Abstract
Background
The aim of the present study is to have a detailed frequency analysis about the effect of balance training with respect to reactive movement strategies and sensory strategies in type 2 diabetic neuropathy (DN) patients. Also understand changes in the role of each postural subsystem for controlling quiet standing after balance training.
Methods
A group of 19 patients were included in the quasi experimental, time- series study. Total frequency power, 99% power frequency, centroidal frequency and frequency spectrum in the intervals between 0.01-0.1, 0.1-0.5, 0.5-1 Hz and 1-3 Hz are reported. The training protocol consisted two patterns of limits of stability trainings, three approaches in weight shifting trainings and one stable standing practice on the biodex stability system. Results: Repeated measure ANOVA analysis and the LSD test indicated significant differences for the eyes open ML- frequency power and ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies.
Conclusions
Decrease in postural sway at low-medium frequencies showed lower reliance on vestibular system. Also, better controlling hip muscles after balance training relieve DN patients’ requirement to more exploratory sway as a compensatory strategy and showed better balance performance after balance training in DN patients.
Trial registration
UMIN-CTR Search Clinical Trials: UMIN000004485.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Complex interactions between postural subsystems which include proprioception, vestibular and visual systems maintain upright posture [1] and manifested in postural sway parameters [2]. Relationship between type 2 diabetic neuropathy (DN) and postural sway show balance problems are attributed to sensory ataxia [3] which is the lack of accurate proprioceptive feedback [4]. Thus, loss or reduction of peripheral sensory information from the feet [5, 6] and the inability of the central nervous system (CNS) to appropriately integrate available postural control information [3, 5, 7] causes postural instability in neuropathic patients with type 2 diabetes. Additionally, switch from an ankle-based to a hip-based balance strategy [7–11], increase in the use of vestibular information [12] and pick up more useful information as exploratory sway at the hip level [5] changes the mechanism of postural control in diabetic neuropathy patients.
Balance training was reported as a useful method to improve DN patients’ inabilities in controlling posture. In this way, studies showed that physiotherapeutic group training such as gait and balance exercises with function orientated strengthening exercises [13], structured balance exercises [14] and distal strength and balance training [15] has the ability to describe balance training effectiveness in DN patients. Also, studies about the effects of dynamic balance training on quiet standing control in DN patients showed improvement in medial-lateral median and mean frequency of postural sway after balance training which showed effective balance training may treat context-specific instabilities of DN patients’ postural control [16]. Context-specific balance training refers to training which recruit reactive movement strategies and sensory strategies by exposing the patients to external perturbations with small movements of sway, like an inverted pendulum as an ankle strategy, and hip strategy by quick and narrow movements of the center of mass [16, 17].
Despite the numerous researches on DN patients’ neuropathy balance training and reported study about alteration in postural sway of DN patients which indicated an increase in sway power density within medium-high frequencies (0.5-1.00 HZ) show lower postural control in diabetic neuropathy patients [18]. We found lack of frequency analyses in the field of alterations in DN patients’ postural control after balance training. For instance, in this study frequency domain analysis expanded in more details to represent the data as a function of frequencies [19] about the postural sway of DN patients after balance training. Benefits of the Fourier Spectral Analysis of postural sway have been explored in several independent studies. These studies have shown that typical ranges of postural frequency (i.e. frequency bands) express the different levels of activity of postural subsystems which may affect postural sway. Study of postural frequency can provide insights into the individual’s use of these postural subsystems. For example, Some Different independent studies in Fourier transform analyses revealed that low frequencies (0.01-0.1 Hz) show visual control, the low-medium frequency band (0.1-0.5 Hz) is sensitive to vestibular stress and disturbance, the medium-high frequencies (0.5-1 Hz) link to somatosensory activity and postural reflexes mediated by lower extremities and over 1 Hz frequency band are induced by dysfunction in the central nervous system [20–24]. Indeed, frequency domain analysis helps us to discriminate between patterns with similar time domain but different frequency domain [19] and might be a valuable tool in clinical diagnosis [18].
