Abstract
This paper examines the growth of undoped and doped thin films with (Co and In) on glass substrate at 350°C using ultrasonic spray technique. We have investigated the influence of doping concentrations ranging from 0 to 4 wt.% on structural, optical, and electrical properties of ZnO thin films. Zinc acetate dehydrate, CoCl3 4H2O or InCl3, ethanol, and monoethanolamine were used as a starting materials, dopant source, solvent, and stabilizer, respectively. The X-ray diffraction analysis indicated that the undoped and doped ZnO thin films have polycrystalline nature and hexagonal wurtzite structure with (002) preferential orientation. The maximum average crystallite sizes of ZnO:Co and ZnO:In were 55.46 and 45.78 nm at concentrations of 2 wt.% Co and 3 wt.% In, respectively, indicating that the crystallinity of doped films improved after doping. The optical absorption spectra showed that all undoped and doped ZnO films are transparent within the visible wavelength region. The band gap energy of ZnO:Co thin films increased after doping from 3.25 to 3.36 eV; however, the optical gap of ZnO:In decreases after doping from 3.25 to 3.18 eV, indicating the increase and decrease, respectively, in the transition tail width. The electrical conductivity of doped films is stabilized after doping. Transparent, conductive Co-doped ZnO thin films deposited by ultrasonic spray technique are of good quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Zinc oxide (ZnO), which is one of the most important binary II-VI semiconductor compounds, has a hexagonal wurtzite structure and a natural n-type electrical conductivity with a direct energy wide band gap of 3.37 eV at room temperature and a large exciton binding energy (approximately 60 meV) [1]. The resistivity values of ZnO films may be adjusted between 10−4 and 10−1 Ω cm by changing the annealing conditions and doping [2]. Transparent conducting oxides are widely used in microelectronic devices, light emitting diodes, thin films, antireflection coatings for transparent electrodes in solar cells [3, 4], and gas sensors in surface acoustic wave devices [5], varistors, spintronic devices, and lasers [6].
ZnO thin films which can be produced by several techniques such as reactive evaporation, molecular beam epitaxy [7], magnetron sputtering technique, pulsed laser deposition [8], sol-gel technique, chemical vapor deposition, electrochemical deposition [9], and spray pyrolysis [10] have been reported. Among these, we will focus more particularly in this paper the spray ultrasonic technique that is a low-cost method suitable for large-scale production. It has several advantages in producing nanocrystalline thin films, such as relatively homogeneous composition, simple deposition on glass substrate because of the low substrate temperatures involved, easy control of film thickness, and fine and porous microstructure. It is possible to alter the mechanical, electrical, optical, and magnetic properties of ZnO nanostructures.
The doped ZnO thin films have various applications such as transparent conductors, in ferromagnetism, semiconductors, and in piezoelectric and solar cells; moreover, the films have low resistivity and good optical gap energy at low temperature and are transparent in the visible region. There are several reports on ZnO nanostructures doped with different elements, such as Al, Ga, Mg, Li, P, N, Ni, In, and Co [11–15]. ZnO:Co and ZnO:In films have been extensively studied because they exhibit high mobility, good optical transparency, and good electrical conductivity and have lower material cost.
In this paper, the CZO and IZO thin films were deposited on glass substrate by ultrasonic spray technique; at a substrate temperature of 350°C, we have studied the effect of the doping concentration on structural, optical, and electrical properties of ZnO thin films. The main goal for this research is to find the optimum doping concentration which gives highly semiconducting properties of ZnO:Co and ZnO:In thin films.
Experimental procedure
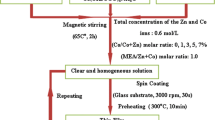
Preparation of spray solution
ZnO solution was prepared by dissolving 0.1 M Zn(CH3COO)2 and 2H2O in the solvent containing equal volumes of ethanol solution (99.995%) of absolute purity; then we added drops of monoethanolamine solution as a stabilizer; and the mixed solution was stirred at 50°C for 120 min to yield a clear and transparent solution.
The doped solution was prepared by adding to the precedent solution CoCl3·4H2O or InCl3 as dopant source. The weight percentages of (Co/Zn) and (In/Zn) were 1, 2, and 3 for ZnO:Co and 2, 3, and 4 for ZnO:In. The solution became clear and homogeneous after stirring for 120 min at 50 to 70°C.
