Abstract
We synthesized a copper rubeanate metal organic framework (CR-MOF) which has the potential to improve the catalytic activity of electrochemical reduction of CO2 due to its characteristics of electronic conductivity, proton conductivity, dispersed reaction sites, and nanopores. Synthesized CR-MOF particles were dropped on carbon paper (CP) to form a working electrode. The onset potential for CO2 reduction of a CR-MOF electrode was about 0.2 V more positive than that observed on a Cu metal electrode in an aqueous electrolyte solution. Our analysis of the reduction products during potentiostatic electrolysis showed formic acid (HCOOH) to be virtually the only CO2 reduction product on a CR-MOF electrode, whereas a Cu metal electrode generates a range of products. The quantity of products from the CR-MOF electrode was markedly greater (13-fold at −1.2 V vs. SHE) than that of a Cu metal electrode. Its stability was also confirmed.
Export citation and abstract BibTeX RIS
The electrochemical reduction of carbon dioxide (CO2) into useful products at ambient pressures and temperatures has been a focus of research interest. A huge number of studies have been carried out in the past several decades especially for metal electrodes in aqueous media.1–4 Most metals have little catalytic activity for CO2 reduction and mainly evolve hydrogen due to electrolysis of water, a competing reaction. Some metals produce carbon monoxide (CO) and formic acid (HCOOH), and, especially for HCOOH formation, a high applied potential of below −1.5 V vs. SHE is generally needed to obtain sufficient current for the process. Copper (Cu) has the unique property of producing hydrocarbons3,5,6 and would appear to be the most hopeful candidate as a catalyst for CO2 electrochemical reduction; however, Cu generates a range of reaction products and the selectivity for each product tends to be low. To improve its selectivity, there have been several explorations with metal alloys.7 The basic idea is to disperse and specify the reaction site and to change the concentration of protons that are needed for electrochemical reduction of CO2. These protons are adsorbed by the atoms adjacent to each Cu site, where they induce a change in local reaction conditions and alter the reduction products of CO2.8
Metal Organic Frameworks (MOFs) which have backbones constructed from metals and organic ligands have also been studied intensively. MOFs are structured and porous materials with nano-scale pores, and have been tested for applications including gas storage,9,10 gas separation,9,10 and heterogeneous catalysis.9,11,12 Interestingly, some MOFs, such as copper rubeanate and its derivatives, show proton conductivity13 that results from the adsorption of a proton to a nitrogen atom in the ligand, accompanied by electron transfer from Cu(II) ions.14 Those MOFs have been applied as electrochemical catalysts in ethanol fuel cell systems and have demonstrated selective formation of acetaldehyde as an oxidant of ethanol.15 In spite of the interest shown in this area, there have until now been no trials on the reduction of CO2 using this system.
Constructing a proton-conductive MOF can thus be expected to implement the advantages of metal alloys. In the MOF structure, metal sites are dispersed and the reaction site needs to be specified. Every reaction site should receive a ready supply of protons due to the proximity of the above-mentioned proton-conductive ligand. Moreover, since the surrounding of the metal reaction sites in MOF materials has very limited space due to the presence of nanopores, the reaction pathway in the MOF catalyst should be very restricted, giving the reaction a high selectivity. Both of these conditions, which are characteristic of MOF materials, should favor the effectiveness of the reaction.
In this study, we report the first trial to employ MOF in the electrochemical reduction of CO2.
Experimental
The experimental compound was prepared from an ethanol solution of 50 mM rubeanic acid (Wako Pure Chemical Industries, Ltd.) and aqueous solution of 50 mM CuSO4 5H2O (Wako Pure Chemical Industries, Ltd.). The precipitate was washed with water and ethanol several times, separated by centrifuge, and air-dried to obtain individual particles of copper rubeanate metal organic framework (hereinafter referred to as CR-MOF). The quality of the sample was checked by elemental analysis, powder X-ray diffraction (XRD; Rigaku RINT2500) and infrared (IR) spectra (JASCO, IR-4200).
To prepare a slurry, 100 mg of CR-MOF particles were then dispersed in 3 mL isopropanol solution. 100 μL of CR-MOF slurry was dropped on a conductive carbon paper (hereinafter referred to as CP) with a thickness of 0.36 mm (Toray Industries Inc., TGP-H-120), and measuring 18 mm square. A CP deposited with CR-MOF particles was air-dried and gripped with a gold-plated electrode holder for use as a cathodic working electrode.
Electrochemical reduction of CO2 was performed in an H-type cell with a Ag/AgCl reference electrode and platinum wire as the counter electrode. Electrochemical control was maintained using a potentiostat (ALS, Model 760). The cathode and anode compartments were separated by a cation exchange membrane (Nafion 117). An aqueous 0.5 M KHCO3 solution was used as the electrolyte. Special-grade chemicals and Millipore water (18.2 MΩcm) were used in all the experiments.