Therefore, we hypothesize that balance training with respect to reactive movement strategies and sensory strategies in DN patients’ can improve postural control and we can specifically show the changes by frequency analysis. Also we can understand differences in the role of each postural subsystem (visual, vestibular and somatosensory) by analyzing the spectral characteristics of center of pressure (COP) fluctuations after balance training.
Methods
Participants
A group of 19 patients (12 women and 7 men) with type 2 diabetic neuropathy were included in the non randomized, quasi experimental and time- series study (Table 1). Patients’ selection was based on several criteria: 1) having controlled type 2 diabetes for more than 5 years, 2) age between 40-70 years, 3) Fasting Blood Sugar (FBS) test results more than 110 mg/dl and less than 200 mg/dl, 4) Valk neuropathy score greater than 2, 5) A1c higher than 8.5, and 6) Snellen visual chart score of more than 16/20 [2]. Patients’ neuropathy was evaluated by recording nerve conduction velocity (NCV), amplitude and latency tests of sensory (sural) and motor (proneal and tibial) nerves. The motor or sensory nerve conduction velocity of 39 m/s was adopted as the lower limit (cut off value: below average – 2 SD of the normal range) [2, 25]. Participants were excluded if they showed orthopedic or neurological disorders related to balance performance, hypotension and autonomic neuropathy [2], retinopathy, scars on the soles of their feet or any prior experience of similar balance trainings using the biodex stability system (BSS), force platform and tilt board.
Patients were completely informed about the purposes of the study and each patient signed a consent form prior to participation. The ethical committee of Tarbiat Modares University which is in accordance with the revised declaration of Helsinki in 2000 approved the study protocol and informed consent forms.
Experimental procedure
Assessments and measurements
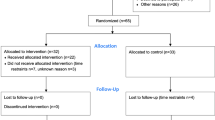
In this study, there was a evaluation and 3-week baseline interval followed with an evaluation (time effect) and by training over 3 weeks with re-evaluation (training effect) (Figure 1). The first evaluation included descriptive information such as age, sex, height, weight, Body mass index (BMI), Valk score, diabetic history and postural strategy assessments with force platform. After the first 3-week period, patients were evaluated for the second time with Valk score and postural strategy assessments. The third evaluation after the patients had been trained by biodex balance exercises for 10 sessions in the second 3-week period again included Valk score and postural strategy assessments. At the beginning of each session, patients’ blood sugar level was tested by a glucometer to be over 7.8 mmol/lit in order to control hyper or hypoglycemia.
The Valk polyneuropathy score assessed the severity of neuropathy based on the frequency of symptom occurrence. This instrument is a 10-item sensory polyneuropathy score that has two different sub-dimensions to evaluate the sensory alteration and neuropathic pain that comprise part of the diabetic symptoms checklist-type 2 (DSC-Type 2) [26].
Force platform measurements (Kistler 9286BA; Winterthur, Switzerland) were used to evaluate the COP fluctuations. The COP is the single point location of the ground reaction force vector [27]. Reliability of COP measures has been confirmed in several previous articles [28]. Assessments were performed in upright stance by instructing the patients to stand on a force platform, touch their heels to each other with 30 degree angle between the medial borders, and focus their eyes on a marker approximately 1.5 m straight ahead while hanging their arms at their sides in comfortable position. Trials were composed of three open- eyes and three closed- eyes conditions. Each trial lasted 30 seconds with sampling rate at 50 HZ with 2 minutes rest period between each trial. The equations were coded and run, using Matlab R2007b software (Math Works Inc. Natick, MA). In the present study, total frequency power (POWER), 99% power frequency (99% FREQ), centroidal frequency (CFREQ) and frequency spectrum are reported. Total power is the integrated area of the power spectrum of COP time series and represents the mean square value of the time series. 99% power frequency is the frequency below which 99% of total power is found and shows the maximum frequency of the main power component and centroidal frequency is the frequency at which the spectral mass in concentrated [29]. Frequency spectrum was calculated by Fast Fourier Transformation (FFT) in the intervals between 0.01-0.1 Hz (low), 0.1-0.5 Hz (low-medium), 0.5-1 Hz (high-medium) and 1-3 Hz (high) of COP signals [20–24, 29]. All of measures were reported for anterior-posterior (AP) and medial- lateral (ML) directions separately.
