Abstract
In the present study, Fe3O4@SiO2 core–shell microspheres were prepared via two steps. First, Fe3O4 nanoparticles were synthesized by co-precipitation of Fe+3 and Fe+2 as reaction substrates and NaOH as precipitant. Second, the surface of Fe3O4 was coated with silica by hydrolysis of tetraethylorthosilicate as the silica source. Subsequently, in order to reduce the amount of interaction and the agglomeration of Fe3O4@SiO2 microspheres, the silica shell of these particles was modified by vinyltriethoxysilane as the silane coupling agent. The structural properties of the samples were characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and Fourier transform infrared spectroscopy analyses. The results indicated that the average sizes of Fe3O4 and Fe3O4@SiO2 particles were about 50 and 500 nm, respectively. Also, the surface characterization of Fe3O4@SiO2 microspheres showed that the silane coupling agent was covalently coupled with the silica surface.
Similar content being viewed by others
Background
Magnetic microspheres and magnetic core-shell structures are highly applied in bio and environmental researches [1–3]. Magnetic microspheres, consisting of an iron oxide core and a silica shell, have attracted more attention for their unique magnetic responsivity, low cytotoxicity, good stability, and chemically modifiable surface [4–7]. The core-shell magnetic silica microspheres have shown good potential for different applications, including bioseparation, enzyme immobilization, and catalysis [8–11].
There are various ways to prepare Fe3O4 nanoparticles, which have been reported in several papers, such as arc discharge, mechanical grinding, laser ablation, microemulsions, high temperature decomposition of organic precursors, etc. [12]. As a simple and cheap method, chemical co-precipitation has the potential to meet the increasing demand for direct preparation of well-dispersed (water-based) Fe3O4 nanoparticles, and it offers a low-temperature alternative to conventional powder synthesis techniques in the production of nanoparticles. Also, through this method, the sizes of nanopaticles can be well controlled by apt surfactant [13, 14].
Surface coating is one of the methods which have often been used in modifying or in improving the material's performances [15]. There are several promising techniques for coating the surface of particles; most of them are chemical processes, such as precipitation, sol-gel, and electrochemistry methods. The heterogeneous nucleation method is usually used in surface coating because of its simple procedure, low cost, and especially, easy controlling of the thickness and composition of the coating layer [16]. The proper shell thickness is favorable to keep the coated particles stable physically and magnetically. Using this method, the surface modification of core-shell composites becomes convenient.
In the magnetic core-shell Fe3O4@SiO2, amorphous silica shells have surfaces decorated with hydroxyl group that renders intrinsically hydrophilic. The hydrophilic surfaces easily adhere to each other through hydrogen bonding and form irregular agglomerations. In order to decrease these agglomerations of particles, the surface of the silica shell modifies with the coupling agents. The modification process is described as a hydrolysis and a condensation reaction between the coupling agents and the silica shell. Consequently, the surface modification removes the silanol groups on the surface of the core-shell particles and changes the hydrophilic surface into a hydrophobic one. The aim of this study is to modify the surface of Fe3O4@SiO2 core-shell microsphere by vinyltriethoxysilane (VTES) in order to remove the silanol groups on the SiO2 shell and decrease the agglomerations of this sample in its applications.
Methods
Materials
Ammonium hydroxide (25 wt% NH3), ethanol (C2H5OH), ferric chloride hexahydrate (FeCl3·6H2O), hydrochloric acid (HCl), tetraethylorthosilicate (TEOS), and VTES were supplied from Sigma-Aldrich (MO, USA). Ferric sulfate heptahydrate (FeSO4·7H2O), sodium hydroxide (NaOH), deionized water, and cetyltrimethylammonium bromide (CTAB) were prepared from Merck & Co., Inc. (NJ, USA).