The substrate was R217102 glass in a size of 1 cm × 1 cm × 0.1 cm; prior to pumping, the substrate (R217102 glass) was cleaned with alcohol in an ultrasonic bath and blow-dried with dry nitrogen gas.
Deposition of thin films
The resulting solutions were sprayed on the heated glass substrates by ultrasonic nebulizer system (Sonics) which transforms the liquid to a stream formed with uniform and fine droplets of 35 μm average diameter (given by the manufacturer). The deposition was performed at a substrate temperature of 350°C with 2 min deposition time [15–17].
Characterization
Crystallographic and phase structures of the thin films were determined by X-ray diffraction (Bruker AXS-8D; Bruker Corporation, Billerica, MA, USA) with CuKα radiation (λ = 0.1541 nm) in the scanning range was between 2θ = 25° and 2θ = 55°. The optical properties of the deposited films was measured in the range of 300 to 800 nm using by an ultraviolet-visible spectrophotometer (UV, Lambda 35; PerkinElmer Inc., Waltham, MA, USA), and the electrical conductivity of the films was measured in a coplanar structure obtained with evaporation of four golden stripes on film surface. All spectra were measured at room temperature in air.
Results and discussion
Crystalline structure
The X-ray diffraction patterns of Co-doped ZnO and In-doped ZnO thin films with doping levels are presented in Figures 1 and 2, respectively. Here the films were deposited on glass substrate at a substrate temperature of 350°C [18]. One can be seen, the diffraction peaks at 2θ = 31.74°, 34.52°, and 36.41 corresponding to the (100), (002), and (101) planes, respectively, of undoped ZnO film (0 wt.%). As discussed in our previous paper [15], this result showed that the thin films were polycrystalline and had a hexagonal wurtzite structure. Moreover, as can be seen in Figures 1 and 2, the ZnO:Co and ZnO:In thin films deposited at different concentrations have a (002) diffraction peak which is the highest one in our publication [15]. The films doped at 2 wt.% Co and 3 wt.% In have higher and sharper diffraction peaks indicating an improvement in (002) peak intensity as compared to other films. The films exhibit polycrystalline structure, which is a hexagonal wurtzite structure, in these spectra [19, 20]. This indicates that all films have preferential c-axis orientation along the (002) plane [21, 22], because of the high-intensity direction and different degrees of substitution of Zn2+ ions by Co2+ and In3+ ions. This result indicates that the doping level of the films improved the structural properties.
In order to attain the detailed structure information (Table 1), the crystallite size G (002) along the c-axis was calculated according to the Scherrer equation [23]:
where G is the crystallite size, λ is the X-ray wavelength (λ = 1.5406 A°), β is the full width at half-maximum (FWHM), and θ is Bragg angle of (002) peak.
According to the hexagonal symmetry, the lattice constant can be calculated by the following formula [24]:
where α, c are the lattice parameters, h, k, l are the Miller indices of the planes, and d hkl is the interplanar spacing.
The lattice mismatch between ZnO film and substrate can result in varying degrees of stress during the deposition process of ZnO thin films. Therefore, the strain also affected significantly the structures and properties of ZnO films to some extent. For ZnO films with wurtzite structure, the strain can be obtained by following formula [10]:
where ϵ is the mean strain in ZnO thin films (Table 1), c is the lattice constant of ZnO thin films, and c0 is the lattice constant of bulk (standard c0 = 0.5206 nm).
In Figure 3 we have reported the variation of the crystallite size as a function of doping level. As can be seen, the crystallite size increased for undoped ZnO and doped films with increasing doping level up to 2 wt.% Co and 3 wt.% In. In this region the values of lattice parameters c decreased (Table 1), and the strain of the films is also decreased, indicating the strain along the c-axis. The increase of the crystallite size has been indicated by the enhancement of the crystallinity and c-axis orientation of ZnO thin films by Zhu et al. [25]. The decrease in the crystallite size with the doping level indicates the deterioration in crystallinity of doped films, as found by Bao et al. [26].
