Abstract
To modify the corrosion protection provided by conversion coated Zn-Al-CO3 layered double hydroxides (LDHs) on electrogalvanized steel sheets (EG steels), methods to intercalate NO3− during post treatment were explored and the effect of intercalation on corrosion resistance was evaluated. EG steels were immersed in a Na2Al2O4 based solution to form a Zn-Al-CO3 LDH conversion coating layer, and were then immersed in a NaNO3 methanol solvent to intercalate NO3−. After 2 h of immersion, it was found that NO3− had completely exchanged with the originally intercalated CO32− anions in the conversion coated Zn-Al-CO3 LDHs. Corrosion resistance measurements of the EG steel surfaces protected by the NO3− intercalated in the LDH layer were performed by a simple immersion test and a droplet test. When the samples were immersed in a large amount of 0.1 M NaCl, the corrosion resistance of the NO3− intercalated LDH layer was inferior to that of the control sample. In contrast, the samples that were exposed to a 100 μl droplet of 0.1 M NaCl displayed improved corrosion resistance. The difference in these results was attributed to the solubility of the LDH layer and the resulting effect on the release process of NO3− from the LDH layer.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Layered double hydroxides (LDHs) are chemical compounds with the general formula M2+(1-X)M3+X(OH)2(An−)x/n·mH2O, where M2+ is a divalent cation, M3+ is a trivalent cation and An− is an anion. LDH compounds have layered crystal structures consisting of a mixed M2+/M3+ hydroxide layer separated by a layer containing hydrated anions An−. Among other cations, Zn2+ and Mg2+ are commonly used for M2+ and Al3+ is commonly used for M3+. It is well known that the interlayer anions are exchangeable with anions present in a surrounding solution or corrosion environment.
This anion exchange is the basis for the storage-and-release inhibitor concept that underlies LDH-based corrosion inhibiting pigments. The effects on corrosion resistance arising from the addition of LDH-based pigments to organic coatings have been widely investigated. R.G. Buchheit and S.P.V. Mahajanam et al. reported that [V10O28]6− intercalated Zn-Al-based LDHs added to an organic coating layer improve the corrosion resistance of an Al alloy substrate.1–4 They also demonstrated that the [V10O28]6− anions were released from the LDHs during an exchange with Cl− anions found in the surrounding Cl−-rich corrosion environment.1–4 G. Williams and H.N. McMurry showed that the addition of CO32−, NO3− or CrO42−-containing Mg-Al-based LDHs to organic coatings improves the filiform corrosion resistance of Al alloy substrates.5–10 These coatings have also been applied to other materials such as galvanized steel sheets and cold-rolled mild steel with promising results.11–14 Regarding galvanized steel sheets, it has been reported that LDH pigments inhibit the delamination of organic coatings. The efficiency of interlayer anions was found to adhere to the following order: CO32− < MoO42− < NO3− < WO42− < VO43− < PO42− < CrO42−.11
Because LDH compounds can be produced easily by hydrothermal synthesis, LDH-based conversion coatings produced by simple immersion processes are also possible. These easily produced coatings have been applied on Al alloys and galvanized steel. For example, it was previously shown that a corrosion-resistant Li-Al-CO3 LDH coating will form on Al alloy substrates by simple immersion of the substrate in a lithium carbonate solution.15–18 It has also been shown that a corrosion-resistant Zn-Al-CO3 LDH coating forms on hot-dip galvanized steel sheets by immersion of the sheets in a high alkaline sodium aluminate solution.19 Coatings produced by these simple immersion processes, however, do not provide a storage-and-release inhibitor effect due to the extreme stability of the intercalated CO32−, which leaves it insusceptible to anion exchange.
The stability of intercalated anions in LDHs is a function of both the anion size and charge. The stability order of typical anions is: I− < NO3− < Br− < Cl− < F− < OH− < SO42− << CO32−.20 Therefore, successful anion exchange in a Cl−-rich corrosive environment requires an anion less stable than Cl−. A possible candidate and the anion chosen for this work is NO3−.
As noted above, CO32− is by far the most stable intercalated anion in LDHs. Due to its omnipresence, it tends to dominate in LDHs formed during immersion processes. This poses problems when considering three common methods customarily employed to intercalate desired anions in LDHs: (1) Anion exchange under aqueous conditions is virtually impossible due to the stability of the CO32− anions. (2) Calcination is also unfeasible because a temperature of 450°C is required to drive off intercalated CO32− and the melting point of Zn is 420°C; and (3) Co-deposition of M2+, M3+ and the desired anion under aqueous conditions often results in CO32− contamination, again due to its omnipresence, especially under the alkaline conditions required for co-deposition. Recently, some new methods for exchanging anions in LDH powders containing a stable CO32− interlayer have been reported. N. Iyi et al. reported that it is possible to intercalate Cl− into Mg-Al-CO3 LDH powder by using dilute HCl as the delivery solution.21 J. Hyeon et al. described using a solvothermal treatment to intercalate aliphatic dicarboxylic acid into Mg-Al-CO3 LDH powder deposited on Si substrates.22 A. Hayashi et al. demonstrated that both NO3− and Cl− can be intercalated into Mg-Al-CO3 LDH powder by using alcohol as the delivery solvent.23 This method seems applicable to LDH conversion coatings on galvanized steels because it can presumably be used to effectively alter the LDH, but it would not affect other parts of the substrate such as the metallic Zn layer.
In this study, a Zn-Al-CO3 LDH conversion coating layer on electrogalvanized steels (EG steels) was modified by the intercalation of NO3− during a post treatment process, and the subsequent corrosion resistance was evaluated. NO3− was chosen as the replacement anion because it has been shown to provide better corrosion resistance than CO32− as an interlayer anion in LDHs added to organic coatings on galvanized steels.20 Furthermore, the storage-and-release concept is expected to provide for the uptake of Cl− and the release of NO3−. Methanol was used as the delivery solvent during the exchange of NO3− for CO32−.
Experimental
Test specimens and treatment procedures
The test samples were EG steels having a thickness of 0.75 mm. The coating weight of Zn on the evaluated side of the test specimens was 64 g/m2. All the specimens were provided from JFE Steel Corporation. The specimens were cut to 50 mm × 70 mm. They were degreased with ethanol before treatment.
In order to form the Zn-Al-CO3 LDH conversion coating layer, 0.25 M Na2Al2O4 solutions containing 0.1 M KNO3, 0.01 M NH4NO3 and Zn(NO3)2·6H2O (hereinafter, the Na2Al2O4 solution) were prepared, and the test specimens were immersed for 16 h at room temperature without stirring. After rinsing with DI water and drying, the specimens were cut to 18 mm × 18 mm.
The specimens were then immersed in a methanol solvent containing 0.24 M NaNO3 (hereinafter, the NaNO3 methanol solvent) to effect the exchange of NO3− in place of CO32−. The temperature was adjusted to 50°C. Five immersion times were chosen: 15 min, 30 min, 45 min, 1 h and 2 h. After rinsing with ethanol and air drying, the specimens were analyzed and evaluated.
Surface observation and analysis
The following surface observations and analyses were carried out in order to determine the crystal structure, appearance and chemical compositions of the resulting LDH conversion coating layers.
The X-ray diffraction (XRD) patterns of the coating layer were determined with an X-ray diffractometer (SmartLab, Rigaku). The incident angle was 3°. The radiation source was a Cu Kα target. A scan range of 3° to 60° was employed with a scan speed and step of 10°/min and 0.01°, respectively. The tube voltage and tube current were 45 kV and 44 mA, respectively. An additional XRD analysis of the specimens was performed after LDH coating was ascertained and the specimens were subsequently immersed in aqueous NaCl. For this additional analysis, it was necessary to focus on the 003 face of the LDH layers. The scanning range was 9° to 13° at a scan speed of 1°/min. All other parameters were the same as in the original XRD analysis.
The specimens were observed with a scanning electron microscope (SEM), (ULTRA PULS, Carl Zeiss) and analyzed by an energy dispersive X-ray spectroscopy (EDX) device incorporated in the SEM. The acceleration voltages in the observation and analysis were 1 kV and 5 kV, respectively.
The Al content of the specimens was measured with an X-ray fluorescence spectrometer (XRF), (ZSX-101E, Rigaku). The amount of Al was calculated from the intensity of the Al peaks observed in the analysis of the specimens versus the intensity observed in the analysis of reference steel sheets on which metallic Al had been vapor deposited, resulting in a known coating layer. The tube voltage, tube current and measured area were 45 kV, 45 mA and a circle with a diameter of 10 mm, respectively. The average value of the amount of Al was 80.8 mg/m2 and a standard deviation was 0.31 mg/m2, when a Zn-Al-CO3 LDH conversion coated specimen was evaluated ten times for this condition.
Evaluation of corrosion resistance
The corrosion resistance of the coated specimens was evaluated using the following three methods.
Electrochemical impedance spectroscopy (EIS): EIS measurements were made in 300 mL of a 0.1 M NaCl solution after 1, 3, and 8 h of immersion at room temperature. Sinusoidal 10 mV voltage signals with frequencies ranging from 10,000 Hz to 0.01 Hz were used as a perturbation. The evaluated area was 1 cm2.
