Abstract
In this work, we used carbon nanotubes (CNTs) as a support for synthesis, size control, and morphology of magnesium oxide using the precipitation method. The morphology of MgO nanoparticles varies by changing the weight percent of carbon nanotubes in the solution. Experimental results indicate that, at optimum condition in a mixture of materials, the surface of purified CNTs is covered by MgO nanoparticles completely. The purified CNTs and MgO/CNTs nanorods had average diameters of about 35 and 65 nm, respectively, and their length was in the order of a few micrometers. The structure of CNTs and MgO/CNTs nanopowders has been characterized by analyzing the X-ray diffraction pattern, Fourier transform infrared spectra, and scanning electron microscopy images.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Carbon nanotubes (CNTs) have been extensively studied and known as materials with outstanding properties. Several applications were proposed for CNTs, many of which are concerned with conductive or high-strength composites, in which the inclusion of CNTs in a ceramic matrix is expected to produce composites possessing high stiffness and improved mechanical properties [1–6]. They have also been widely used as support for the controlled synthesis of nanostructures [7].
However, there are major obstacles to overcome in using CNTs as support additives, mainly the difficulty of the uniform dispersion of CNTs, wettability of the CNTs surface, and the load transfer from the matrix to the CNTs [8]. Due to the chemically ‘inert’ characteristic and the highly hydrophobic nature of the CNT surface, it is difficult to make an attachment with metal precursors [7]. Then, surface modification is required so that efficient tube-matrix interactions are established. The extensive dispersion of various functional groups such as hydroxyl, carboxyl, and carbonyl on the CNT surface could provide active sites for metal ions to attach [9]. Unfortunately, some of the previous studies indicated that these chemical treatments resulted in an extreme degradation of the tubes and the decrease in Young's modulus [10]. The creation of stable nanotube coatings, which do not significantly alter the tube surface, is expected to exhibit improving mechanical properties when compared with heavily degraded nanotubes [11]. Hernadi et al. [7] have obtained homogeneous coverage of multi-walled carbon nanotubes (MWCNTs) using inorganic materials implementing organometallic compounds. SiO x -coated CNTs [12] have also been fabricated through a sol–gel technique at room temperature. Chen et al. [13] obtained SnO-CNT composites, using a sol–gel method, as anode-active material for lithium-ion batteries. Sun et al. [14] have coated CNTs with a thin SnO2 layer using a chemical solution route.
Nanoscale magnesium oxide exhibits unique physical and chemical properties due to its structural characteristic [15]. Several novel methods have been reported in literature for the preparation of nanosized MgO particles [15, 16]. The particle size and surface morphology of synthesized MgO by wet chemical procedure depend upon several factors such as the rate of hydrolysis of magnesium salts, temperature, type of base, concentration of the salt, and drying and calcination steps. Proper choice of these parameters can lead to particles of uniform morphology and size [16, 17].
The coated nanotubes may improve the adhesion between the nanotubes and the matrix when CNTs are used as fillers in the composite materials. However, the mechanical behavior of CNTs is more complicated. Whether or not the strength and stiffness of CNTs can be transferred to the MgO depends on the amount of interfacial bonding between the two phases, which is affected by the CNTs' wettability and interfacial area [18]. Thus, it is important to synthesize CNT-MgO composites and to characterize the interfacial bonding action between the CNTs and the MgO without disruption of the local order of CNTs [9].
In this work, we describe our successful attempt with a simple precipitation method to achieve homogeneous coverage of CNTs with MgO nanoparticles, during which the CNTs were well dispersed in an aqueous solution. The results show that carbon nanotubes can play an important role in controlling the morphology and size of MgO nanoparticles.
Results and discussion
Morphology
The morphology and microstructure of CNTs and MgO/CNT nanopowders were carried out by analyzing scanning electron microscopy (SEM) images. The SEM images of the CNT sample before and after purification are shown in Figure 1. The images show the large amount of impurities attached to the CNT surface (Figure 1a). After the purification steps and the acid treatment, the impurity materials (metal catalyst particles and amorphous carbon) are efficiently removed (Figure 1b). The purification disclosed intertwined CNTs which form a dense 3D-network, extending for several micrometers.
We synthesized nanocomposite powders with different concentrations of CNTs to determine the optimized condition. For now, we discuss the obtained results for the following four concentrations of CNTs in the solution: 0, 0.02, 0.04, and 0.06 g. Figure 2a shows the SEM images of the as-synthesized MgO nanoparticles without any use of CNTs. These nanoparticles are like tiny aggregated nanoparticles which make them bulky. For the 0.02- and 0.04-g concentrations of CNTs, because of the high concentration of Mg(NO3)2·6H2O, the CNTs are invisible (Figure 2b,c). By comparing Figure 2a with Figure 2b,c, we can see that nanoparticles have different morphologies due to the presence of CNTs. Though larger MgO aggregates still exist, many individual nanoparticles are attached onto the CNT surfaces. Thus, the presence of CNTs in the solution can cause the change of MgO nanoparticle morphology.