Current – Potential (j-U) curves were measured under magnetic stirring with N2 and CO2 (99.999%) gas bubbling, respectively.
Before electrolysis, CO2 was bubbled into the stirred catholyte to purge the residual gas components and fill the cathode compartment with CO2. Potentiostatic electrolysis for CO2 reduction was performed under sealed conditions.
After electrolysis, the gaseous and liquid products were analyzed by gas and liquid chromatography, respectively. Hydrogen was determined using a TCD detector, and CO and hydrocarbons were determined using an FID detector attached to the gas chromatograph (GL Sciences Inc., GC-4000, using packed columns). A high performance liquid chromatograph (Shimadzu, LC-2010) was used for detecting HCOOH using a TSK-gel SCX(H+) column (Tosoh) with UV detectors.
Results and Discussion
The results of elemental analysis for the obtained CR-MOF particles (Found: C, 10.0; H, 1.8; N, 11.9; S, 30; Cu,34) showed good agreement with the values obtained from the literature (C, 11.08; H, 1.66; N, 12.07; S, 33.19; Cu, 34.9).16 Incidentally, the difference between the calculated and founded value in the literature was caused by problems with removing water content. Our obtained CR-MOF particles therefore had the desired composition, similar to that in the literature.
XRD measurements showed broad peaks around 2θ = 15.3 and 27.8 degrees, indicating that our CR-MOF particles were crystalline, not amorphous. The positions of the peaks were similar to those in the literature.17
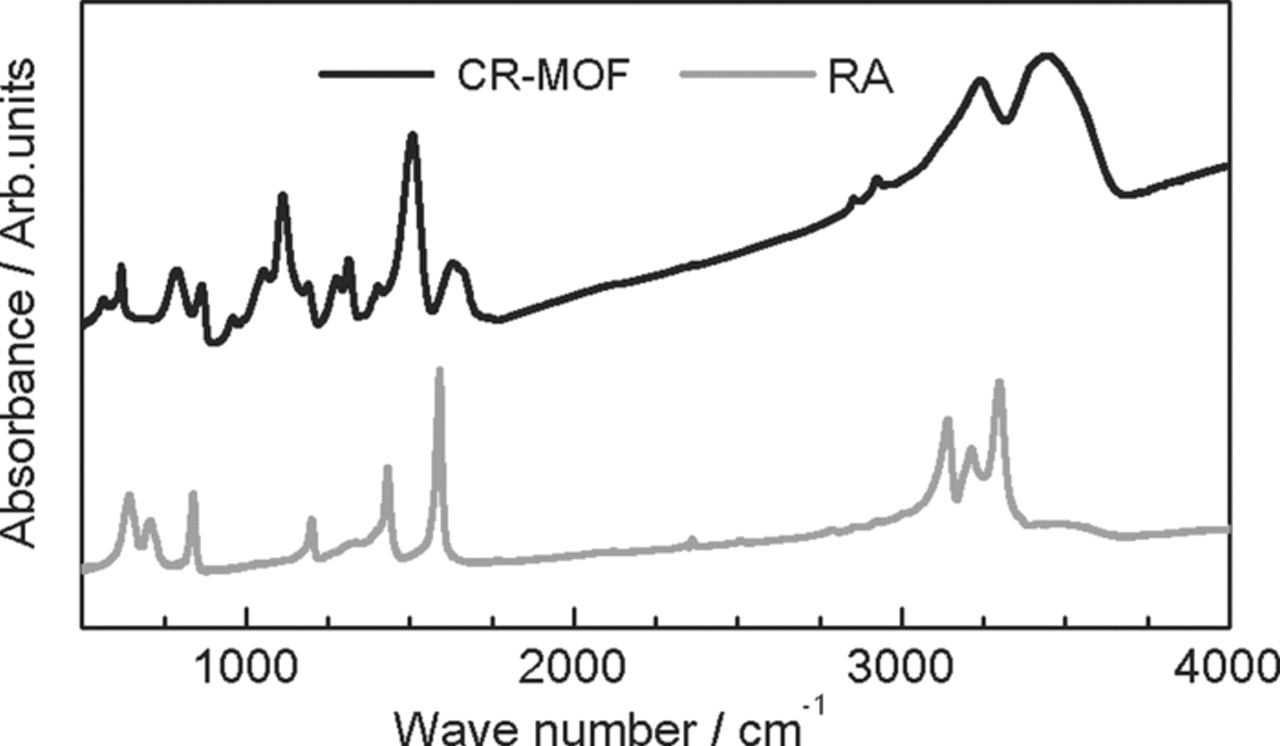
Figure 1 shows the IR spectra of the prepared CR-MOF particles and rubeanic acid (RA) as a constituent. The CR-MOF spectrum appeared broader than that of RA, clearly suggesting the formation of a polymer structure. The absorption peaks of the CR-MOF (3241, 1508, 1278, 1112, 865 and 788 cm−1, respectively) were very similar to the values recorded in the literature.16
Figure 1. IR spectra for CR-MOF and rubeanic acid (RA).