Immersion test: The specimens were immersed in 200 mL of a 0.1 M NaCl solution for a certain time period at room temperature. After rinsing with DI water and air drying, visual observations of the specimens were noted. The experiments were performed twice.
Droplet test: 100 μL of a 0.1 M NaCl solution was dropped on the specimens. The specimens were then kept in a small humidity-controlled chamber for 8 h at room temperature. The relative humidity of the chamber was kept at >98% using a saturated K2SO4 solution. The experiments were performed twice.
In order to investigate the effect of the composition change of the NaCl solution on the corrosion resistance of the EG steels, both anodic and cathodic polarization were conducted using a potentiostat in a 0.10 M NaCl solution, 0.10 M NaCl + 0.01 M NaNO3 solution and 0.09 M NaCl + 0.01 M NaCO3 solution after 1 h of immersion. Polarization started from open circuit potential (OCP) and was performed in each direction at a scan rate of 0.5 mV/s. The reference electrode was a saturated calomel electrode (SCE).
Results
Intercalation behavior
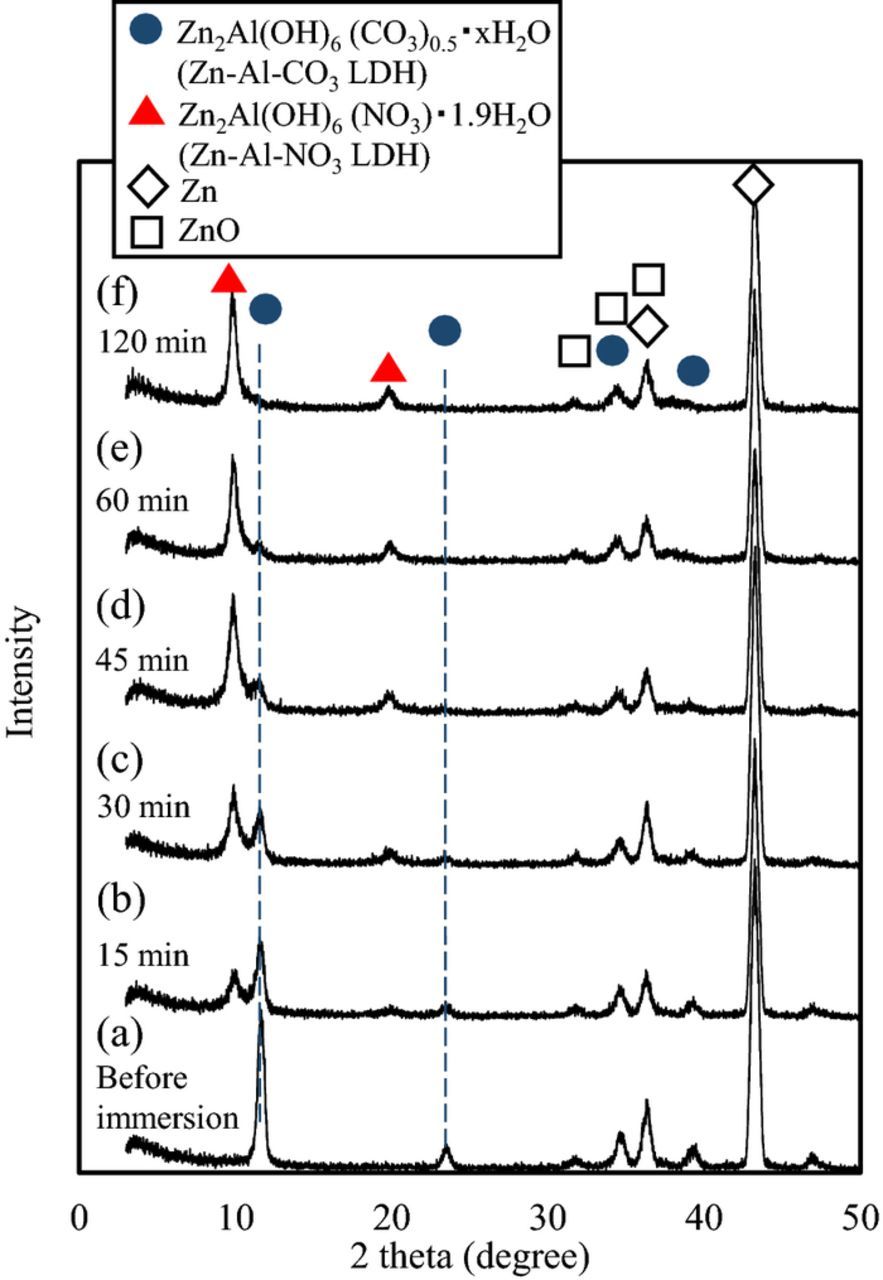
The XRD spectra of the specimens before immersion in the NaNO3 methanol solvent (after immersion in the Na2Al2O4 solution) and with increasing immersion time are shown in Fig. 1. The spectrum of the specimen that was not immersed in the NaNO3 methanol solvent displays clear evidence of the presence of Zn, ZnO and Zn2Al(OH)6 (CO3)0.5·xH2O (Zn-Al-CO3 LDH) [PDF Card No.: 00-048-1023]. As the immersion time increased, the peaks attributable to Zn-Al-CO3 LDH decreased, and