The SEM image of MgO/CNT nanorods with the 0.06-g concentration of CNTs in the solution is shown in Figure 2d. The images show the development of a large amount of MgO nanoparticles attached to the surfaces of CNTs, making dense and entangled MgO/CNT hybrid nanostructures forming a three-dimensional network structure. For the prepared composites, all of the MgO nanoparticles were firmly deposited on the surface of CNTs, and all of the CNTs are covered by well-dispersed MgO nanoparticles.
In order to obtain the average diameters of CNTs and MgO/CNT nanorods and their range of distribution, we have selected more than 30 per samples and measured their outer diameters from their respective SEM images. It can be seen that the purified CNTs have diameters in the range of 20 to 50 nm (Figure 1b), and MgO/CNT nanorods have diameters within 45 to 150 nm (Figure 2d); their average diameters are about 35 and 65 nm, respectively. Then, the thickness of the MgO layer is about 30 nm.
FT-IR spectroscopy
The nanoparticle loading process was dependent on the functionalization of CNTs. The positive Mg ions may be adsorbed on some sites due to imperfections of the CNT surface and act as preferential nucleation sites for the formation of larger crystals. The functionalization of CNTs using oxidizing acid is one of the most crucial factors, as prerequisite, which would introduce a large number of carboxyl groups on the surface of CNTs, and the high density of functional groups in these restrictive positions would provide great opportunity for further position-selective modification of CNTs with MgO.
A Fourier transform infrared (FT-IR) spectrum was mainly used to identify the presence or absence of functional groups. Figure 3 shows the FT-IR spectra of initial and functionalized CNTs using HNO3. As shown on the FT-IR spectra, acid treatment can result in the appearance of some important absorption peaks approximately at 1,210.9, 1,461.8, and 1,514.4 cm−1 which correspond to O-C=O, C-O, and C=O, respectively. These peaks are, however, barely noticeable in the functionalization of the CNTs using HNO3 [6, 19–21]. The FT-IR spectra indicate that oxygen with high density is generated from functionalized groups on the surface of CNTs during the acid treatment.
As a result, we herein conjecture that the MgO/CNT nanorods form under precipitation conditions; the redox reaction between Mg ions will take place on the surface of CNTs. Consequently, MgO nucleated heterogeneously via precipitation deposition because CNTs have prevented MgO from growing into aggregated nanoparticles [22, 23].
XRD analysis
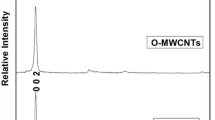
The multi-walled carbon nanotubes, MgO nanoparticles, and MgO/CNT nanopowders were analyzed using X-ray diffraction (XRD) analyses. The XRD spectra of CNTs before and after purification with the 2θ range of 20° to 80° are shown in Figure 4. The purified CNTs present a characteristic diffraction peak at about 26.5°, corresponding to the interlayer spacing of multi-walled CNTs, which indicates that the structure of CNTs was not destroyed during the purification process.
Also, the XRD pattern of MgO nanoparticles and MgO/CNT nanopowders (for 0.06 g of CNTs in the solution), in the 2θ range of 20° to 100°, is shown in Figure 5. For both XRD patterns (Figure 5a,b), the intense peaks were observed at 36.8°, 42.7°, 62.1°, and 75.5°, indicating the crystalline nature of the synthesized MgO nanoparticles with fcc structure [16, 24]. MgO nanoparticles are composed of pure crystalline MgO since no other impurity peaks are observed in the XRD patterns. In addition, we can see that the characteristic peak of CNTs still exists at 2θ = 26.5° (Figure 5b), but the peak intensity decreases after decorating. The average MgO nanoparticle size for both syntheses (with and without the use of CNTs) was determined from the X-ray diffraction line broadening using the Scherrer formula [25], and their average values are about 11 and 7.1 nm, respectively. The results indicated that the presence of carbon nanotubes in the solution can not only change the morphology of MgO nanopowders, but also decreased the average size of the MgO nanoparticles. We believe that the nucleation and growth processes of MgO were significantly influenced by the introduction of CNTs, which have altered the size and shape of MgO.
Conclusions
The results of the synthesis of carbon nanotubes decorated with MgO nanoparticles using the precipitation method were demonstrated in this work. High efficiency of the proposed method was confirmed as well as the possibility of the coating of MgO nanoparticles onto CNTs, without aggregation of these particles. Magnesium ions were attached on CNTs due to the coordination reaction between magnesium ions and polar oxygenated functional groups in the precipitation process. Calculations show that MgO particles appear to have a smaller average size due to the presence of CNTs, and they are durably attached to the nanotubes.
