Retraction
This article was mistakenly published twice. For this reason this duplicate article has now been retracted. For citation purposes please cite the original:http://www.inljournal.com/?_action=articleInfo&article=21
Abstract
Al-doped and un-doped ZnO thin films deposited on quartz substrates by the nebulized spray pyrolysis method were studied to investigate the wettability of the surface. The main objective of the present study was to investigate the wettability of ZnO thin film by changing the concentration of Al doping. Microstructure and water contact angles of the films were measured by scanning electron microscopy (SEM) and using a contact angle goniometer. SEM studies revealed that the grain size within the film increases with the doping concentration. The contact angles were studied to see the effect of aluminum doping on the hydrophilicity of the film. ZnO films were found to be hydrophobic in nature. A good correlation was observed between the SEM micrographs and contact angle results. The nature of the film was found to change from being hydrophobic to hydrophilic after the treatment in low-pressure DC glow discharge plasma, which, however, was reversible with the storage time.
Similar content being viewed by others
Background
ZnO-based nanostructures are of peculiar interest among the semiconductor oxides due to their superior optical and electronic properties and several potential applications in nanoscale transistors, sensors, and UV laser diodes[1, 2]. ZnO films are now considered to be a suitable prospect to replace ITO with many experiments still being carried out to get more information[3].
During the past decade, there has been an increasing interest in controlling the wettability of solids which depends both on surface energy and roughness. ZnO has been a popular compound among the scientific community to study regarding the wetting properties of metal oxides[4]. Wettability is a very important characteristic of a solid surface. It is strongly affected by both the chemical composition and geometrical structure of the solid surface. Hydrophobic and super-hydrophobic surfaces have been extensively investigated because they have great value on basic research and potential application in industries producing self-cleaning materials. One of the reasons for the extensive study of wetting properties of metal oxide is due to the fact that wettability of such material is significantly altered by UV irradiation and dark storage. There are different ways to prepare ZnO thin films ranging from the sol-gel method to pulsed laser deposition. In the present study, nanostructured ZnO thin films were deposited on quartz substrates by spray pyrolysis technique. The films thus obtained and further modified by a non-equilibrium plasma treatment were characterized by scanning electron microscopy (SEM) and contact angle analysis.
Experimental methods
In the present study, the ZnO thin films were deposited on quartz substrates, using a nebulizer, by spray pyrolysis. First, the un-doped ZnO film was prepared on the substrate followed by ZnO doping with 3% and 7% concentrations of Al. The reactants used were zinc acetate (98.5% purity, Qualigens Fine Chemicals, GlaxoSmith Kline Pharmaceuticals Ltd., Mumbai, India), aluminum acetate (99.0% purity, Sigma, Sigma-Aldrich Corporation, St. Louis, MO, USA), and ethanol (pure, Bengal Chemicals and Pharmaceuticals Ltd., Calcutta, India). Spray pyrolysis of heated (500°C) zinc acetate-ethanol solution (mole concentration of Al acetate within 0% to 10%) yielded thin deposits. Wettability of the film was studied by the measurement of the contact angles of water drops on the surface of the film. An investigation of the effect of plasma treatment on the hydrophobicity of the ZnO films was carried out by measuring the contact angles before and after the plasma treatment. Plasma processing was performed in a DC glow discharge at a low pressure in the range of 2 to 10 Pa and at the applied voltage of 3.5 kV. In order to study the reversible nature of plasma surface modification, contact angles were measured for various storage time starting from the time just after the treatment to 2 days.
Deposition of thin films
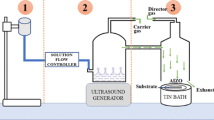
The synthesis of ZnO and Al-doped ZnO was done with the use of an ultrasonic spray nebulizer. This device sprays a gel-like solution at a continuous and constant flow rate to get a film with a smooth surface. The details of the film deposition system are presented elsewhere[5]. Dehydrated zinc acetate was the starting material with quartz as the substrate. A 0.1 M aqueous solution of zinc acetate in ethanol was prepared as the precursor of the thin film. For the aluminum doping, the aluminum acetate solution prepared in ethanol was mixed with 0.1 M zinc acetate with different molar concentrations. A few drops of acetic acid were added finally to get a complete dissociation of zinc acetate. The resulting solution was stirred with a magnetic stirrer at 60°C for 10 min to get a clear homogeneous solution. The solution was then kept inside an ultrasonic vibrator for 10 min just before the spraying.