Training and intervention
Standing balance trainings were carried out by Biodex Stability System (Biodex 945-302; Biodex Medical Systems Inc, 20 Ramsay Rd, Shirley, New York, USA) according to balance training program in our previous study [16]. This device measures the degree of tilt about each axis of ML and AP during dynamic conditions by two under-platform potentiometers which record tilting. It moves in a 360° range of motion by a balance platform which provides up to 20° of surface tilt. At first, patients were familiarized with the device by standing on the platform with their hands at their sides. Then, in that position, the stability platform was unlocked to allow motion. Afterwards, the patients were instructed to adjust feet positions until they found a position at which they were able to maintain moving point in the center or near the center of the circles with the difficulty level of 8 (level of 8 is the easiest level and the most difficult level is 1). Then, the platform locked and the place and angles of feet were fixed for all trainings sessions. Trainings were three times a week with one day interval between each session for 30 minutes and progressed from easy to difficult by lowering the stability level through sessions with the same standing method and feet placing. Each patient started training from level 8 and progressed to more difficult and less stable levels through 10 sessions. Level progressions selected according to the quality of exercise done by patients and limits that were assigned.
Our balance training methods borrowed from reactive movement strategies and sensory strategies to provoke somatosensory information regarding the guiding effects of external visual biofeedback. For this purpose, the physical therapy team asked the patients with DN to control perturbations due to the instability and gravity effects of an unstable platform based on targets providing external visual biofeedback for balance training. These targets cued slow and small movements to provoke ankle strategy, and they indicated fast and large movements to activate hip strategy.
The training protocol consisted of three stages:
-
a)
Limits of stability trainings were prescribed in two patterns of practices; simple and intermediate difficulty pattern. In these practices the patients were instructed to focus on visual external feedback while guiding moving point into the middle of the target. These practices required coordinated contractions of ankle muscles to make precise and small movements. Gravity accompanied with an unstable platform produce perturbations against ankle coordinated movements for pursuing and achieving the targets.
-
b)
Weight shifting trainings were performed in three approaches. In the first approach, patients were instructed to guide moving point from quarter one to quarter three (a), In the second approach they guided moving point from quarter two to quarter four (b), and in the third approach they guided moving point in parallel to the vertical line in the feedback screen (anterior-posterior direction) (c). These practices required both ankle and hip muscles in both AP and ML directions against the perturbing effect of gravity and unstable platform. These factors affect the coordinated participation of muscles that control postural strategies and guidance of movements according to the biofeedback.
-
c)
Stable standing practice on the BSS platform was carried out with no visual feedback and the patients instructed to focus on covered screen and hold the platform in horizontal position. Gravity in accompany with unstable platform produce perturbations against ankle coordinated muscles contractions were the result of patients estimation about their postural orientation and equilibrium.
Data analysis
The patients’ normal distribution baseline means were analyzed with the Kolmogorov–Smirnov test. Repeated measure ANOVA was performed to test mean differences in three sessions of assessments. Mean differences of each pair of sessions were compared with the least significant differences (LSD) test. SPSS software version 15 was used for all statistical analyses.
Sample size calculation
Based on the study by Kim et al. POWER for a healthy young women group in anterior-posterior direction of COP signals was 11.1 ± 5.3 [30] compared with 8.7 ± 4.3 in DN patients in our study. With respect to DN patients’ differences in their AP-POWER from 8.7 to 7.4 after balance trainings, the effect size was 0.6. Therefore, assuming the effect size of 0.5 (medium) to have a larger group of study, a significant alpha 0.05 and a statistical nominal power of 0.88, 19 participants were needed to test these relationships as indicated in Cohen’s Table [31].