after 2 h of immersion, no evidence of the Zn-Al-CO3 LDH was observed. Furthermore, with increasing immersion time, new peaks attributable to Zn2Al(OH)6 (NO3)·1.9H2O (Zn-Al-NO3 LDH) [PDF Card No.: 00-055-0193] appeared. The peaks attributable to Zn-Al-NO3 LDH were visible on the spectra of samples immersed for only 15 min, and the intensity of these peaks continued to increase with time. Immersion time did not seem to have any significant effect on the presence of Zn and ZnO.
Figure 1. XRD patterns of Zn-Al-CO3 LDH conversion coated EG steels. (a) before and (b-f) after immersion in NaNO3 methanol solvent at 50°C. Times refer to immersion in the NaNO3 methanol solvent.
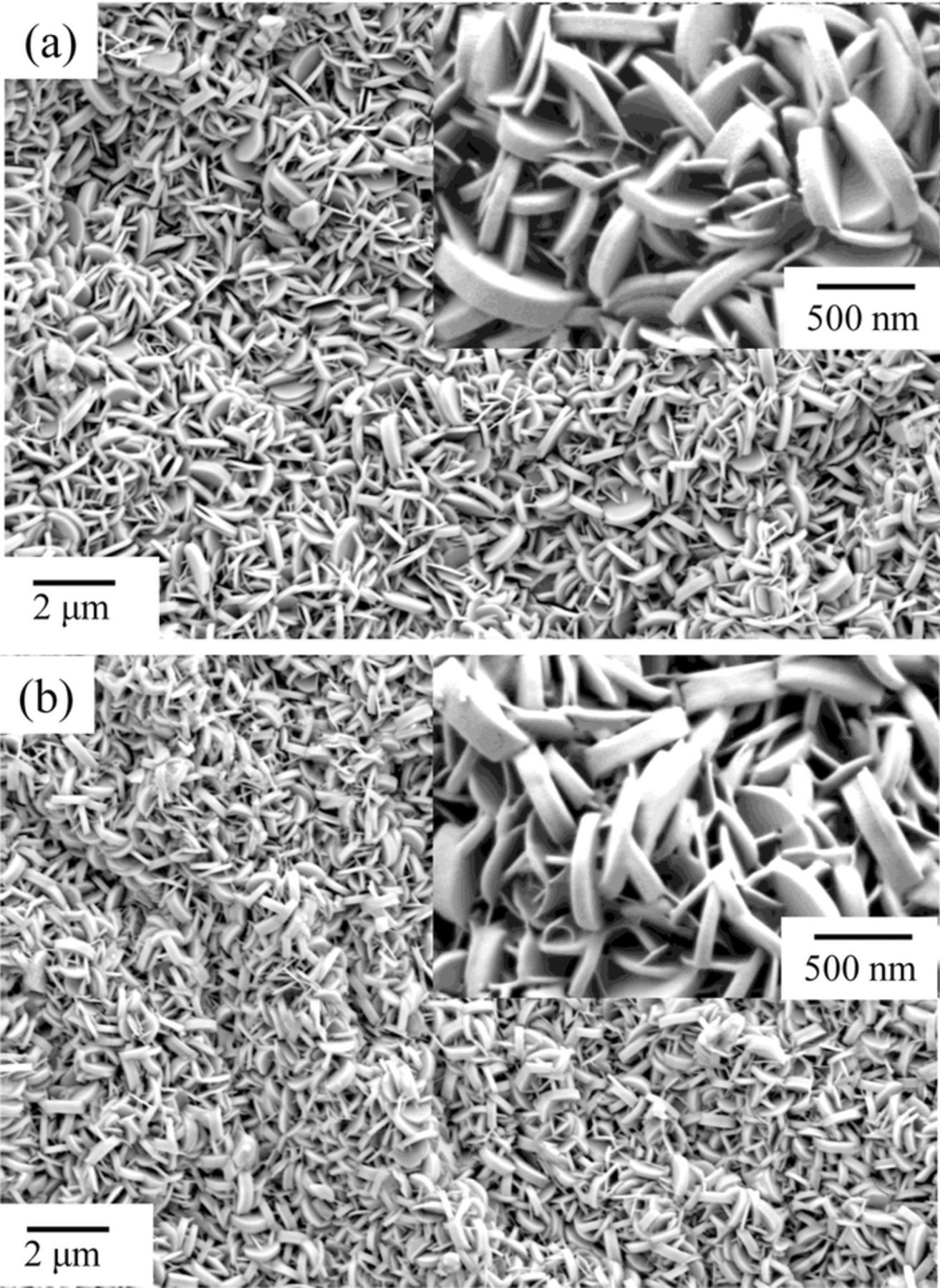
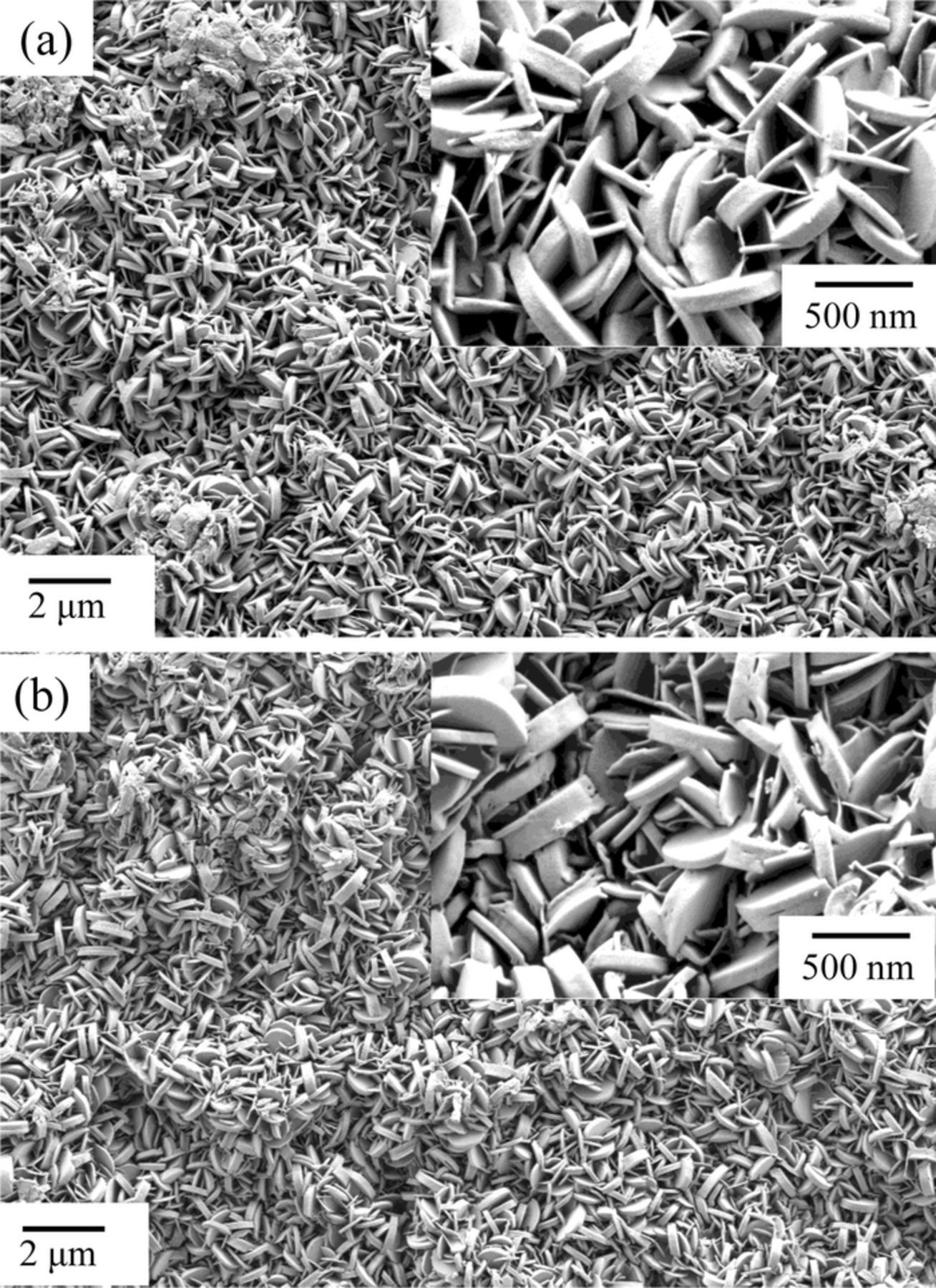
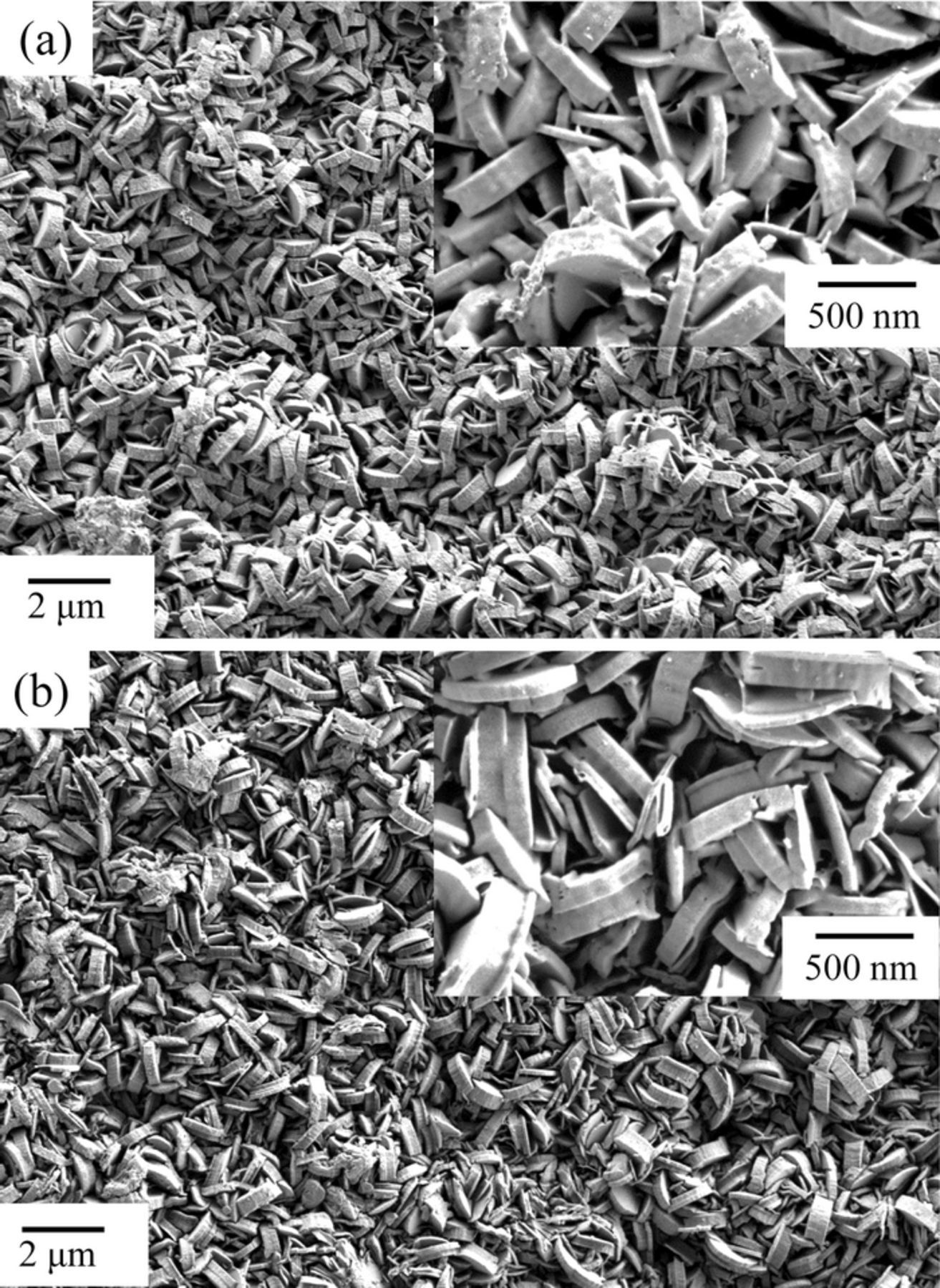
SEM images of the specimens before and after the 2 h immersion in the NaNO3 methanol solvent are shown in Fig. 2. Before immersion, plate-like crystals, typical of the shape of LDH crystals, covered the surface. After the 2 h immersion, the crystal shape and size were exactly the same as before the immersion. The crystals both before and after immersion were analyzed with EDX, and the results are shown in Table I. Before immersion, Zn, Al, O and C, corresponding to the composition of Zn-Al-CO3 LDH, were detected on the surface. Alternatively, Zn, Al, O, N and a greatly diminished C signal, corresponding to the composition of Zn-Al-NO3 LDH, were detected on the surface subjected to immersion. The small amount of C detected on the specimen after immersion most likely arises from adventitious C.