The quartz substrates went through ultrasonic cleaning with ethanol. The un-doped and Al-doped zinc acetate solutions were then deposited on the substrate with the initial temperature of 500 ± 10°C. However, during the process of deposition, the temperature was reduced to 325 ± 10°C. The measurement of the substrate temperature was done using a K-type thermocouple probe along with a laser temperature detector to maintain constant temperature with electric heater. The film thickness was maintained constant within a certain range with a controlled flow rate of 5 ml/min and constant sprayed volume of the solution. The spray nozzle was kept at a fixed distance of 20 cm away from the substrate. Finally, the films were dried at 500°C for 2 min immediately after the deposition to evaporate the solvent and increase the surface smoothness.
Contact angle measurement
The quantitative evaluation of the wetting of a solid by a liquid is made in terms of the contact angle θ. Geometrically, it is the angle formed by a liquid at the three-phase boundary intersection between liquid, gas, and solid. Figure 1 shows the schematic diagram of the contact angle and the interfacial tensions.
The most important relation regarding the contact angle is Young’s equation that relates the contact angle θ, liquid-vapor surface tension γlv, solid-vapor surface tension γsv, and solid-liquid surface tension γsl as expressed in Equation 1[6]
Drops of distilled water of 4 μl volume were made using a micro-syringe. The contact angles of the water drops on the thin films were measured using a contact angle goniometer. The images of the drops were analyzed with the standard software to compute contact angles.
Results and discussion
Microstructure and surface wettability
The static contact angle for the clean and pure quartz substrate was found to be 45°. However, after the deposition of the ZnO thin films, the surface was found to be hydrophobic with a contact angle greater than 90°. Figure 2 shows the images of water drops on the surface of the ZnO thin films with different Al concentrations and also the corresponding SEM images, showing the hydrophobic nature of the ZnO films as a whole.
From Figure 2, it can be observed that ZnO coating alone can increase the hydrophobicity of the surface significantly. It is interesting to note that the degree of hydrophobicity also depends on the concentration of aluminum added to the film. The contact angles measured on different surfaces are presented in Table 1.
From the values of the contact angle and the SEM images, we can observe that the value of contact angle is directly correlated with the surface structure of the film. The dependence of hydrophobicity of the film on its structure has been explained in terms of the amount of air spaces observed in the nanoscale in an earlier work[7, 8]. The paper reports that the trapped air in the film surface plays an important role in the surface properties. The trapped air pressure balances the gravity of the water droplet, and the surface tension of the water tries to keep the shape of the droplet spherical. So, the film with high volume of air displays higher hydrophobic characteristics. Our experiments also indicated similar results as can be seen from Figure 2. The largest value of contact angle in the case of Figure 2c can be attributed to the high volume of air trapped in the film surface.
The values in Table 1 also give an idea about the smoothness of the surface. The high error in the un-doped ZnO film indicates that the film was not uniform over the substrate. With the Al doping, the increasing uniformity could be observed as the error was less or equal to 1°. This is an important observation as this could lead to many applications regarding the increase in smoothness of the surfaces. The change in morphology of the ZnO film by the doping of Al can be attributed to the fact that crystallite sizes are significantly increased due to Al incorporation[9].
Reversible wettability of the ZnO thin films after plasma treatment
Figure 3 shows the change in hydrophobicity of the ZnO film after the plasma treatment. It is evident that the plasma treatment induces a remarkable change in the surface properties of the films. A treatment of the film for 5 min in the plasma turns a hydrophobic ZnO film to a nearly perfect hydrophilic one. The significant decrease in the contact angles is clearly evident from the images of the drop. The un-doped ZnO sample showed a decrease of about 59% in the values of contact angle.
The decrease in the contact angles for the Al-doped ZnO film was even much higher, 80% for both samples. Lu et al.[4] reported similar results of change in hydrophobicity of the ZnO films after UV irradiation. Since plasma is a good source of UV radiation, plasma treatment can also be considered to be similar regarding the effect of UV irradiation. However, there are also other additional effects of plasma treatment such as the bombardment of the surface by energetic ions and electrons in addition to UV photons.
It is important to note that the hydrophilic nature thus induced to the thin film by plasma treatment is not permanent. The surface modification induced by plasma treatment is reversible in nature. Figure 4 shows the evolution of contact angle with the storage time after treatment.
The plasma-treated films were stored at room temperature in a dust-free environment. The contact angles were measured up to 3 days after the treatment with the interval of 1 day. It can be observed that this reversible process for the ZnO films is quite similar to the case of polymers as reported in our earlier study of the stability of plasma-modified surface[10].