Results
Kolmogrov-Smirnov analysis of normality showed that all three sessions of assessments data distribution were normal.
Means and standard deviations of the scores of three sessions (Table 2) showed reduction in the Valk scores of the third session. Repeated measure ANOVA analysis indicated that within-subject effects failed to reach significance (p = 0.065).
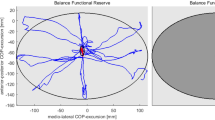
In the analysis of Force platform variables (Table 2, Figure 2) by repeated measure ANOVA analysis, within-subject effects of open eyes conditions reached significant differences for the eyes open ML-POWER, ML-99%FREQ and ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies of COP signals while failed to reach any significant differences for other FFT sway power ranges and frequency domain analyses in AP direction or in eyes closed conditions (Table 2). The LSD test indicated significant differences for the eyes open ML- POWER and ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies of COP signals between paired sessions second and third (p = 0.019, p = 0.005), but failed to reach any significant differences between paired sessions first and second (p = 0.626, p = 0.256). Eyes open ML-99%FREQ did not show any significant differences between paired sessions in LSD test (p> 0.05).
Discussion
In the present study, we tried to have a detailed frequency analyses about the effectiveness of balance training which should treat context-specific instabilities of DN patients’ postural control by placing more emphasis on somatosensory information. By means of these trainings, our methods recruited reactive movement strategies and sensory strategies for provoking somatosensory information with respect to the guiding contributions of external visual biofeedback. In this study, DN patients improved their balance control by reduction in eyes open ML-POWER and ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies. Furthermore, the Valk severity score did not show any significant difference in the severity of diabetic neuropathy, indicating that the better balance performance in DN patients was probably improved by other mechanisms than changes in the severity of neuropathy (Table 2).
In the present study, spectral characteristics of postural sway in DN patients were added to confirm better balance performance of DN patients. Frequency domain analyses help us to discriminate between patterns with similar time domain but different oscillation patterns, specifically, in Fourier transform [19]. In this way, the data can be expressed as a sum of simple sinusoids, each having specific frequency [19]. Some Different independent studies in Fourier transform analyses revealed that low frequencies (0.01-0.1 Hz) show visual control, the low-medium frequency band (0.1-0.5 Hz) is sensitive to vestibular stress and disturbance, the medium-high frequencies (0.5-1 Hz) link to somatosensory activity and postural reflexes mediated by lower extremities and over 1 Hz frequency band are induced by dysfunction in the central nervous system [18, 20–24]. In our results we found significant reduction in eyes open ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies which is a band related to modifications in vestibular. In fact, low-medium Frequency sway is invoked in patients with peripheral vestibular pathology [21, 24, 32]. Moreover, Horak et al. demonstrated that DN patients with somatosensory loss, showed more dependence on vestibular information and increased vestibulospinal sensitivity as a result of somatosensory loss. Also, they implied that DN patients are unstable because the importance accredited to the vestibular system does not supply enough information for controlling balance instead of deficient somatosensory system [12]. In summary, Bonnet et al. indicated that an intact vestibular system in DN patients compensates incompletely for impairment of perceptual subsystems grounded in the somatosensory system and the efficiency of the information for about head control does not match the information from mechanoreceptors, tendons, skin and etc [5]. Thus, decrease in postural sway at eyes open low-medium frequencies (0.1-0.5 HZ) in our study shows lower reliance on vestibular system in DN patients after balance training that may show better postural control. So, DN patients get sufficient information from other subsystems such as visual or somatosensory. On the other side, the study by Oppenheim et al. who expressed precisely Fourier transform in DN patients in different bands, power of sway at medium-high frequencies (0.5-1 Hz) was reduced which is under the effects of somatosensory feedback [18]. As a result, while we did not found any differences in 0.5-1 Hz band power which may reveal that balance training had no significant improvement on somatosensory feedback, but it is probable that changes in the medium-high band power in our results provide enough information to reduce the role of vestibular subsystem and helped the CNS to appropriately integrate available information for postural control.
