied to the detection of acetone at room temperature and low concentrations. A limit of detection of 10 ppm was obtained for the developed sensor after repeated exposure of acetone, showing its suitability for room temperature sensing of acetone without the major shortcomings of larger systems required by high operating temperatures.76
Relative humidity (R.H) or moisture is one of the environmental conditions that affect the performance of gas sensors. In this way, the acetone sensing should be imperatively be optimized at around 80%–98% (R.H) before measurement because of the high humidity of breath acetone. Therefore, consistent acetone detection needs to be insensitive to relative humidity or moisture. For example, Righettoni et al.,140 have developed a new breath acetone sensing material based on Si:WO3. A significant decrease in sensitivity over humidity of 4.5% between 80 and 90% R.H was obtained. In 2014, Salehi et al.,141 have measured the effect of competition between acetone and water adsorption on Carbon nanotubes–SnO2 nanocomposite based human breath gas sensor at 85% RH as well at low temperature of 37 °C. The response of sensor was within tolerable range, while it was decreasing. Early research was done by Yu et al.,142 which confirmed irrelevant relative humidity effect to their Polypyrrole based sensor with unfortunate maximum investigated relative humidity effect of 45%.
Conclusions and Future Perspectives
Different conventional and sophisticated techniques, such as, GC-MS, SIFT-MS, PTS-MS, HPLC, etc are used for the detection of diabetes as mentioned in this review. Unfortunately, those techniques are invasive and expensive. Development of chemiresistive metal oxide-based acetone sensors are the future of non-invasive point of care diabetes management. However, use of metal oxides present some challenges in chemiresistive sensing applications, i.e. sensitivity, selectivity and stability. The improvement of those challenges will lead to better diabetes management. In this review article, challenges and better strategies to enhance the chemiresistive behaviour of metal oxides for acetone detection have been presented as a guide for future perspective. In addition, the need for more work on selected doped metal oxide materials, are highlighted.
Acknowledgments
The authors would like to thank the National Research Foundation South Africa for funding the project (Grant UID).