The above-mentioned results of elemental analysis, XRD and IR indicate our samples to have been stoichiometric crystalline polymer compounds.
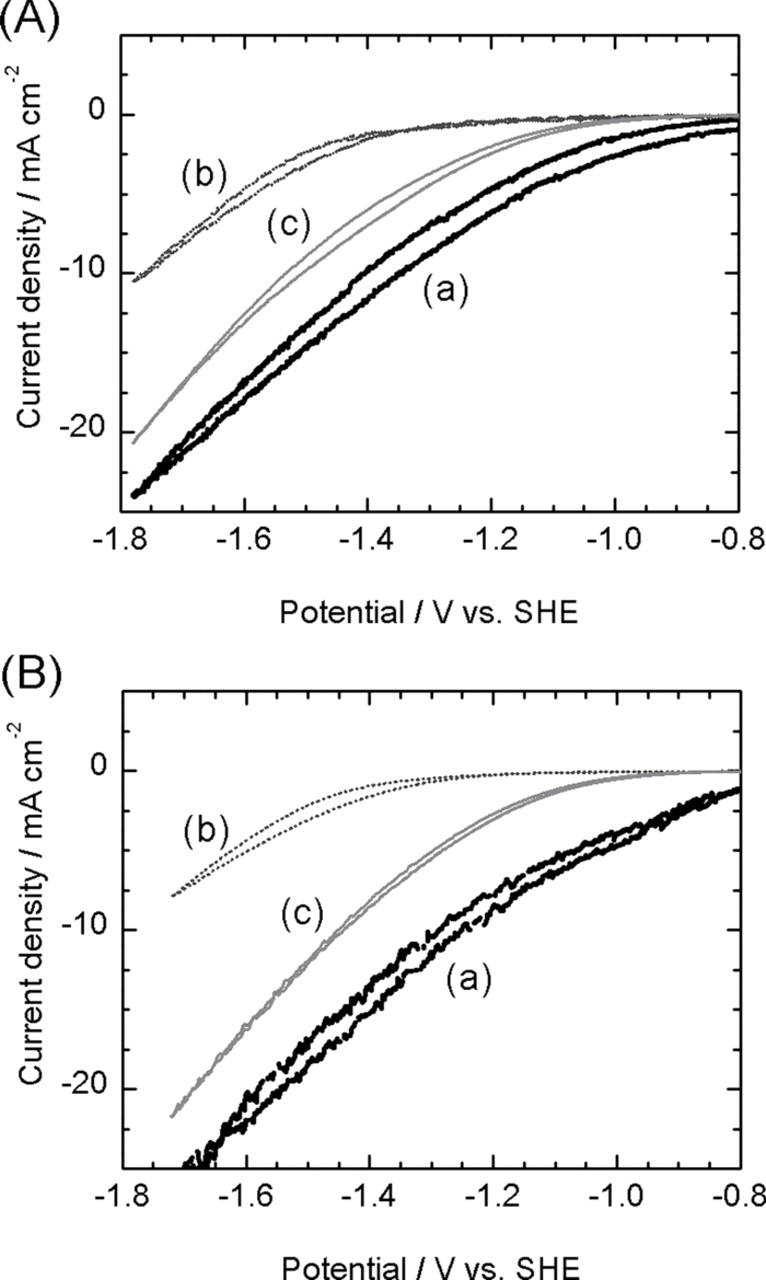
Figure 2 shows the results of the Cyclic voltammetry (CV) of the CP electrode with CR-MOF (a), comparing the data for a bare CP (b) electrode and Cu metal electrode (c) in 0.5 M KHCO3 aqueous solution; (A) is the CVs after CO2 saturation and (B) is the CVs before CO2 saturation which was deaerated by N2 gas. The pH of the solution (A) was 8.7, and that of (B) was 7.7, respectively. Current density in Fig. 2 was calculated by dividing the measured current of each electrode by the apparent surface area. Potential values are translated to SHE (Standard Hydrogen Electrode) values. To compensate the pH difference between (A) and (B) solutions, the potential of (B) was corrected by +59 mV to make it equivalent to the pH 7.7 of CO2 saturated solution (A).
Figure 2. Cyclic voltammetry in 0.5 M KHCO3 aqueous solution on (a) CR-MOF, (b) bare CP, and (c) Cu metal electrode. (A): CO2 saturated, (B): N2 bubbled solution.