Lateral instability was introduced by some studies as a marker of impaired balance in DN patients [33]. Also, alterations in the balance parameters in ML direction recorded by force platform demonstrated that DN patients are less stable in the ML direction of postural sway parameters [11] and suggested the crucial role of vestibular system in the control of hip strategy in ML direction [12]. In fact, the biomechanical and sensory problems of these patients at the ankle level lead to compensation with the more available postural control information at the hip level (abductor/adductor muscles) which increases DN patients’ sensitivity to information in the ML axis [5]. Thus the reliance on the hip joint in DN patients does not result in better postural control [34]. In the present study, results exhibited decrease in eyes open ML-POWER and ML-FFT sway power within low-medium (0.1-0.5 HZ) frequencies of COP signals in DN patients after balance training (Table 2, Figure 2). Subsequently, the first possibility may be reduction in the reliance on the vestibular system which crucially controls hip strategy in ML direction. So, the modifications in the ML results shows better controlling of hip muscles after balance training. The later possibility may be related to the speculation which analyzes the higher minimum sway in DN patients as an exploratory sway and useful noise which try to pick up more useful information for postural control at the hip level. In this speculation, although increase in minimum sway negatively lead to more falls but reliance on a postural mechanism at the hip level make them to sway more in ML direction [5]. As a result, when that need provided by information from trained postural control subsystems in DN patients, the sway was decreased which was observed in our results. The study by Pripatla et al. confirmed the possibility of compensation by supplying enough information from subsensory noise in their study [35].
The last possibility was confirmed by our previous study which showed that balance training allowed patients to regain control of a degree of freedom of their hip joint [16]. Subsequently, lower usage of vestibular system and better controlling hip muscles after balance training relieve DN patients’ requirement to more exploratory sway as a compensatory strategy and showed better balance performance after balance training in DN patients. These findings are in agreement with the study by Nagy et al. which reported the effectiveness of balance training by evaluating spectral characteristics in elderly participants. The subjects showed improvement in balance confidence and in control of ML balance in response to the 8 weeks training, and the higher ML direction frequency power exhibited after the training may be indicative of better balance performance [36]. Moreover, other studies which recruited DN patients in physiotherapeutic gait and balance group training [13], structured balance exercises [14, 15] confirmed the effects of balance training in DN patients.
Conclusion
Finally, frequency and spectral analyses of postural sway in DN patients, specifically in ML direction are useful methods for evaluating postural control subsystems after balance trainings. But, further studies needs to investigate the underlying physiology of these possible alterations to precisely discriminate and decide about the role of each subsystem in the improvements after balance training.
Competing of interests
The authors declare that they have no competing interests.
Abbreviations
- CNS:
-
Central nervous system
- DN:
-
Diabetic neuropathy
- FBS:
-
Fasting Blood Sugar
- NCV:
-
Nerve conduction velocity
- BSS:
-
Biodex stability system
- FFT:
-
Fast Fourier Transformation
- POWER:
-
Total frequency power
- 99% FREQ:
-
99% power frequency
- CFREQ:
-
Centroidal frequency
- AP:
-
Anterior-posterior
- ML:
-
Medial- lateral.