Optical characteristics
Figures 4 and 5 show the absorbance spectra of ZnO:Co and ZnO:In samples. The thin films were deposited at different concentrations at a temperature of 350°C for 2 min. It is clear that the absorbance at higher than 400 nm wavelength is low. This confirms that the absorbance value and the tail height of doped films were obtained at different concentrations and reach a minimum concentration of 0 wt.%, followed by an increase and then decrease, with the doping level further increasing. As seen from this figure, the absorption edge shifts in ZnO:Co thin films are better than in ZnO:In. We found that the values of absorption edge shifts are decreased and increased with increasing doping level, which shows that the optical band gap of the CZO films is broadened after doping with cobalt [15] and the optical band gap of the IZO films narrowed after doping with indium. The films obtained at concentrations of 2 wt.% Co and 3 wt.%. In show lower absorption after doping at lower wavelength, which indicated the enhancement of the crystallinity [18, 27]. In addition, the difference of the absorbance between undoped and doped films can be observed clearly at wavelength smaller than 400 nm. These results show that the produced ZnO:Co and ZnO:In thin films could be used in photovoltaic applications due to the sharp increase of absorbance in the strong absorption region.
In order to further investigate the effect of doping level on doped films, the optical band gap energy Eg was measured from the absorbance spectra using the following relations [28–30]:
where A is the absorbance, and d is the film thickness; T the transmittance spectra of thin films; α is the absorption coefficient values; B is a constant; hν is the photon energy and Eg is the band gap energy of the semiconductor. Besides, we have used the Urbach energy (Table 2), which is related to the disorder in the film network, expressed as follows [31]:
where A is the absorbance, A0 is a constant, and Eu is the Urbach energy.
Figure 6 shows the typical variation of (A hν)2 as a function of photon energy (hν) used for optical band gap, which is determined by extrapolation of the linear region to (A hν)2 = 0 [15, 32].
Figure 6 shows a plot of (A hν)2 vs. photon energy hν for undoped ZnO thin films. A linear dependence of (A hν)2 on hν at higher photon energies indicates that the films are essentially direct transition-type semiconductors. The inset shows the drawn ln A as a function of photon energy (hν) to deduce the Urbach energy.
As clearly seen in the Figure 7, the optical gap energy increased with increasing Co doping from 0 to 2 wt.% and the value is found to be 3.25 to 3.36 eV, which results in the narrowing of the conduction band EC and the valence band EV and causes the motion of EC upwards and EV downwards; hence, Co doping causes the band gap broadening [15].
In the four elaborated films (Figure 7), it could be noticed that the band gap energy of IZO films decreases after doping from 3.25 to 3.18 eV with In doping increase from 0 to 3 wt.%. This decrease in the band gap with the increasing indium concentration might be explained in terms of electron concentration dependence of the band gap shift in In-doped ZnO films. The optical band gap is related to the transition of the electrons from valance band to Fermi energy levels in the conduction band for degenerate semiconductors. According to Moss-Burstein effect, the band values should increase with In concentration, but the Eg value decreased with doping. This can be explained by the increase of the defects (such as an increase of the Urbach energy in Figure 8), impurities, or incomplete substitution of dopant with the host atoms [33–36]. The same phenomena are carried out by Tubtimtae and Lee, where they have observed a red shift of the absorption edges from 3.37 eV (undoped ZnO) to 3.34 eV (In-doped ZnO) [37].
From Figure 8 one can see that the Urbach energy decreases after doping with cobalt and increases after doping with indium reaching a minimum of about 0.108 and 0.328 eV for 2 wt.% Co and 3 wt.% In, respectively, as expressed in the literature [15, 18, 38].
Electrical conductivity
Figure 9 shows the variation of the electrical conductivity σ of undoped ZnO and doped films with Co and In as a function of doping level. As can be seen, the electrical conductivity decreases first and then increases, reaching a maximum of about 7.63 and 7.82 Ω cm−1for 2 wt.% Co and 4 wt.% In, respectively. The increase in the conductivity of the films with doping has been explained by the displacement of the electrons [39]; the latter are coming from the ions In3+ and Co2+ donors in the substitutional sites of Zn2+, which resulted in an increased carrier density. The decrease of the electrical conductivity with the increase of the doping level are explained by increasing of the disorder in the films; hence, the potential barriers are increased [40, 41] because the introduced atoms are segregated into the grain boundaries.