Synthesis of Fe3O4 nanoparticles
In this work, Fe3O4 nanoparticles were prepared by co-precipitation method. At first, 0.04 mol of FeCl3·6H2O and 0.02 mol of FeSO4·7H2O were dissolved in 50 ml of 0.5 M HCl solution. Then, 500 ml of 1.5 M NaOH was added dropwise to the solution under vigorous stirring at 80°C. The obtained Fe3O4 precipitant was separated with a magnet and was repeatedly washed with deionized water. Finally, the precipitant was dried at 50°C for 4 h [17]. The chemical reaction of Fe3O4 precipitation is expected as follows (Equation 1):
Synthesis of Fe3O4@SiO2 core-shell microspheres
Fe3O4@SiO2 core-shell microspheres were prepared through the hydrolysis of TEOS, using sol-gel process. The details were as follows: 0.50 g Fe3O4 nanoparticles was added in 50 ml of 0.1 M HCl aqueous solution, and the mixture was subjected to ultrasonic treatment for 10 min. Then, the precipitant was separated with a magnet and was washed with deionized water three times. The obtained precipitant was added to a mixture of 80 ml ethanol, 20 ml deionized water, 2.0 ml ammonia solution (25 wt %), and 0.2 ml TEOS. The resulting mixture was stirred for 6 h at room temperature. Then, the precipitant was separated with a magnet and was washed with deionized water three times. The obtained precipitant was dispersed in a mixture of 80 ml ethanol, 60 ml deionized water, 2.0 ml ammonia solutions (25 wt%), and 0.50 g of CTAB. The reaction mixture was stirred for 1 h; then, 1.0 ml TEOS was added. After stirring for 6 h, the resulting Fe3O4@SiO2 precipitant was separated with a magnet and was washed with deionized water and ethanol. Finally, the precipitant was dried at 50°C for 12 h [18].
Surface modification of Fe3O4@SiO2 core-shell microspheres
The VTES as silane coupling agent was used to covalently couple with the silica surface of the Fe3O4@SiO2 microspheres. At first, 0.1 g Fe3O4@SiO2 microspheres was added in a mixture of 26.6 ml ethanol and 6.6 ml deionized water; then, 1.76 ml VTES was added to this mixture. After subjecting the mixture to ultrasonic treatment for 30 min, 3 μl ammonia solution (25 wt%) was dripped into the reaction mixture, and the solution was shaken for 2 h at room temperature. The solution was heated for 1 h at 50°C. At the end, the modified Fe3O4@SiO2 precipitant was separated with a magnet and was washed with ethanol three times in order to remove the excessive coupling agent. The resulting precipitant was dried at room temperature. The overall schematic is presented in Figure 1.
Results and discussion
X-ray diffraction
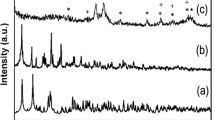
The X-ray diffraction (XRD) patterns of (a) Fe3O4 and (b) Fe3O4@SiO2 are shown in Figure 2. In Figure 2 curve a, weak diffraction peaks with 2θ at 30.0°, 35.6°, 48.3°, 57.2°, and 62.5° are observed, which indicate that the Fe3O4 particles have an amorphous structure. From Figure 2 curve b, we can observe that the XRD pattern of the Fe3O4@SiO2 microspheres is similar to the pattern of Fe3O4 nanoparticles because the coating of the SiO2 layer does not change the structure of the Fe3O4 nanoparticles [19].
Scanning electron microscopy
To determine the morphology and the average size of Fe3O4 particles, scanning electron microscopy (SEM) was used. In Figure 3, the SEM image of the Fe3O4 particles shows that these particles have an irregular morphology, and the average size is about 50 nm.
Transmission electron microscopy
Figure 4 shows the transmission electron microscopy (TEM) images of the Fe3O4@SiO2 microspheres. We can clearly see that the Fe3O4@SiO2 particles are spherical with a smooth surface. The average particle size is about 500 nm, and it was because of the agglomeration of Fe3O4 inside the microspheres and the surface growth of SiO2 on the shell [20]. The internal structure cannot be clearly seen due to the larger particle size of the microspheres, which cannot be broken down by electron beam from transmission electron microscopy [18].
Fourier transform infrared spectroscopy
The Fourier transform infrared (FTIR) spectra of (a) Fe3O4, (b) Fe3O4@SiO2, and (c) Fe3O4@SiO2 modified by VTES are shown in Figure 5. In all curves in Figure 5, the bands at around 1,621 and 3,423 cm−1 can be assigned to the H-O-H stretching modes and bending vibration of the free or adsorbed water, respectively.