References
Nashner LM, McCollum G: The organization of human postural movements: A formal basis and experimental synthesis. Behav Brain Sci 1985, 8: 135–172. 10.1017/S0140525X00020008
Yamamoto R, Kinoshita T, Momoki T, Aria T, Okamura A, Hirao K, Sekihara H: Postural sway and diabetic peripheral neuropathy. Diabetes Res Clin Pract 2001, 52: 213–221. PMID: 11323091 10.1016/S0168-8227(01)00236-4
Boucher P, Bard C, Teasdale N, Fleury M, Courtemanche R: Postural stability in diabetic polyneuropathy. Diabetes Care 1995, 18: 638–645. PMID: 8586001 10.2337/diacare.18.5.638
Horak FB, Nashner LM, Diener HC: Postural strategies associated with somato-sensory and vestibular loss. Exp Brain Res 1990, 82: 167–177. PMID: 2257901 10.1007/BF00230848
Bonnet C, Carello C, Turvey MT: Diabetes and postural stability: Review and Hypotheses. J Mot Behav 2009, 41: 172–190. PMID: 19201687 10.3200/JMBR.41.2.172-192
Horak FB, Dickestein R, Peterka RJ: Diabetic neuropathy and surface sway-referencing disrupt somatosensory information for postural stability in stance. Somatosens Mot Res 2002, 19: 316–326. PMID: 12590833 10.1080/0899022021000037782
Ahmmed AU, Mackenzie IJ: Postural changes in diabetes mellitus. J Laryngol Otol 2003, 117: 358–364. PMID: 12803785 10.1258/002221503321626393
Uccioli L, Giacomini P, Monticone G, Magrini A, Durols A, Bruno E, Parisi L, Di Grilamo S, Menzinger G: Body sway in diabetic neuropathy. Diabetic care 1995, 18: 339–344. PMID: 7555477 10.2337/diacare.18.3.339
Giacomini P, Bruno E, Monticone G, Digirolamo S, Magrini A, Parisi L: Postural rearrangement in IDDM patients with peripheral neuropathy. Diabetic Care 1996, 19: 372–374. PMID: 8729163 10.2337/diacare.19.4.372
Dickestein R, Shupert CL, Horak FB: Fingertip touch improves postural stability in patients with peripheral neuropathy. Gait Posture 2001, 14: 238–247. PMID: 11600327 10.1016/S0966-6362(01)00161-8
Lafond D, Corriveau H, Prince F: Postural control mechanisms during quiet standing in patients with diabetic sensory neuropathy. Diabetes Care 2004, 17: 173–178. PMID: 14693985 10.2337/diacare.27.1.173
Horak FB, Hlavacka F: Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol 2001, 86: 575–585. PMID: 11495933
Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, Staal JB, de Bruin ED: The gait and balance of patients with diabetes can be improved: a randomized controlled trial. Diabetologia 2010, 53: 458–466. PMID: 19921145 10.1007/s00125-009-1592-4
Morrison S, Colberg SR, Mariano M, Parson HK, Vinik AI: Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care 2010, 33: 748–750. PMID: 20097781 10.2337/dc09-1699
Richardson JK, Sandman D, Vela S: A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil 2001, 82: 205–209. PMID: 11239311 10.1053/apmr.2001.19742
Slasabili H, Bahrpeyma F, Forogh B, Rajabali S: Dynamic stability training improves standing balance control in neuropathic patients with type 2 diabetes. J Rehabil Res Dev 2011,48(7):775–786. 10.1682/JRRD.2010.08.0160
Horak FB: Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Aging 2006,3(Suppl 2):ii7-ii11. PMID: 16926210 10.1093/ageing/afl077
Oppenheim U: Kohen_Raz R, Alex D, Kohen_Raz A, Azarya M: postural characteristics of diabetic neuropathy. Diabetes Care 1999, 22: 328–333. PMID: 10333953 10.2337/diacare.22.2.328
Stergiou N: Innovative analyses of human movement. Champaign: Human Kinetics; 2004.
DeWit G: Optic versus vestibular and properoceptive impulses, measured by posturography. Agressologie 1972, 13: 79–82. PMID: 4538702
Taguchi K: Spectral analysis of the movement of the center of gravity in vertiginous and ataxic patients. Agessologie 1978, 19: 69–70. PMID: 727365
Patat A, Foulhoux P, Klein MJ: Residual effects on the equilibrium of three hypnotics (loprazolam, flunitrazepam, triazolam) assessed by spectrum analysis of postural oscillations. Therapie 1986, 41: 443–447. PMID:2880405
Ferdjallah M, Harris GF, Wertsch JJ: Instantaneous spectral characteristics of postural stability, using time-frequency analysis. In Proceedings of the 19th Annual Conference of the IEEE Engineering in Medicine and Biology 1997, 19: 1675–1678.
