Abstract
Background
The depletion of fossil fuel reserves has stimulated the search for sustainable sources of energy that are carbon-neutral or renewable. In this context, microalgae are a promising energetic resource. They are photosynthetic microorganisms that use CO2 as carbon source, with high specific growth rates. Furthermore, some species present high lipid content that can be easily converted into biodiesel. Accordingly, this study aims to analyze the effect of light supply (one of the most important culture parameters) on lipid production of selected microalgae, Chlorella vulgaris and Pseudokirchneriella subcapitata.
Methods
Both microalgal species were cultured under different light irradiance values (36, 72, 96, and 126 μE m−2 s−1) and for each light irradiance value, three light/dark ratios (10:14, 14:10, and 24:0) were tested. Lipid contents of both microalgae were then determined using a recently developed colorimetric method.
Results/Conclusions
P. subcapitata presented higher lipid productivity than C. vulgaris. High lipid concentration was achieved in microalgal cultures with higher light irradiance values. However, for 96 and 126 μE m−2 s−1, lipid productions of both microalgae were not significantly higher than with 72 μE m−2 s−1, which means that microalgal light saturation point may be achieved.
Similar content being viewed by others
Background
Economic development and increased standards of living have resulted in an increased demand for energetic sources. In the last decades, fossil fuels have been extensively used as a source of energy. Consequently, there are now concerns about the low availability of this non-renewable energy resource and about the environmental impacts associated to burning of fossil fuels [1–3]. Attentions are now focused on the production of renewable and carbon-neutral energetic sources, such as biodiesel. Biodiesel consists of a mixture of esters with long-chain fatty acids, such as lauric, palmitic, stearic, and oleic [2, 4]. This renewable energy source can be produced from vegetable oils, animal fats, microorganisms' oils, or waste cooking oil [2, 4–7].
Microalgae are known for their high lipid contents (20% to 50% of dry weight) and high growth rates, being a promising oil source for biodiesel production [8, 9]. However, producing biodiesel from microalgal lipids is not economically viable, requiring several optimizations to reduce production costs and to achieve current demand for biofuels [10]. Different studies have reported that lipid contents differ according to the microalgal strain and their cultivation conditions [11, 12]. To analyze the lipid content of microalgae, the most applied method is organic solvent extraction followed by chemical characterization of lipid profiles using gravimetric analysis [13], thin-layer chromatography, high-performance liquid chromatography, or gas chromatography [14, 15]. However, this procedure is costly, time-consuming, and requires large amounts of toxic and harmful solvents and biomass [5, 9, 10, 16]. Additionally, extraction efficiencies of these methods strongly depend on water content present in microalgal biomass, requiring a previous drying process [9].
Microscopic and colorimetric methods showed to be simple and rapid procedures for screening lipid content of microalgae. Microscopic methods require the use of dyes, such as triptan blue or Nile red, which are selectively incorporated in cells and bind to the target compounds, while colorimetric ones, also known as spectrophotometric methods, are based on the color development of the analyzed solution after reaction of the molecules of interest with specific ions [17–19]. Application of these methods requires little amounts of biomass and reagents [20, 21]. Nile red (a lipid-soluble fluorescent dye) has been frequently used in the determination of neutral lipid contents in animal cells [22], bacteria [23], and microalgae [20, 21, 24]. This method has received much attention due to the rapid and extraction-free determination of neutral lipids and also due to its sensitivity [21, 25]. However, this method is not universal as the applied concentrations of Nile red should be defined for each organism. The establishment of a specific amount of Nile red for each organism is required because this dye acts by penetrating the cells and binding to cytosolic neutral lipids, which means that different affinities of cell membranes to uptake Nile red will result in false determination of lipid contents [18, 21]. Another methodology used to easily determine microalgal lipids is to quantify free fatty acids (FFA). Lipids can be accumulated in free or bound forms; in microalgae, they are mainly stored as bound fatty acids (FA). Thus, previous saponification (hydrolysis of FA) will allow the quantification of FFA as they can react with copper or cobalt salts, forming colored complexes that can be detected by colorimetric/spectrophotometric methods [26–28]. The method applied in this study is based on the fact that FFA can react with copper or cobalt salts with the formation of colored complexes that can be extracted by chloroform in the presence of triethanolamine (TEA). The amount of copper in the chloroform phase is then colorimetrically determined by adding diethyldithiocarbamate to develop a yellow-colored product that can be detected by measuring absorbance at 440 nm. This method was derived from the methods proposed by Wawrik and Harriman [19] and Chen and Vaidyanathan [18].
The aim of this study was to evaluate the influence of different light supplies on microalgal lipid productivities. The colorimetric method derived from those proposed by Wawrik and Harriman [19] and Chen and Vaidyanathan [18] was applied to cultures of Chlorella vulgaris and Pseudokirchneriella subcapitata grown under different light supplies: irradiance values of 36, 72, 96, and 126 μE m−2 s−1 and photoperiods of 10:14, 14:10, and 24:0.