Figure 2. SEM images of Zn-Al-CO3 LDH conversion coated EG steels. (a) before immersion and (b) after 120 min immersion in the NaNO3 methanol solvent at 50°C.
Table I. Results of EDX analysis of conversion coated Zn-Al-CO3 LDH applied to EG steels. (a) before immersion and (b) after 120 min immersion to the NaNO3 methanol solvent at 50°C.
| at % | |||||
|---|---|---|---|---|---|
| C | N | O | Al | Zn | |
| a) before immersion | 2.5 | 0.0 | 65.9 | 9.7 | 21.9 |
| b) after 120 min immersion | 1.5 | 3.4 | 61.9 | 10.5 | 22.7 |
These results suggests that a Zn-Al-CO3 LDH conversion coating layer containing a small amount of ZnO successfully formed on the metallic Zn surface with immersion in the Na2Al2O4 solution and that additional immersion in the NaNO3 methanol solvent for 2 h at 50°C resulted in complete replacement of the CO32− interlayer with a new NO3− interlayer.
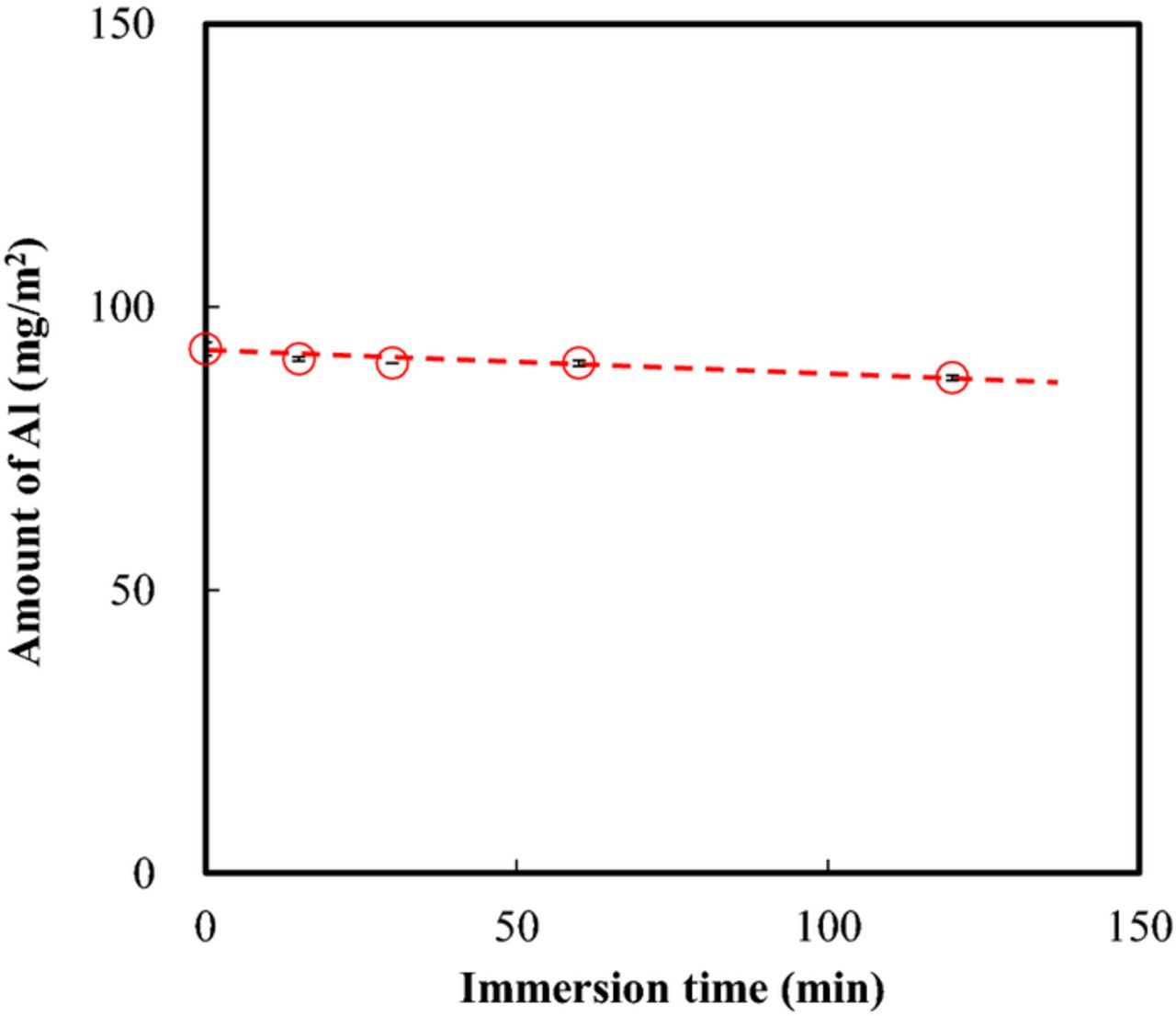
Relationship between the amount of Al present in the coating (as measured with XRF) and immersion time in the NaNO3 methanol solvent are shown in Fig. 3. The experiments were replicated twice. The average values were plotted in the figure and, the maximum and minimum values were shown as error bars. As can be seen in Fig. 3, the amount of Al present in the coating decreases slightly with increasing immersion time in the NaNO3 methanol solvent. Because Al is a constituent element in both Zn-Al-CO3 LDH and Zn-Al-NO3 LDH, this can be interpreted as an indication that the LDH layer dissolved slightly during immersion in the NaNO3 methanol solvent. However, the amount of decrease of Al was quite small and negligible.
Figure 3. Amount of Al present in the coating (measured with XRF) as a function of immersion time in the NaNO3 methanol solvent at 50°C.
Corrosion resistance
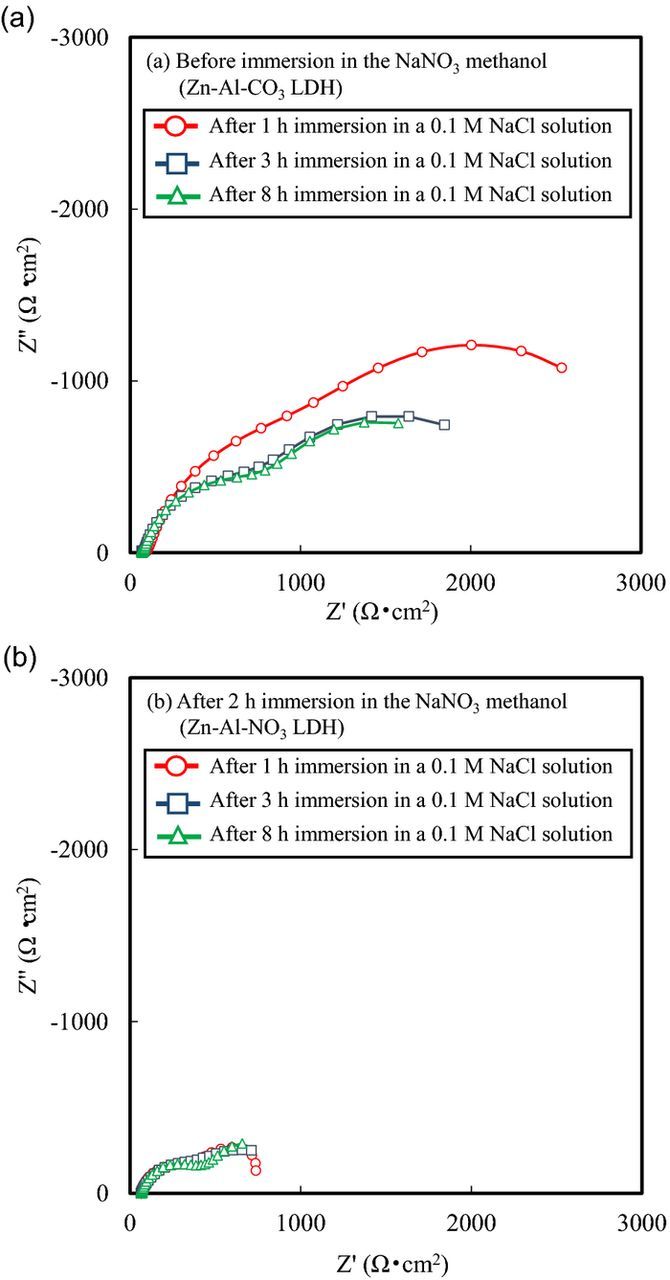
Nyquist plots from the EIS analysis of LDH conversion coated specimens before and after 2 h immersion in the NaNO3 methanol solution are shown in Figs. 4a and 4b, respectively. The plots indicate that corrosion resistance tends to decrease with increasing immersion in the NaNO3 methanol solution. These results, therefore, indicate that the intercalation of NO3− leads to a deterioration in corrosion resistance as measured with EIS.
Figure 4. Results of EIS of LDH conversion coated EG steels (a) before and (b) after 2 h immersion in the NaNO3 methanol solvent. The measurements were carried out in a 0.1 M NaCl solution after 1 h, 3h and 8 h immersion.