Methods
Synthesis, purification, and functionalization of CNTs
CNTs were synthesized by the catalytic decomposition of ethylene at 930°C over commercial Fe nanoparticles (Sigma-Aldrich, St. Louis, MO, USA; purity was 99% and the average size of nanoparticles was about 25 nm), using catalytic chemical vapor deposition method. It is well known that most produced CNTs contain some impurities such as amorphous carbon, fullerenes, and catalyst particles that could significantly affect the CNT properties, which are a serious impediment for CNTs to be directly used as functional filler in composites. Therefore, such impurities of carbon and metal catalyst particles are encapsulated in the CNT closed tips and walls and need to be removed, trying to avoid a concurrent damage of the tubular structures. In order to remove the impurity, at first, the as-produced CNTs were heated in a horizontal furnace under air flow condition at 500°C for 2 h, sonicated in a mixed solution of HNO3 (5 M) and HCl (5 M) for 30 min, and stirred for 4 h. This suspension was filtered using a membrane having a 0.2-μm pore size, then washed several times with distilled water until acid-free, and finally dried at 120°C for 2 h. Under these treatments, the product was purified from the impurities, and the surfaces of CNTs were modified, which could alter the surface interactions significantly during the coating procedure.
Synthesis of MgO/CNT nanopowders
The MgO/CNT nanopowders were synthesized by precipitation of the magnesium hydroxide gels in aqueous solution containing CNTs using Mg(NO3)2·6H2O as a salt and liquid ammonia as the precipitating agent. At first, certain amounts of purified CNTs (0, 0.02, 0.04, and 0.06 g) were dispersed into the distilled water solution of ammonia (50 cm3; pH = 10.5) by ultrasonication for 10 min. Then 1.8 g of Mg(NO3)2·6H2O was dissolved in 100 cm3 of distilled water. With constant magnetic stirring, the solution of Mg(NO3)2·6H2O was added dropwise to the above solution at 50°C through a dropping funnel. The rate of addition of the salt solution was kept approximately at 20 ml/h. During the addition, the pH of the mixture decreased due to hydrolysis of the salt. The pH was maintained at 10.5 by controlling the addition of the liquid ammonia solution. After completion of the precipitation procedure, the mixture was stirred at room temperature for 12 h, washed and filtered continually in distilled water, and dried at 120°C followed by calcination at 450°C for 2 h. During the calcination procedure, the temperature of the furnace was steadily raised from room temperature to 450°C with an increment of 10°C/min. At 450°C, the temperature was maintained for 2 h to achieve the final material.
Characterization of CNTs and MgO/CNT nanopowders
The resulting CNTs and MgO/CNT nanopowders were characterized by SEM images (Philips MAG 15 kV, ×30,000; FEI Co., Hillsboro, OR, USA), FT-IR spectra (8400 S, Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan), and XRD pattern (CuKα spectra, λ = l.54 Å; GBC Scientific Equipment LLC, Hampshire, IL, USA). Also, the MgO nanoparticle size was determined using the Debye-Scherrer equation.
References
Thostenson ET, Ren Z, Chou TW: Advances in the science and technology of carbon nanotubes and their composites: a review. Composites Science and Technology 2001, 61: 1899–1912. 10.1016/S0266-3538(01)00094-X
Paradise M, Gogswami T: Carbon nanotubes – production and industrial applications. Materials and Design 2007, 28: 1477–1489. 10.1016/j.matdes.2006.03.008
Raffaelle RP, Landi BJ, Harris JD, Bailey SG, Hepp AF: Carbon nanotubes for power applications. Materials Science and Engineering B 2005, 116: 233–243. 10.1016/j.mseb.2004.09.034
Wei T, Fan Z, Luo G, Wei F: A new structure for multi-walled carbon nanotubes reinforced alumina nanocomposite with high strength and toughness. Mater Lett 2008, 62: 641–644. 10.1016/j.matlet.2007.06.025
Xia ZH, Lou J, Curtin WA: A multiscale experiment on the tribological behavior of aligned carbon nanotube/ceramic composites. Scr Mater 2008, 58: 223–236. 10.1016/j.scriptamat.2007.09.039
Chen L, Zhang BL, Qu MZ, Yu ZL: Preparation and characterization of CNTs–TiO2 composites. Powder Technology 2005, 154: 70–72. 10.1016/j.powtec.2005.04.028