Conclusions
In conclusion, highly transparent, conductive ZnO:Co and ZnO:In thin films have been deposited on glass substrate by ultrasonic spray at a substrate temperature of 350°C. The crystalline structure, conductivity, and optical properties were investigated. All the films are polycrystalline structure wurtzite with (002) preferential orientation and, thus, are favorable to the diffusion of atoms absorbed on the substrate. The maximum values of crystallite size G = 33.28, 55.46, and 45.78 nm are attained in ZnO, ZnO:Co, and ZnO:In films at concentrations of 0, 2, and 3 wt.%, respectively. The absorption edge shifts in ZnO:Co thin films are better than in ZnO:In. We found that the values of absorption edge shifts are decreased and increased with the increasing doping level, which shows that the optical band gap of the ZnO:Co films is broadened after doping with cobalt from 3.25 to 3.36 eV and the optical band gap of the ZnO:In films is narrowed after doping with indium from 3.25 to 3.18 eV. The electrical conductivity of doped films is stabilized after doping with cobalt and indium. Transparent, conductive C-doped ZnO thin films deposited by ultrasonic spray technique are of good quality. The amount of doping concentration is 2 wt.% Co and 3 wt.% In, in ZnO thin films.
Methods
Authors did not provide this information.
References
Ma L, Ai X, Huang X, Ma S: Effects of the substrate and oxygen partial pressure on the microstructures and optical properties of Ti-doped ZnO thin films. Superlattice. Microst. 2011, 50: 703–712. 10.1016/j.spmi.2011.09.012
Ko YD, Kim KC, Kim YS: Effects of substrate temperature on the Ga-doped ZnO films as an anode material of organic light emitting diodes. Superlattice. Microst. 2012, 51: 933–941. 10.1016/j.spmi.2012.03.012
Benramache S, Benhaoua B, Chabane F, Lemadi FZ: Influence of growth time on crystalline structure. Conductivity and optical properties of ZnO thin films. J. Semicond 2013, 34: 023001–1.
Khomchenko VS, Kryshtab TG, Savin AK, Zavyalova LV, Roshchina NN, Rodionov VE, Lytvyn OS, Kushnirenko VI, Khachatryan VB, Adame JAA: Fabrication and properties of ZnO:Cu and ZnO:Ag thin films. Superlattice. Microst. 2007, 42: 94–98. 10.1016/j.spmi.2007.04.016
Venkatachalam S, Iida Y, Kanno Y: Preparation and characterization of Al doped ZnO thin films by PLD. Superlattice. Microst. 2008, 44: 127–135. 10.1016/j.spmi.2008.03.006
Rahmane S, Djouadi MA, Aida MS, Barreau N, Abdallah B, Zoubir NH: Power and pressure effects upon magnetron sputtered aluminum doped ZnO films properties. Thin Sol. Film. 2010, 519: 5–10. 10.1016/j.tsf.2010.06.063
Wang ZY, Hu LZ, Jie Z, Jie S, Wang ZJ: Effect of the variation of temperature on the structural and optical properties of ZnO thin films prepared on Si (1 1 1) substrates using PLD. Vacuum 2005, 78: 53–57. 10.1016/j.vacuum.2004.12.014
Mosbah A, Moustaghfir A, Abed S, Bouhssira N, Aida MS, Tomasella E, Jacquet M: Comparison of the structural and optical properties of zinc oxide thin films deposited by d.c. and r.f. sputtering and spray pyrolysis. Surf. Coat. Technol 2005, 200: 293–296. 10.1016/j.surfcoat.2005.02.012
Mosbah A, Abed S, Bouhssira N, Aida MS, Tomasella E: Preparation of highly textured surface ZnO thin films. Mater. Sci. Eng. B 2006, 129: 144–149. 10.1016/j.mseb.2006.01.005
Bahsi ZB, Oral AY: Effects of Mn and Cu doping on the microstructures and optical properties of sol-gel derived ZnO thin films. Opt. Mater. 2007, 29: 672–678. 10.1016/j.optmat.2005.11.016