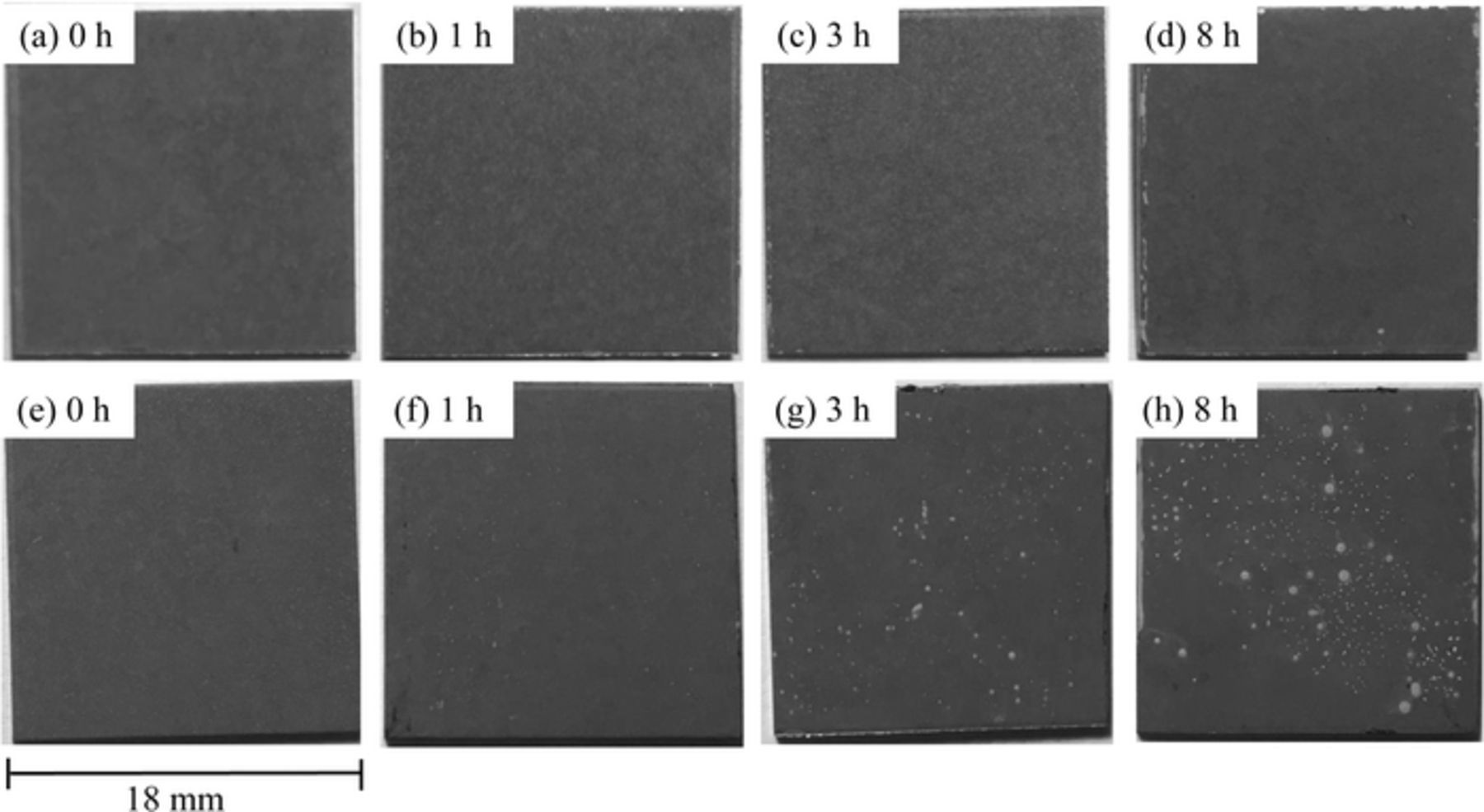
Specimens with either the Zn-Al-CO3 LDH coating or the Zn-Al-NO3 LDH coating (formed after 2 h in the NaNO3 methanol solvent) were subjected to immersion in 0.1 M NaCl for 0 (control), 1, 3 and 8 h. The physical appearance of the specimens is shown in Fig. 5. No obvious pitting corrosion was observed on the Zn-Al-CO3 LDH coated specimen after immersion for up to 8 h, whereas some small pitting corrosion was observed on the Zn-Al-NO3 LDH coated specimen after 1 h of immersion. Additionally, pitting corrosion increased with immersion time in the NaCl solution, as is obvious in the case of the specimen subjected to 3 and 8 h of immersion. These results are in agreement with the EIS results and, again, indicate that the corrosion resistance of specimens coated with Zn-Al-CO3 LDH is higher than that of the Zn-Al-NO3 LDH coated specimens.
Figure 5. Physical appearance of specimens with either Zn-Al-CO3 LDH coating (a-d) or Zn-Al-NO3 LDH coating (e-h) after subsequent immersion in a 0.1 M NaCl solution. Times refer to immersion in the NaCl solution.
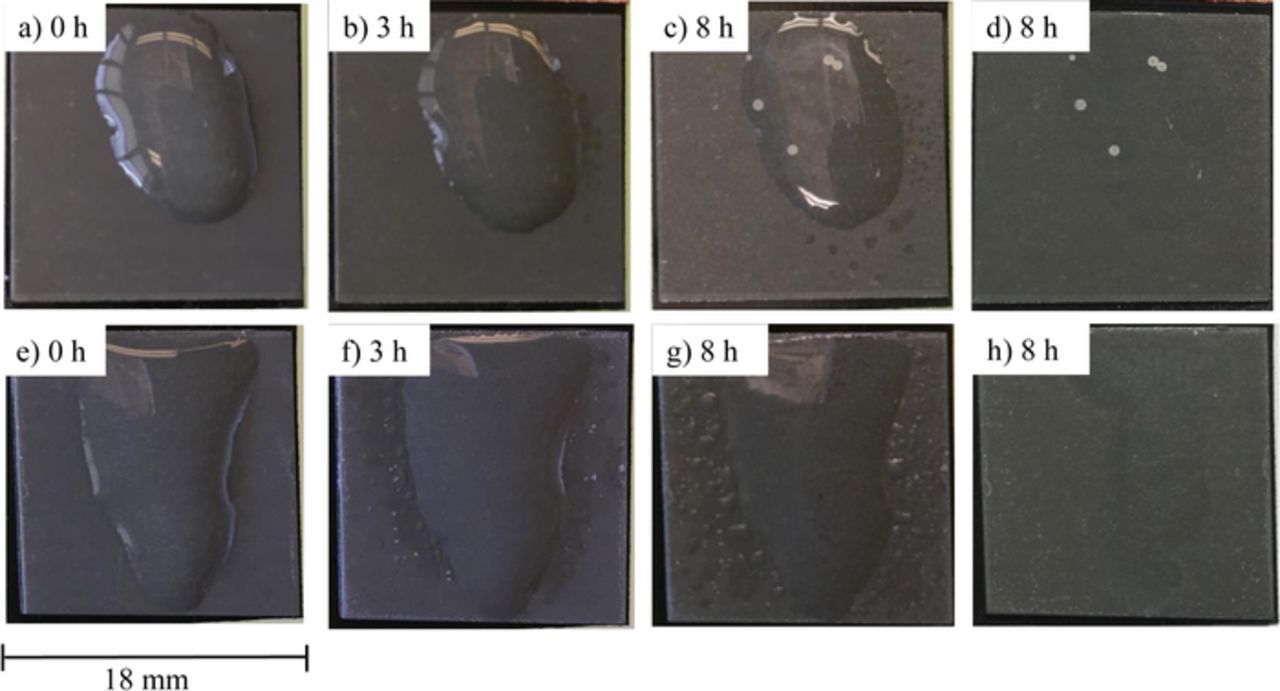
Likewise, specimens with both coatings were subjected to droplet testing, in which 100 μL of 0.1 M NaCl was dropped on the surface and left in place for 8 h. The physical appearance of the specimens after this test is shown in Fig. 6. The results of the droplet tests were the opposite of those observed in the immersion tests. After 8 h of exposure, some pitting corrosion was observed on the Zn-Al-CO3 LDH coated specimen, whereas no obvious pitting corrosion was observed on the Zn-Al-NO3 LDH coated specimen although some white dots which might be initial sites of pitting corrosion were observed on the surface (Fig. 6h). An effort was made to control the droplet shape, however it was difficult due to the different wettability. These differences, which result in different surface areas, might affect the corrosion resistance slightly because of different cathodic oxygen reduction reaction. However, the results that the corrosion resistance of Zn-Al-NO3 LDH coated specimen in the droplet tests was better than that of the immersion tests and the opposite was seen for Zn-Al-CO3 LDH coated specimen suggest a different mechanism is at play in droplet testing versus immersion testing and/or the EIS experiments.
Figure 6. Physical appearance of specimens with either Zn-Al-CO3 LDH coating (a-d) or Zn-Al-NO3 LDH coating (e-h) after subsequent exposure to 100 μl of 0.1 M NaCl droplet in 98% HR chamber. Times refer to exposure. (d, h) after washing and drying.
These immersion and droplet tests were repeated twice, respectively. The duplicate results were not presented as these results were similar to those shown in Figs. 5 and 6.