In Figure 5 curve a, the band at 567 cm−1 is related to the Fe-O bending vibration. In Figure 5 curve b, the broad high-intensity band at 1,061 cm−1 is associated with the motion of oxygen in Si-O-Si antisymmetric stretch, due to the asymmetric stretching bonds of Si-O-Si in SiO2. The band at 793 cm−1 is assigned to the Si-O-Si symmetric stretch, while the band at 451 cm−1 corresponds to the Si-O-Si or O-Si-O bending modes. The band at 964 cm−1 is assigned to the Si-O symmetric stretch. The band at 570 cm−1 is an indication of the presence of Si-O-Fe. In Figure 5 curve c, the broad band at 1,076 cm−1 is for asymmetric stretching bonds of Si-O-Si, the band at 771 cm−1 is for Si-O-Si symmetric stretch, the band at 454 cm−1 is for Si-O-Si or O-Si-O bending modes, the band at 954 cm−1 is for Si-O symmetric stretch, and the band at 578 cm−1 is for Si-O-Fe. The C=C band cannot be seen in the Figure 5 curve c because it appears at around 1,640 cm−1, and it overlaps with the O-H stretching vibration of H2O. The absorption band at 2,971 cm−1 is associated with the C-H of vinyl groups. These results show that the surface modification of Fe3O4@SiO2 by VTES was successful.
Conclusions
The Fe3O4 nanoparticles were prepared by co-precipitation from Fe+3 and Fe+2, and silica particles were coated on the surface of Fe3O4 nanoparticles through the hydrolysis of TEOS in a sol-gel process. The characterization results of SEM and TEM showed that the average diameters of Fe3O4 and Fe3O4@SiO2 were about 50 and 500 nm, respectively. The analysis of the XRD patterns showed that the structures of these samples were amorphous. In the FTIR spectra, we showed that the chemical bonds of Fe-O-Si happened at the surface of the Fe3O4 nanoparticles. Also, VTES was covalently coupled with the silica surface of the Fe3O4@SiO2 microspheres. Consequently, it was proved that the surface modification happened. Surface chemical modification is one of the various methods to enhance the compatibility between the hydrophobic polymer and the hydrophilic fillers [21, 22]. Therefore, the hydrophobic surface of Fe3O4@SiO2-Si-CH=CH2 microspheres will be advantageous to be used as the filler in polymeric materials.
Authors’ information
FA is a MSc student of Applied Chemistry. AH is an associate professor of Physical Chemistry, SN is an associate professor of Applied Chemistry.
References
Chan Y, Zimmer JP, Stroh M, Steckel JS, Jain RK, Bawendi MG: Incorporation of luminescent nanocrystals into monodisperse core-shell silica microspheres. Adv. Mater. 2004, 16: 2092–2097. 10.1002/adma.200400237
Deng YH, Yang WL, Wang CC, Fu SK: A novel approach for preparation of thermal responsive polymer magnetic microsphere with core–shell structure. Adv. Mater. 2003, 15: 1729–1732. 10.1002/adma.200305459
Salgueiriño-Maceira V, Correa-Duarte MA, Spasova M, Liz-Marzán LM, Farle M: Composite silica spheres with magnetic and luminescent functionalities. Adv. Mater. 2006, 16: 509–514.
Lu Y, Yin Y, Mayers BT, Xia Y: Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol–gel approach. Nano. Lett. 2002, 2: 183–186. 10.1021/nl015681q
Deng YH, Wang CC, Hu JH, Yang WL, Fu SK: Investigation of formation of silica-coated magnetite nanoparticles via sol–gel approach. Colloid. Surf. A. 2005, 26: 87–93.