Laughlin PJ, Redfern MS: Spectral analysis of visually induced postural sway in healthy elderly young subjects. IEEE Trans Neural Syst Rehabil Eng 2001, 9: 24–30. PMID: 11482360 10.1109/7333.918273
Tankisi H, Pugdahl K, Fuglsang-Fredriksen A, Johnsen B, de Carvalho M, Fawcett PRW, Labarre-Vila A: Pathophysiology inferred from electro diagnostic nerve tests and classification of polyneuropathies. Suggested guidelines. Clin Neurophysiol 2005, 116: 1571–1580. PMID: 15907395 10.1016/j.clinph.2005.04.003
Valk GD, Grootenhuis PA, van Eijk JTM, Bouter LM: Methods for assessing diabetic polyneuropathy: validity and reproducibility of the measurement of sensory symptom severity and nerve function tests. Diabetes Res Clin Pract 2000, 47: 87–96. PMID: 10670907 10.1016/S0168-8227(99)00111-4
Winter DA: Human Balance and posture control during standing and walking. Gait Posture 1995, 3: 193–214. 10.1016/0966-6362(96)82849-9
Lin D, Seol H, Nussbaum MA, Madigan ML: Reliability of Cop- based postural sway measures and age- related differences. Gait Posture 2008, 28: 337–342. PMID: 18316191 10.1016/j.gaitpost.2008.01.005
Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM: Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng 1996, 43: 956–966. 10.1109/10.532130
Kim JW, et al.: Sex differences in the postural sway characteristics of young and elderly subjects during quiet natural standing. Geriatr Gerontol Int 2010, 10: 191–198.
Cohen J: Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum associates; 1988.
Golomer E, Cremieux J, Dupui P, Isableu B, Ohlmann T: Visual contribution to self-induced body sway frequencies and visual perception of male professional dancers. Neurosci Lett 1999, 267: 189–192. 10.1016/S0304-3940(99)00356-0
Kim BJ, Robinson CJ: Effects of diabetic neuropathy on body sway and slip perturbation detection in older population. Int J Occup Saf Ergon 2006, 12: 241–254. PMID: 16984784
Maki BE, Holliday PJ, Topper AK: A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol 1994, 49: M72-M84. PMID: 8126355 10.1093/geronj/49.2.M72
Nagy E, Feher-Kiss A, Barnai M, Domja’n-Preszner A, Angyan L, Horvath G: Postural control in elderly subjects participating in balances Training. Eur J Appl Physiol 2007, 100: 97–104. PMID: 17333243 10.1007/s00421-007-0407-x
Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipstiz LA, et al.: Noise-enhanced balance control with diabetes and patients with stroke. Ann Neurol 2006, 59: 4–12. 10.1002/ana.20670
Acknowledgments
Funding/Support: This material was based on work supported by Tarbiat Modares University, School of Medical Sciences, Tehran, Iran. This study was extracted from a Master of Physical therapy project and supported by the Tarbiat Modares University, School of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
Study concept and design: HS, FB. Acquisition of data: HS, FB. Analysis and interpretation of data: HG, AE, HS. Drafting of manuscript: HS. Critical revision of manuscript for important intellectual content: HS, FB. Statistical analysis: MK. Obtained funding: FB. Administrative, technical, or material support: FB. Study supervision: HS, FB, AE. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Salsabili, H., Bahrpeyma, F., Esteki, A. et al. Spectral characteristics of postural sway in diabetic neuropathy patients participating in balance training. J Diabetes Metab Disord 12, 29 (2013). https://doi.org/10.1186/2251-6581-12-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-12-29