Behavior involving uptake of Cl− in exchange for release of NO3− in NaCl solution
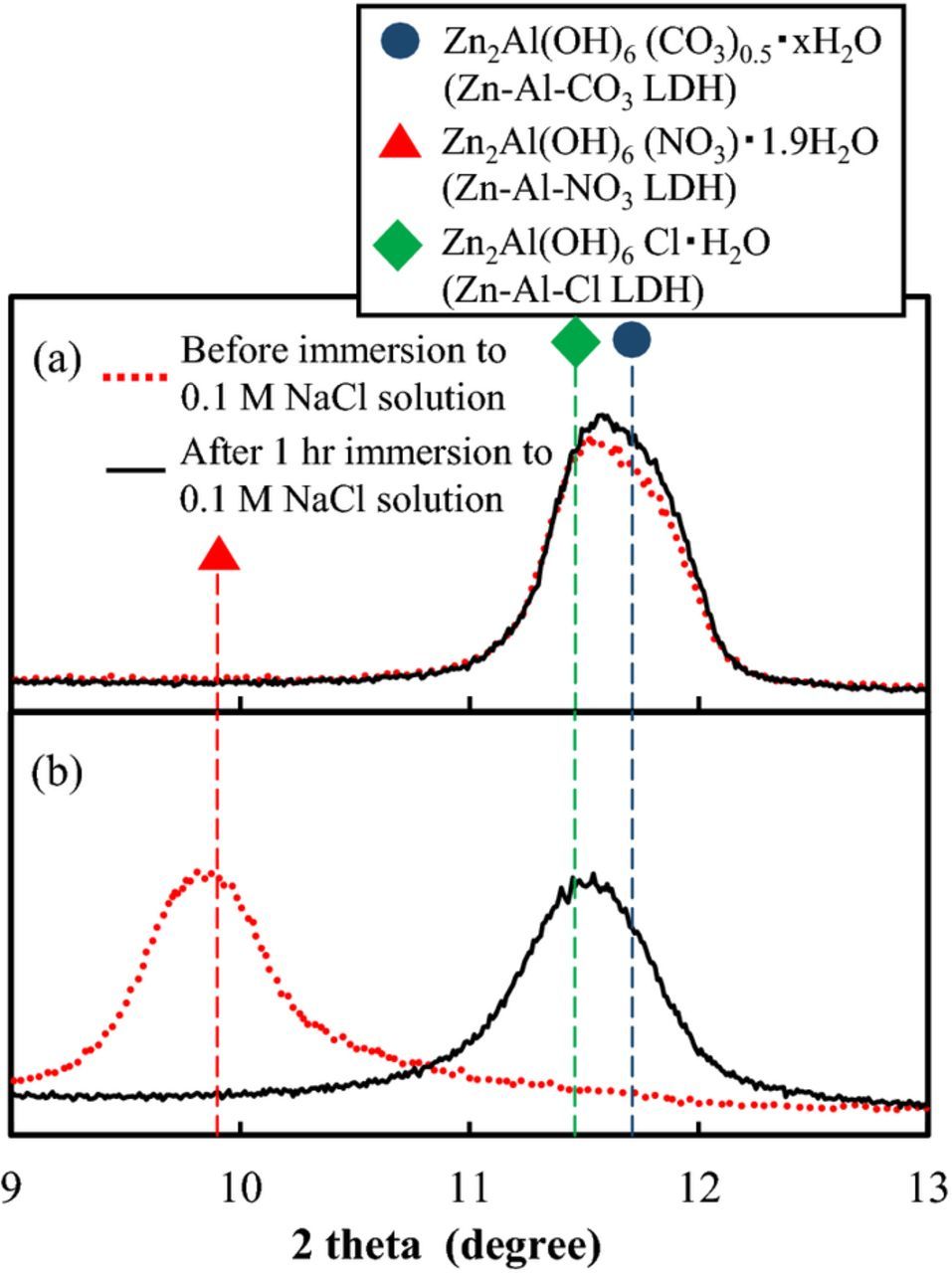
Ideally, the LDH coating would uptake Cl− in exchange for the release of the originally intercalated ion when the treated specimens were exposed to a corrosive NaCl solution. In order to obtain data on this exchange process, an additional XRD analysis of Zn-Al-CO3 LDH coated specimens and Zn-Al-NO3 LDH coated specimens was performed after immersion in 0.1 M NaCl for 1 h. The XRD patterns obtained from these specimens after immersion in aqueous NaCl are shown in Fig. 7. The spectra (Fig. 7a) indicate that when the Zn-Al-CO3 LDH coated specimen was exposed to aqueous NaCl, no anion exchange occurred because the peak is exactly the same as before exposure. On the other hand, when the Zn-Al-NO3 LDH coated specimen was immersed in aqueous NaCl, the peak corresponding to the 003 face of Zn-Al-NO3 LDH (9.90°) disappeared and a new peak appeared at 11.56° (Fig. 7b), indicating the successful release of NO3−. The exchanged anion would be Cl− or CO32− because the peak at 11.56° is between Zn2Al(OH)6Cl·H2O (Zn-Al-Cl LDH) [PDF Card No.: 01-075-2982] and Zn-Al-CO3 LDH. Unfortunately, the XRD peak arising from a Zn-Al-CO3 LDH is very similar to that arising from Zn-Al-Cl LDH, so that it is difficult to identify the exchanged anion from only the XRD results. To clarify the exchanged anion, the specimens were observed with SEM and analyzed with EDX. The results are shown in Fig. 8 and Table II, respectively. As in the specimens before immersion (Fig. 2), plate-like crystals, typical of the shape of LDH crystals, were also observed after immersion, meaning the crystal shape and size did not change during the 1 h immersion in the NaCl solution. However, Cl was detected instead of N on the surface of the Zn-Al-NO3 LDH after immersion in the NaCl solution (Table IIb), whereas Cl was not detected on the surface of the Zn-Al-CO3 LDH coated specimen after immersion in the NaCl solution (Table IIa). These results suggest that the exchanged anion includes at least Cl in the case of the Zn-Al-NO3 LDH, and anion exchange did not occur in the Zn-Al-CO3 LDH. The exchanged layer formed on the original Zn-Al-NO3 LDH most likely includes some CO32− from air, but the use of deionized water and a neutral pH suggests that the intercalated layer consists primarily of Cl−. This result indicates that when a specimen with a Zn-Al-NO3 LDH coating is exposed to aqueous NaCl, the NO3− interlayer is released and replaced with Cl− or CO32−.
Figure 7. XRD patterns of (a) Zn-Al-CO3 LDH and (b) Zn-Al-NO3 LDH conversion coated EG steels before and after 1 h of immersion in a 0.1 M NaCl solution.
Figure 8. SEM images of (a) Zn-Al-CO3 LDH and (b) Zn-Al-NO3 LDH conversion coated EG steels after 1 h of immersion in a 0.1 M NaCl solution.
Table II. Results of EDX analysis of (a) Zn-Al-CO3 LDH and (b) Zn-Al-NO3 LDH conversion coated EG steels after 1 h of immersion in a 0.1 M NaCl solution.
| at % | ||||||
|---|---|---|---|---|---|---|
| C | N | O | Al | Cl | Zn | |
| a) Zn-Al-CO3 LDH | 2.6 | 0.0 | 65.0 | 9.8 | 0.0 | 22.6 |
| b) Zn-Al-NO3 LDH | 2.0 | 0.0 | 58.0 | 10.4 | 4.4 | 25.2 |
The specimens after 1 h exposure to to 100 μl of 0.1 M NaCl droplet were also observed with SEM and analyzed with EDX. The results are shown in Fig. 9 and Table III, respectively. Similar results to those of 1 h immersion were obtained for the droplet test. These results suggest that the replacement of NO3− with Cl− or CO32− had also occurred in the droplet test as same as the immersion test.
Figure 9. SEM images of (a) Zn-Al-CO3 LDH and (b) Zn-Al-NO3 LDH conversion coated EG steels after 1 h exposure to 100 μl of 0.1 M NaCl droplet.