This phenomenon of reversible wettability of UV-treated ZnO films was described in detail by Lu et al.[4]. Using the same reasoning, we can explain the reversible nature for the films treated in plasma, too. Plasma treatment may possibly generate electron-hole pairs in the ZnO surface. The electrons thus produced can react with lattice metal ions to form Zn+ defective sites which are favorable for hydroxyl adsorption. This results to an increase in the surface hydrophilicity. However, after adsorption of hydroxyl radical, the surface becomes energetically unstable. This unstable surface gradually loses its hydrophilicity as the previously adsorbed hydroxyl group is replaced gradually by oxygen atoms. This may be the cause of reversible wettability. Another reason may be the thermodynamically driven reorientation of the polar species from the surface to the subsurface as reported to be observed in the case of plasma-treated polymer surfaces[11].
Conclusion
A relatively good hydrophobic surface can be formed by depositing ZnO nanofilms on a glass substrate. Hydrophobocity of ZnO thin films can be well tailored by changing the doping concentration of Al. The surface homogeneity was increased with the Al doping when compared to the un-doped one. With the plasma treatment, the behavior of the films changed from being hydrophobic to hydrophilic. With the aging of the films after plasma treatment, the hydrophobicity was found to be recovered after 2 days. This behavior has many different practical consequences. This process can be used to control the surface topography. The industrial applications could include self-cleaning coatings and anti-fogging materials.
References
Yin LW, Li MS, Bando Y, Golberg D, Yuan X, Sekiguchi T: Tailoring the optical properties of epitaxially grown biaxial ZnO/Ge, and coaxial ZnO/Ge/ZnO and Ge/ZnO/Ge heterostructures. Adv. Funct. Mater. 2007,17(2):270–276. 10.1002/adfm.200600065
Richters J, Dev A, Müller S, Niepelt R, Borschel C, Ronning C, Voss T: Influence of metallic coatings on the photoluminescence properties of ZnO nanowires. Phys. Status Solidi RRL 2009, 3: 166–168. 10.1002/pssr.200903176
Madhup DK, Subedi DP, Chimouriy SP: Optical characterization and thickness estimation of Al3+ ion doped ZnO nano-films from transmittance spectra. J. Optoelectron. Adv. Mater. 2010, 12: 1035–1044.
Lü J, Huang K, Chen X, Zhu J, Meng F, Song X, Sun Z: Reversible wettability of nanostructured ZnO thin films by sol–gel method. Appl. Surf. Sci. 2010, 256: 4720–4723. 10.1016/j.apsusc.2010.02.080
Huczko A, Dabrowska A, Madhup DK, Subedi DP, Chimouriya SP: Al-doped ZnO nanofilms: synthesis and characterization. Phys. Status Solidi 2010, 247: 3035–3038. 10.1002/pssb.201000164
Kwok DY, Lam CNC, Li A, Leung A, Wu R, Mok E, Neumann AW: Measuring and interpreting contact angles: a complex issue. Colloids Surf., A Physicochem. Eng. Asp. 1998, 142: 219–232. 10.1016/S0927-7757(98)00354-9
Sun M, Du Y, Hao W, Xu H, Yu Y, Wang T: Fabrication and wettability of ZnO nanorod array. J. Mater. Sci. Technol. 2009, 25: 53–57.
Fang X, Yu Z, Sun X, Liu X, Qin F: Formation of superhydrophobic boehmite film on glass substrate by Sol-gel method. Front. Chem. Eng. China 2009, 3: 97–101. 10.1007/s11705-009-0148-y
Mondal S, Kanta KP, Mitra P: Preparation of Al-doped ZnO (AZO) thin film by SILAR. J. Phys. Sci 2008, 12: 221–229.
Subedi DP, Madhup DK, Adhikari K, Joshi UM: Plasma treatment at low pressure for the enhancement of wettability of polycarbonate. Indian J. Pure. Appl. Phys. 2008, 46: 540–544.
Rangel EC, Gadioli GZ, Cruz NC: Investigations on the stability of plasma modified silicone surface. Plasmas Polymers 2004, 9: 35–48.
Acknowledgment
This work was supported by the Third World Academy of Sciences (TWAS), ICTP, Italy, through a research grant no. 06–016 RG/PHYS/AS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DPS prepared the design of the study, facilitated the plasma treatment of the samples, guided the contact angle analysis, and prepared the manuscript. DKM and AS carried out the deposition of the ZnO film. UMJ performed the contact angle measurement. AH participated in the SEM analysis. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Subedi, D.P., Madhup, D.K., Sharma, A. et al. Retracted: Study of the wettability of ZnO nanofilms. Int Nano Lett 2, 1 (2012). https://doi.org/10.1186/2228-5326-2-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-2-1