Benramache S, Benhaoua B, Chabane F: Effect of substrate temperature on the stability of transparent conducting cobalt doped ZnO thin films. J. Semicond. 2012, 33: 093001–1. 10.1088/1674-4926/33/9/093001
Yamada T, Nebiki T, Kishimoto S, Makino H, Awai K, Narusawa T, Yamamoto T: Dependences of structural and electrical properties on thickness of polycrystalline Ga-doped ZnO thin films prepared by reactive plasma deposition. Superlattice. Microst. 2007, 42: 68–73. 10.1016/j.spmi.2007.04.080
Duclère JR, Novotny M, Meaney A, O'Haire R, McGlynn E, Henry MO, Mosnier PJ: Properties of Li-, P- and N-doped ZnO thin films prepared by pulsed laser deposition. Superlattice. Microst 2005, 38: 397–405. 10.1016/j.spmi.2005.08.011
Abed S, Aida MS, Bouchouit K, Arbaoui A, Iliopoulos K, Sahraoui B: Non-linear optical and electrical properties of ZnO doped Ni thin films obtained using spray ultrasonic technique. Opt. Mater. 2011, 33: 968–972. 10.1016/j.optmat.2011.01.018
Benramache S, Benhaoua B: Influence of substrate temperature and cobalt concentration on structural and optical properties of ZnO thin films prepared by ultrasonic spray technique. Superlattice. Microst. 2012, 52: 807–815. 10.1016/j.spmi.2012.06.005
Djelloul A, Aida MS, Bougdira J: Photoluminescence, FTIR and X-ray diffraction studies on undoped and Al-doped ZnO thin films grown on polycrystalline α-alumina substrates by ultrasonic spray pyrolysis. J. Lumin 2010, 130: 2113–2117. 10.1016/j.jlumin.2010.06.002
Zebbar N, Kheireddine Y, Mokeddem K, Hafdallah A, Kechouane M, Aida MS: Structural, optical and electrical properties of n-ZnO/p-Si heterojunction prepared by ultrasonic spray. Mater. Sci. Semicond. Process. 2011, 14: 229–234. 10.1016/j.mssp.2011.03.001
Benramache S, Benhaoua B: Influence of annealing temperature on structural and optical properties of ZnO:In thin films prepared by ultrasonic spray technique. Superlattice. Microst. 2012, 52: 1062–1070. 10.1016/j.spmi.2012.08.006
Kavak H, Tuzemen ES, Ozbayraktar LN, Esen R: Optical and photoconductivity properties of ZnO thin films grown by pulsed filtered cathodic vacuum arc deposition. Vacuum 2009, 83: 540–544.
Wang Y, Chu B: Structural and optical properties of ZnO thin films on (111) CaF 2 substrates grown by magnetron sputtering. Superlattice. Microst. 2008, 44: 54–61. 10.1016/j.spmi.2008.01.024
Rolo AG, de Campos Ayres J, Viseu T, de Lacerda-Arôso T, Cerqueira MF: The annealing effect on structural and optical properties of ZnO thin films produced by r.f. sputtering. Superlattice. Microst 2007, 42: 265–269. 10.1016/j.spmi.2007.04.069
Zhang Z, Bao C, Yao W, Ma S, Zhang L, Hou S: Influence of deposition temperature on the crystallinity of Al-doped ZnO thin films at glass substrates prepared by RF magnetron sputtering method. Superlattice. Microst. 2011, 49: 644–653. 10.1016/j.spmi.2011.04.002
Rani S, Suri P, Shishodia PK, Mehra RM: Synthesis of nanocrystalline ZnO powder via sol-gel route for dye-sensitized solar cells. Sol. Energ. Mat. Sol. C 2008, 92: 1639–1645. 10.1016/j.solmat.2008.07.015
Benramache S, Benhaoua B, Khechai N, Chabane F: Elaboration and characterisation of ZnO thin films. Matériaux & Techniques 2012, 100: 573–580.
Zhu BL, Zhao XZ, Su FH, Li GH, Wu XG, Wu J, Wu R: Low temperature annealing effects on the structure and optical properties of ZnO films grown by pulsed laser deposition. Vacuum 2010, 84: 128–130.