Table III. Results of EDX analysis of (a) Zn-Al-CO3 LDH and (b) Zn-Al-NO3 LDH conversion coated EG steels after 1 h exposure with 100 μL droplet of a 0.1 M NaCl solution.
| at % | ||||||
|---|---|---|---|---|---|---|
| C | N | O | Al | Cl | Zn | |
| a) Zn-Al-CO3 LDH | 2.5 | 0.0 | 65.2 | 10.0 | 0.0 | 22.4 |
| b) Zn-Al-NO3 LDH | 1.9 | 0.0 | 57.6 | 10.6 | 5.6 | 24.2 |
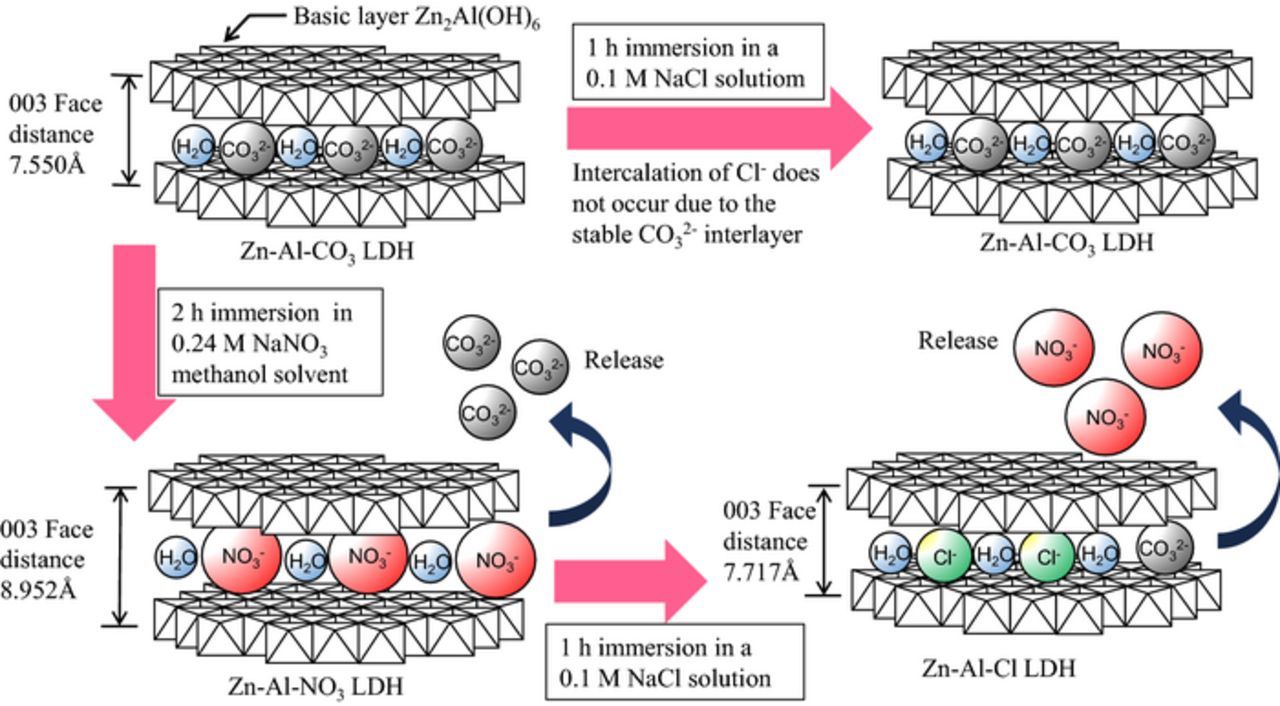
The intercalation behavior of the conversion coated LDH layers on EG steels is schematically summarized in Fig. 10. As shown, the CO32− interlayer of the conversion coated Zn-Al-CO3 LDH which has 7.550 Å of 003 face distance [PDF Card No.: 00-048-1023] can be replaced with NO3− by immersion in the NaNO3 methanol solvent at 50°C for 2 h. The 003 face distance of the crystal changes to the 8.952 Å of the Zn-Al-NO3 LDH [PDF Card No.: 00-055-0193]. This is in good agreement with previous work which reported that it is possible to intercalate NO32− into Mg-Al-CO3 LDH powder by using alcohol as a solvent,23 even on EG steels with a conversion coated LDH. As also shown in Fig. 10, no exchange occurs between CO32− and Cl− when the Zn-Al-CO3 LDH coated specimen is immersed in aqueous NaCl. In contrast, when the Zn-Al-NO3 LDH coated specimen is immersed in aqueous NaCl, the NO3− interlayer is replaced with Cl− or CO32− and the 003 face distance changes to around the 7.717 Å of Zn-Al-Cl LDH [PDF Card No.: 01-075-2982]. This can be explained by the difference in the stability of the interlayer anion, as described by S. Miyata.20 The change in the composition of the surrounding NaCl solution due to the storage of Cl− in exchange for the release of NO3− would be one of the factors which affect corrosion resistance.
Figure 10. Schematic summary of storage-and-release behavior of Zn-Al-CO3 LDH and Zn-Al-NO3 LDH conversion coated EG steels, subsequently immersed in a 0.1 M NaCl solution.
Discussion
Effect of NO3− intercalation on corrosion resistance
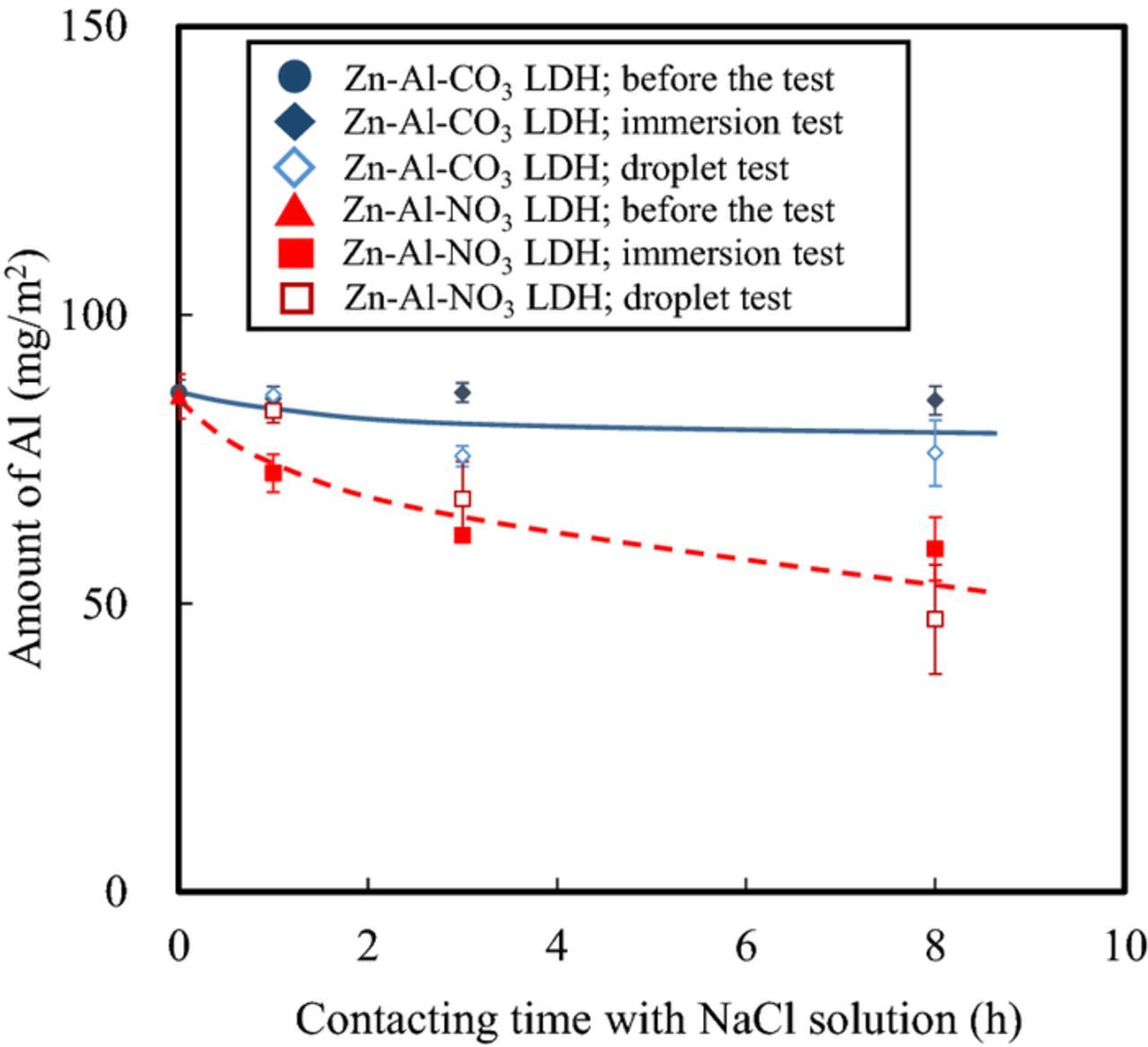
In order to better understand the NO3− intercalation process and how the altered LDH responds to an aqueous NaCl environment, specimens with both LDH layers, which were subjected to both immersion in aqueous NaCl and the aqueous NaCl droplet test, were analyzed with XRF (Fig. 11). The experiments were replicated twice. The average values were plotted in the figure and, the maximum and minimum values were shown as error bars. As can be seen in Fig. 11, the amount of Al detected on the Zn-Al-CO3 LDH coated specimen remained relatively stable in the specimens subjected to the immersion test and the droplet test. In the case of the Zn-Al-NO3 LDH coated specimen, however, the amount of Al decreased with time after being subjected to both the immersion test and the droplet test. Because Al is one of the constituent elements of both LDHs, this suggests that at least some of the Zn-Al-NO3 LDH layer dissolved during immersion or when exposed to a droplet, whereas the Zn-Al-CO3 LDH layer remained stable. It is well established that the solubility of carbonate salts is lower than that of nitrate salts, which are essentially completely soluble. Similarly, the solubility of an LDH could depend on the identity of the anion present in its interlayer. Therefore, it is reasonable to assume that these differences in solubility could affect the corrosion resistance values measured in the EIS and immersion testing analyses (Figs. 4 and 5), resulting in lower resistance due to the dissolution of the Zn-Al-NO3 LDH layer during immersion.