In the CO2 saturated solution (A), the cathodic current of the CR-MOF electrode (a) rose to a more positive potential by about 0.6 V than that of CP (b); and a significant increase in the cathodic current was observed, clearly demonstrating that CR-MOF acts as a catalyst for CO2 reduction. The CR-MOF electrode showed a more positive onset potential by about 0.2 V than that of the Cu metal electrode (c). CR-MOF should thus have better catalytic activity for CO2 reduction than Cu metal.
Regarding the cathodic currents of the CR-MOF electrode (a) and Cu metal electrode (c), those in the CO2 saturated solution (A) were smaller than those in the N2 bubbled one (B), whereas CP electrode (b) showed similar CV curve in (A) and (B). The decrease of the current in CO2 saturated solution should be caused by the slow reaction rate of CO2 reduction.3 Although H2 production only occur in the N2 bubbled solution (B), the reaction substitute slow CO2 reduction for fast H2 evolution in the CO2 saturated solution (A), resulting in the decrease of the cathodic current. On the CP electrode (b), the main reaction was H2 evolution (as describe later) even in the CO2 saturated solution (A), therefore CVs are similar both (A) and (B).
Figure 3 shows a product analysis of the CO2 reducion for potentiostatic electrolysis on (a) Cu metal electrodes and (b) CR-MOF electrodes, respectively. Copper metal electrodes produced HCOOH, CO, and hydrocarbons such as methane, ethylene and ethane. The quantity of these products rapidly decreased at −1.2 V vs. SHE. On the other hand, the CR-MOF electrodes produced HCOOH with a current efficiency of 30% at every potential. The remaining product was hydrogen. The selectivity of HCOOH among the CO2 reduction products was more than 98%. It clearly indicate that the CR-MOF have high selectivity for HCOOH formation on electrochemical reduction of CO2. One of the reason of this high selectivity should be considered that the CO2 adsorption to the CR-MOF become weaker than that to Cu. HCOOH is known as easy to produce at weak adsorption of CO2,3 The metallic site of the CR-MOF is ionic14 and the density of electrons on the site of the CR-MOF is smaller than that on Cu metal. Decrease of density of electrons should cause weak adsorption of CO2 on the reaction site, resulting in the selective formation of HCOOH. Concerning of nano-pore effect, we haven't realized yet because the pore size of our synthesized CR-MOF hasn't been well-controlled and a little bit large to appear some nano-sized pore effect. Further examination is now proceeding to clarify the reasons for high selectivity of HCOOH.
Figure 3. Product analysis of CO2 reduction for potentiostatic electrolysis on (a) Cu metal and (b) CR-MOF electrodes.
Furthermore, the amount of HCOOH on CR-MOF was 13.4 μmol/cm2/h, whereas that on Cu was 1.1μmol/cm2/h at −1.2 V vs. SHE. In brief, the quantity of products at the CR-MOF electrode was greater than that at the Cu metal electrode, notably 13-fold greater at low applied potential. A bare CP electrode, by contrast, evolves only hydrogen and very small quantities of CO2 reduction products. The CR-MOF electrode shows special catalytic activity for HCOOH formation, even at low applied potential. The reason of the low applied potential is assumed that MOF is a kind of the metal complex. Some investigations have shown that control of the reaction steps by use of appropriate organic ligands in certain metal complex materials can decrease the applied potential needed for CO2 reduction.18,19
Figure 4 shows the formation of HCOOH by CO2 reduction on the CR-MOF electrode with elapse of time. The amount of HCOOH was in proportion to time, indicating that the reaction of CO2 to HCOOH proceeded constantly during electrolysis, without any degradation of the electrode.
Figure 4. Formation of HCOOH by CO2 reduction on CR-MOF electrode at −1.3 V vs. SHE with elapse of time.
Conclusion
Effective conversion of CO2 into HCOOH can be achieved using CR-MOF in aqueous media by means of potentiostatic electrolysis.
We successfully prepared particles of CR-MOF that analysis confirmed to be the intended polymer compound, and we applied these particles to the electrochemical reduction of CO2 in aqueous electrolyte solution. The onset potential for CO2 reduction of a CR-MOF electrode occurred at potentials approximately 0.2 V more positive than those observed with a Cu metal electrode. Our analysis of the products of potentiostatic electrolysis showed formic acid (HCOOH) to be almost the only reduction product of CO2 on the CR-MOF electrode, whereas the Cu metal electrode generated a range of products. Furthermore, the quantity of products on the CR-MOF electrode was greater than that of Cu metal electrode, specifically 13-fold, at −1.2 V vs. SHE. Electrolysis over time showed that a constant reaction proceeded at the CR-MOF electrode.
These results clearly show that good selectivity and applied potential can be achieved by adopting CR-MOF as a catalyst for the electrochemical reduction of CO2.
Acknowledgment
The authors gratefully thank Prof. Hiroshi Kitagawa for useful discussions about copper rubeanate metal organic frameworks.