Zhang F, Wang CC: Fabrication of one-dimensional iron oxide/silica nanostructures with high magnetic sensitivity by dipole-directed self-assembly. J. Phys. Chem. C. 2008, 112: 15151–15156. 10.1021/jp804452r
Yang D, Hu JH, Fu SK: Controlled synthesis of magnetite-silica nanocomposites via a seeded sol-gel approach. J. Phys. Chem. C. 2009, 113: 7646–7651. 10.1021/jp900868d
Xu X, Deng C, Gao M, Yu W, Yang P, Zhang X: Synthesis of magnetic microspheres with immobilized metal ions for enrichment and direct determination of phosphopeptides by matrix-assisted laser desorption ionization mass spectrometry. Adv. Mater. 2006, 18: 3289–3293. 10.1002/adma.200601546
Deng Y, Qi D, Deng C, Zhang X, Zhao D: Superparamagnetic high-magnetization microspheres with an Fe 3 O 4 @SiO 2 core and perpendicularly aligned mesoporous SiO 2 shell for removal of microcystins. J. Am. Chem. Soc. 2008, 130: 28–29. 10.1021/ja0777584
Levy L, Sahoo Y, Kim KS, Bergey EJ, Prasad PN: Synthesis and characterization of multifunctional nanoclinics for biological applications. Chem. Mater. 2002, 14: 3715–3721. 10.1021/cm0203013
Ge J, Zhang Q, Zhang T, Yin Y: Core-satellite nanocomposite catalysts protected by a porous silica shell: controllable reactivity, high stability, and magnetic recyclability. Angew. Chem. Int. Ed. 2008, 47: 8924–8928. 10.1002/anie.200803968
Sun YK, Ma M, Zhang Y, Gu N: Synthesis of nanometer-size maghemite particles from magnetite. Colloids. Surf. A. 2004, 245: 15–19. 10.1016/j.colsurfa.2004.05.009
Cornell RM, Schertmann U: Iron Oxides in the Laboratory: Preparation and Characterization. VCH: Weinheim; 1991.
Chen S, Feng J, Guo X, Hong J, Ding W: One-step wet chemistry for preparation of magnetite nanorods. Mater. Lett. 2005, 59: 985–988. 10.1016/j.matlet.2004.11.043
Kim TK, Lee MN, Lee SH, Park YC, Jung CK, Boo JH: Development of surface coating technology of TiO 2 powder and improvement of photocatalytic activity by surface modification. Thin. Solid. Films. 2005, 475: 171–177. 10.1016/j.tsf.2004.07.021
Ma Q: Heterogeneous nucleation on potent spherical substrates during solidification. Acta. Mater. 2007, 55: 943–953. 10.1016/j.actamat.2006.09.016
Wang J, Zheng S, Shao Y, Liu J, Xu Z, Zhu D: Amino-functionalized Fe 3 O 4 @SiO 2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid. Interf. Sci. 2010, 349: 293–299. 10.1016/j.jcis.2010.05.010
Ji J, Zeng P, Ji S, Yang W, Liu H, Li Y: Catalytic activity of core-shell structured Cu/Fe 3 O 4 @SiO 2 microsphere catalysts. Catal. Today. 2010, 158: 305–309. 10.1016/j.cattod.2010.03.074
Huang X, Wang G, Yang M, Guo W, Gao H: Synthesis of polyaniline-modified Fe 3 O 4 /SiO 2 /TiO 2 composite microsphere and photocatalytic application. Mater. Lett. 2011, 65: 2887–2890. 10.1016/j.matlet.2011.06.005
Kim J, Lee JE, Lee J, Yu JH, Kim BC, An K, Hwang Y, Shin CH, Park JG, Kim J, Hyeon T: Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J. Am. Chem. Soc. 2006, 128: 688–689. 10.1021/ja0565875
Hussain F, Hojjati M, Okamoto M, Gorga RE: Review article: polymer-matrix nanocomposites, processing, manufacturing, and application. J. Compos. Mater. 2006,40(17):1511–1575. 10.1177/0021998306067321
Schadler LS, Laul KO, Smith RW, Petrovicova E: Microstructure and mechanical properties of thermally sprayed silica/nylon nanocomposites. J. Therm. Spray. Technol. 1997,6(4):475. 10.1007/s11666-997-0034-4
Acknowledgments
The authors are grateful to the Department of Chemistry and Nanotechnology Research Center of Urmia University for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FA prepared the samples, the characterized of the results, and prepared the manuscript. AH and SN carried out the characterization and the interpretation of the results. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ahangaran, F., Hassanzadeh, A. & Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int Nano Lett 3, 23 (2013). https://doi.org/10.1186/2228-5326-3-23
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-23