Bao D, Gu H, Kuang A: Sol-gel-derived c -axis oriented ZnO thin films. Thin Sol. Film. 1998, 312: 37–41. 10.1016/S0040-6090(97)00302-7
Nian H, Hahn SH, Koo KK, Kim JS, Kim S, Shin EW, Kim EJ: Preparation and characterization of sol-gel Li and Al codoped ZnO thin films. Mater. Lett. 2010, 64: 157–160. 10.1016/j.matlet.2009.10.030
Verma A, Khan F, Kumar D, Kar M, Chakravarty BC, Singh SN, Husain M: Sol-gel derived aluminum doped zinc oxide for application as anti-reflection coating in terrestrial silicon solar cells. Thin Solid Films 2010, 518: 2649–2653. 10.1016/j.tsf.2009.08.010
Wu C, Shen J, Ma J, Wang S, Zhang Z, Yang X: Electrical and optical properties of molybdenum-doped ZnO transparent conductive thin films prepared by dc reactive magnetron sputtering. Semicond. Sci. Technol. 2009, 24: 125012–125014. 10.1088/0268-1242/24/12/125012
Bouraiou A, Aida MS, Meglali O, Attaf N: Potential effect on the properties of CuInSe2 thin films deposited using two-electrode system. Curr. Appl. Phys. 2011, 11: 1173–1178. 10.1016/j.cap.2011.02.014
Benouis CE, Benhaliliba M, Juarez AS, Aida MS, Chami F, Yakuphanoglu F: The effect of indium doping on structural, electrical conductivity, photoconductivity and density of states properties of ZnO films. J. Alloys Compd. 2010, 490: 62–67. 10.1016/j.jallcom.2009.10.098
Liu J, Ma SY, Huang XL, Ma LG, Li FM, Yang FC, Zhao Q, Zhang XL: Effects of Ti-doped concentration on the microstructures and optical properties of ZnO thin films. Superlattice. Microst. 2012, 52: 765–773. 10.1016/j.spmi.2012.06.021
Chen KJ, Hung FY, Chang SJ, Hu ZS: Microstructures, optical and electrical properties of In-doped ZnO thin films prepared by sol-gel method. Appl. Surf. Sci. 2009, 255: 6308–6312. 10.1016/j.apsusc.2009.02.007
Keskenler EF, Turgut G, Dogan S: Investigation of structural and optical properties of ZnO films co-doped with fluorine and indium. Superlattice. Microst. 2012, 52: 107–115. 10.1016/j.spmi.2012.04.002
Jung MN, Lee ES, Jeon TI, Gil KS, Kim JJ, Murakami Y, Lee SH, Park SH, Lee HJ, Yao T, Makino H, Chang JH: Synthesis and investigation on the extrinsic carrier concentration of indium doped ZnO tetrapods. J. Alloys Compd. 2009, 481: 649–653. 10.1016/j.jallcom.2009.03.065
Yousefi R, Sheini FJ, Zak AK, Mahmoudian MR: Effect of indium concentration on morphology and optical properties of In-doped ZnO nanostructures. Ceram. Int. 2012, 38: 6295–6301. 10.1016/j.ceramint.2012.04.085
Tubtimtae A, Lee MW: ZnO nanorods on undoped and indium-doped ZnO thin films as a TCO layer on nonconductive glass for dye-sensitized solar cells. Superlattice. Microst. 2012, 52: 987–996. 10.1016/j.spmi.2012.08.002
Daranfed W, Aida MS, Hafdallah A, Lekiket H: Substrate temperature influence on ZnS thin films prepared by ultrasonic spray. Thin Solid Films 2009, 518: 1082–1084. 10.1016/j.tsf.2009.03.227
Hafdallah A, Yanineb F, Aida MS, Attaf N: In doped ZnO thin films. J. Alloys Compd. 2011, 509: 7267–7270. 10.1016/j.jallcom.2011.04.058
Mosbah A, Aida MS: Influence of deposition temperature on structural, optical and electrical properties of sputtered Al doped ZnO thin films. J. Alloys Compd. 2012, 515: 149–153.
Lucio-Lopez MA, Luna-Arias MA, Maldonado A, Olvera ML, Acost DR: Preparation of conducting and transparent indium-doped ZnO thin films by chemical spray. Sol. Energ. Mat. Sol. C. 2006, 90: 733–741. 10.1016/j.solmat.2005.04.010
Acknowledgments
The authors would like to thank Prof. A. Si Ahmed, Prof. A. Chari, Dr. S. Rahmane, Mr. B. Gasmi, Mr. H. Bentrah, Dr. O. Belahssen, Dr. D. Bensahal, and Mr. C. Mahboub for their helpful counseling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Authors did not provide this information.
Authors’ contributions
Authors did not provide this information.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Benramache, S., Benhaoua, B. & Bentrah, H. Preparation of transparent, conductive ZnO:Co and ZnO:In thin films by ultrasonic spray method. J Nanostruct Chem 3, 54 (2013). https://doi.org/10.1186/2193-8865-3-54
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-54