Hernadi K, Ljubovic E, Seo JW, Forro L: Synthesis of MWNT-based composite materials with inorganic coating. Acta Mater 2003, 51: 1447–1452. 10.1016/S1359-6454(02)00539-6
Esawi AMK, Borady MAE: Carbon nanotube-reinforced aluminum strips. Composites Science and Technology 2008, 68: 486–492. 10.1016/j.compscitech.2007.06.030
Zhao L, Gao L: Novel in situ synthesis of MWNTs–hydroxyapatite composites. Carbon 2004, 42: 423–426. 10.1016/j.carbon.2003.10.024
Andrews R, Jacques D, Minot M, Rantell T: Fabrication of carbon multi-wall nanotube/polymer composites by shear mixing. Macromolecular Materials and Engineering 2002, 287: 395–403. 10.1002/1439-2054(20020601)287:6<395::AID-MAME395>3.0.CO;2-S
Jia ZJ, Wang ZY, Xu CL, Liang J, Wei BQ, Wu DH, Zhu SW: Study on poly(methyl methacrylate)/carbon nanotube composite. Mater Sci Eng A 1999, 271: 395–400. 10.1016/S0921-5093(99)00263-4
Seeger T, Redlich P, Grobert N, Terrones M, Walton DRM, Kroto HW, Rühle M: SiOx-coating of carbon nanotubes at room temperature. Chemical Physics Letters 2001, 339: 41–46. 10.1016/S0009-2614(01)00256-1
Chen MH, Huang ZC, Wu GT, Zhu GM, You JK, Lin ZG: Synthesis and characterization of SnO–carbon nanotube composite as anode material for lithium-ion batteries. Mater Res Bull 2003, 38: 831–836. 10.1016/S0025-5408(03)00063-1
Sun Z, Liu Z, Han B, An G: Supercritical carbon dioxide-assisted deposition of tin oxide on carbon nanotubes. Mater Lett 2007, 61: 4565–4568. 10.1016/j.matlet.2007.02.052
Wang W, Qiao X, Chen J, Li H: Facile synthesis of magnesium oxide nanoplates via chemical precipitation. Mater Lett 2007, 61: 3218–3220. 10.1016/j.matlet.2006.11.071
Kumar D, Reddy VB, Mishra BG, Rana RK, Nadagouda MN, Varma RS: Nanosized magnesium oxide as catalyst for the rapid and green synthesis of substituted 2-amino-2-chromenes. Tetrahedron 2007, 63: 3093–3097. 10.1016/j.tet.2007.02.019
Wang JA, Novaro O, Bokhimi X, Lopez T, Gomez R, Navarrete J, Llanos ME, Lopez-Salinas E: Characterizations of the thermal decomposition of brucite prepared by sol–gel technique for synthesis of nanocrystalline MgO. Mater Lett 1998, 35: 317–323. 10.1016/S0167-577X(97)00273-5
White AA, Best SM: Hydroxyapatite-carbon nanotube composites for biomedical applications: a review. International Journal of Applied Ceramic Technology 2007, 4: 1–13. 10.1111/j.1744-7402.2007.02113.x
Osorio AG, Silveria ICL, Bueno VL, Bregmann PC: H2SO4/HNO3/HCl—functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl Surf Sci 2008, 255: 2485–2489. 10.1016/j.apsusc.2008.07.144
He B, Wang M, Sun W, Shen Z: Preparation and magnetic property of the MWNT-Fe2+ composite. Mater Chem Phys 2006, 95: 289–293. 10.1016/j.matchemphys.2005.06.038
Sun W, Huang Z, Zhang L, Zhu J: Luminescence from multi-walled carbon nanotubes and the Eu(III)/multi-walled carbon nanotube composite. Carbon 2003, 41: 1685–1687. 10.1016/S0008-6223(03)00148-9
Ma SB, Ahn KY, Lee ES, Oh KH, Kim KB: Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45: 375–382. 10.1016/j.carbon.2006.09.006
Yue H, Huang X, Yang Y: Preparation and electrochemical performance of manganese oxide/carbon nanotubes composite as a cathode for rechargeable lithium battery with high power density. Mater Lett 2008, 62: 3388–3390. 10.1016/j.matlet.2008.03.014
Soumitra K, Subhadra C: Synthesis and characterization of one-dimensional MgO nanostructures. J Nanosci Nanotechnol 2006, 6: 1447–1452. 10.1166/jnn.2006.307
Bergeret G, Gallezot P, Ertl G, Knözinger H, Weitkamp J: Handbook of Heterogeneous Catalysis. Weinheim: Wiley; 1997.
Acknowledgment
The authors would like to acknowledge the University of Mazandaran, Azad University of Qaemshahr and the Iranian National Nanotechnology Initiation Council (INNIC) for their financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interest.
Authors' contributions
All authors provided the same contributions in this article. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Taleshi, F., Hosseini, A.A. Synthesis of uniform MgO/CNT nanorods by precipitation method. J Nanostruct Chem 3, 4 (2012). https://doi.org/10.1186/2193-8865-3-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-4