Figure 11. Relationship between amount of Al remaining on conversion coated surface and contact time in a 0.1 M NaCl solution (based on XRF data).
The difference in solubility between Zn-Al-CO3 LDH and Zn-Al-NO3 LDH in the droplet test is similar to that in the immersion test (Fig. 11), even though the corrosion resistance of the Zn-Al-NO3 LDH coated specimen was higher than that of the Zn-Al-CO3 LDH coated specimen (Fig. 6). It appears that the results of the droplet test cannot be explained by the difference in the solubility of the two LDH layers.
To clarify this contradiction, the concentration change in the NaCl solution attributable to the exchange with NO3− was considered. The chemical formula of Zn-Al-NO3 LDH is Zn2Al(OH)6 (NO3)·1.9H2O. From this formula, it is clear that the ratio of Al to NO3− is 1. Based on the XRF data, the amount of Al was assumed to be approximately 80 mg/m2 (Fig. 3). Since the specimen size is 18 mm × 18 mm, approximately 1 μmol of NO3− exists on each side of a specimen. In the immersion tests (conducted in 200 mL of an originally 0.1 M NaCl solution), no significant change is expected in the concentration of Cl− and NO3−. Clearly, any change that did occur would be quite small and negligible. However, with the droplet size of 100 μL, in spite of the smaller surface area, the decrease in the Cl− and NO3− concentrations in the surrounding NaCl solution would be much greater than that in the immersion tests. Using the values noted above, approximate calculations predict a decrease of up to 10% in the concentration of Cl− and a corresponding increase in NO3− in the droplet experiments after exchange.
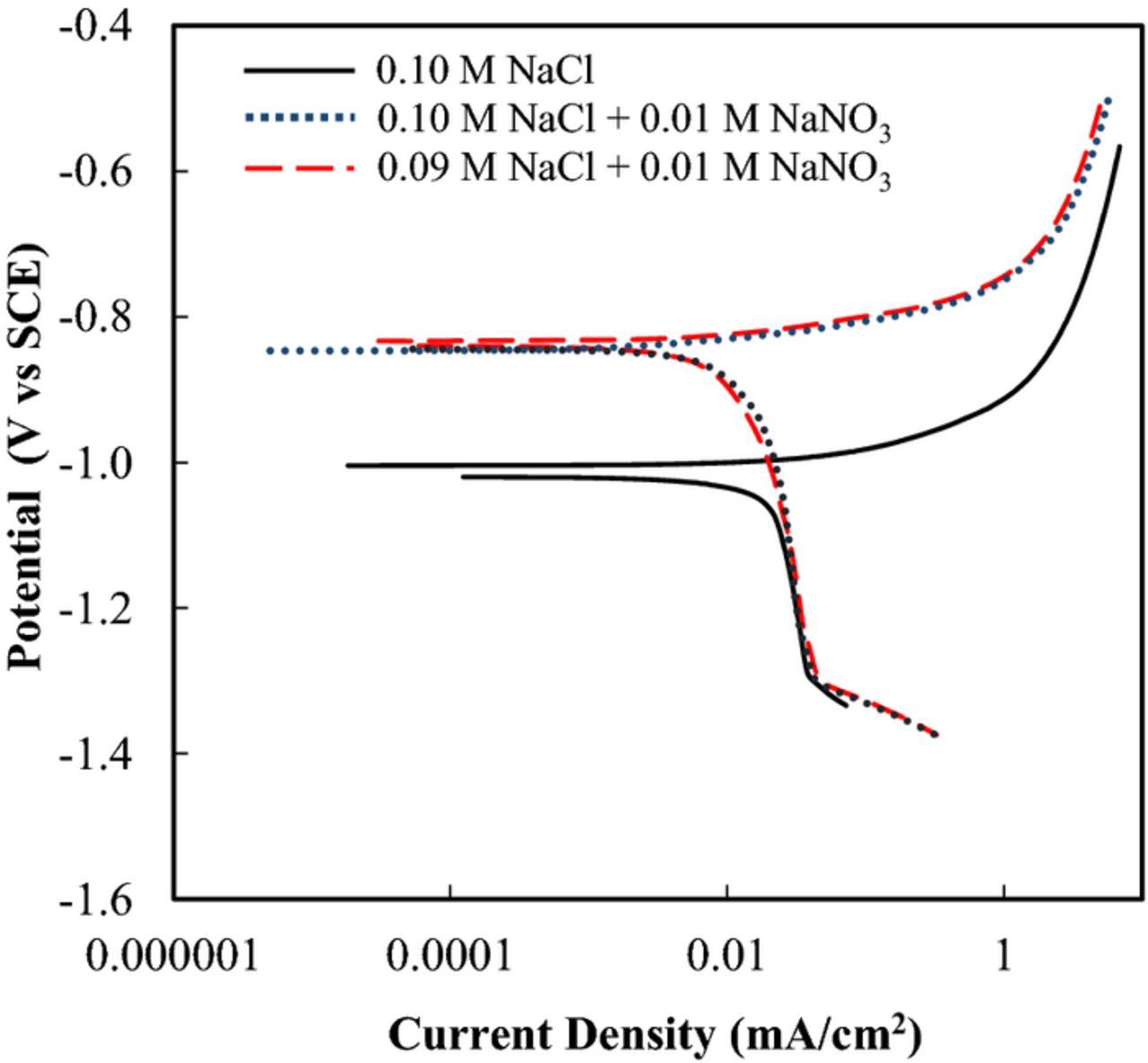
To investigate the effect of this Cl− and NO3− concentration difference on the corrosion resistance of EG steels, polarization experiments were performed in a 0.10 M NaCl solution, 0.10 M NaCl + 0.01 M NaNO3 solution and 0.09 M NaCl + 0.01 M NaNO3 (Fig. 12). These results show a reduced anodic current in both the 0.10 M NaCl + 0.01 M NaNO3 solution and the 0.09 M NaCl + 0.01 M NaNO3 solution. In this condition, the corrosion rate is determined by the cathodic reaction (diffusion limit of oxygen reduction reaction), because a bare EG steel was used as specimens and this corrodes uniformly. However, in the case for droplet test of LDH coated specimens, the corrosion occurred as pitting corrosion. It is assumed that pit growth rate is determined by both anodic dissolution of Zn and cathodic oxygen reduction in this case, because anodic reaction occurs localized area and it is surrounded by large cathodic sites. Furthermore, oxygen diffusion is accelerated due to the thin wet film of droplets. If the kinetics of cathodic reaction of the Zn-Al-CO3 LDH and Zn-Al-NO3 LDH coated specimens is the same because the thickness of the wet film and the area of cathodic sites are similar, pit growth rate is determined by differences in the anodic reaction. If the process is driven by the anodic dissolution of Zn, these polarization results support the differences observed in the EIS and immersion tests versus the droplet tests. As previously shown (Figs. 4 and 5), more pitting was observed on the Zn-Al-NO3 LDH coated specimens in immersion testing, and the opposite was seen in the droplet tests (Fig. 6), in which more pitting was seen on the Zn-Al-CO3 LDH coated specimens. The differences in pitting activity observed in the immersion tests versus the droplet tests can be, in part, explained by the increased NO3− concentration in the NaCl solution.
Figure 12. Polarization curves of EG steels in 0.1 M NaCl, 0.1 M NaCl + 0.01 N NaNO3 and 0.09 M NaCl + 0.01 N NaNO3 solutions.
Conclusions
A method to intercalate NO3− into conversion coated Zn-Al-CO3 LDH on electrogalvanized steel sheets (EG steels) was explored and its effect on corrosion resistance was evaluated.
- (1)It was found that NO3− could successfully exchange with CO32− in conversion coated Zn-Al-CO3 LDH when the conversion coated substrate was immersed in a 0.24 M NaNO3 methanol solvent at 50°C for 2 h.
- (2)The results of EIS and immersion testing in 0.1 M NaCl showed that the converted Zn-Al-NO3 LDH coated specimens provide lower corrosion resistance than that of the Zn-Al-CO3 LDH coated specimens. However the Zn-Al-NO3 LDH coated specimen showed higher corrosion resistance than that of the Zn-Al-CO3 LDH coated specimen in 100 μL droplet testing with 0.1 M NaCl.
- (3)The solubility of the Zn-Al-NO3 LDH layer was found to be greater than that of the Zn-Al-CO3 LDH. This could affect the corrosion resistance of the coating when exposed to large amounts of solution.
- (4)Exchange of Cl− for NO3− in Zn-Al-NO3 LDH was observed, but a similar exchange with CO32− in Zn-Al-CO3 LDH was not observed. This was attributed to the different stabilities of the interlayer anions.
- (5)The replacement of NO3− with Cl− or CO32− affects the corrosion resistance of an LDH when the coating is exposed to a small amount of solution because the exchange process has a greater effect on the solution concentrations.
Acknowledgment
The authors thank Prof. Belinda Hurley and Prof. Gerald S. Frankel for their discussion of this work and help with grammatical editing. We also wish to acknowledge financial support received from JFE Steel Corporation, Japan.
ORCID
Katsuya Hoshino 0000-0002-1793-4593