Methods
Microorganisms cultivation and harvesting
C. vulgaris and P. subcapitata (obtained from the American Type Culture Collection) were cultured in modified Organisation for Economic Co-operation and Development test medium [29], with the following composition (per liter): 24 mg MgCl2·6H2O, 36 mg CaCl2·2H2O, 30 mg MgSO4·7H2O, 3.2 mg KH2PO4, 0.16 mg FeCl3·6H2O, 0.2 mg Na2EDTA·2H2O, 0.37 mg H3BO3, 0.83 mg MnCl2·4H2O, 6 μg ZnCl2, 3 μg CoCl2·6H2O, 0.02 μg CuCl2·2H2O, 14 μg Na2MoO4·2H2O, and 100 mg NaHCO3. Nitrogen was supplied in the form of NaNO3 for C. vulgaris and in the form of NH4Cl for P. subcapitata (30 mg L−1). Experiments were performed in 500-mL flasks operating in batch with a working volume of 400 mL. The cells were cultivated for 12 days using the growth medium described above. The experimental conditions were the following: (1) initial cell concentration of 0.05 to 0.08 g L−1, (2) room temperature (22.2 ± 1.2°C), and (3) continuous aeration with the injection of atmospheric air at the bottom of the flasks. The assays were carried out under different irradiance values: 36, 72, 96, and 126 μE m−2 s−1. For each value, different photoperiods were evaluated: 10:14, 14:10, and 24:0 (light/dark). All the experiments were conducted in triplicates.
Growth monitoring
Duplicate samples were collected at 24-h intervals, and biomass concentration was determined by measuring optical density at 683 nm, OD683[30], using a V-1200 spectrophotometer (VWR International China Co., Ltd, Shanghai, China). The relationship between OD683 (y) and cell dry weight (x) of C. vulgaris and P. subcapitata was established by linear regression: y = 1.841x(R2 = 0.997) and y = 2.732x(R2 = 0.993), respectively.
Standard curves
Sodium salts of different fatty acids (decanoic acid, lauric acid, myristic acid, sarkosyl acid, and palmitic acid) were purchased from Sigma-Aldrich, St. Louis, MO, USA (≥98% purity). Stock solutions (5 mM) of each salt were prepared in a solvent mixture of chloroform and methanol (2:1), according to the method proposed by Chen and Vaidyanathan [18]. Serial dilutions were prepared, and 300 μL of each dilution was used for lipid quantification, as described below.
Saponification of cellular lipids
For each experiment performed, 1.5 mL was collected on the first and last day of culturing. These algal suspensions were centrifuged at 12,000 rpm for 10 min in an Eppendorf 5424 centrifuge (Eppendorf, Hamburg, Germany), and cell pellets were stored at -20°C until lipid extraction. The cell pellets were thawed and resuspended in 20 μL of Tris-HCl (1 M, pH 8.0). Then, 480 μL of saponification reagent (25% methanol in 1 M NaOH) and approximately 50 mg of 0.1-mm glass beads (Sigma-Aldrich) were added. Cell disruption was promoted by vigorous vortexing for 3 min. Then, another 500 μL of saponification reagent was added, and the mixture was saponified at 90°C for 30 min and vortexed every 5 min to hydrolyze ester bonds of membrane lipids and triglycerides [18, 19].
Lipid extraction and colorimetric detection
After saponification, the samples were cooled down to room temperature, and 600 μL of the saponified mixture was added to an Eppendorf tube (Eppendorf) containing 900 μL of a solvent mixture of chloroform and methanol (2:1). After vortexing for 2 min, the samples were centrifuged at 5,000 rpm for 2 min. A volume of 500 μL of the organic phase was then transferred to a new Eppendorf tube containing copper reagent (9 vol. aq. 1 M TEA, 1 vol. 1 M acetic acid, 10 vol. 6.45% w/v Cu(NO3)2·3H2O). The procedure applied to standards was similar. To 300 μL of standard solutions, 300 μL of saponification reagent and 300 μL of chloroform and methanol mixture (2:1) were added. After vortexing and centrifugation in the same conditions, 500 μL of the organic phase was added to a new Eppendorf tube, containing the same volume of copper reagent. Samples and standards were vortexed and centrifuged again. After this step, 200 μL of the organic phase was carefully transferred to a quartz cuvette, where 200 μL of color-developing reagent (1% w/v sodium diethyldithiocarbamate in 2-butanol) was added. In this step, it is important that none of the aqueous phase is transferred since it contains copper ions that will produce false signal during colorimetric detection. Absorbance at 440 nm was then measured using a V-1200 spectrophotometer (VWR International China Co., Ltd). A solution containing 200 μL of chloroform and methanol (2:1) mixture supplied with 200 μL of color-developing reagent was used as a blank [18, 19].
Statistical analysis
Lipid quantification was performed in triplicate, and data were analyzed using paired samples t test from the statistical software SPSS 17.0 (Statistical Package for the Social Sciences Inc., Chicago, IL, USA). Significance level was set at p < 0.05.
Results and discussion
Microalgal growth
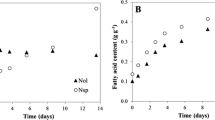
Figure 1 shows the growth curves of C. vulgaris and P. subcapitata corresponding to different light supplies at room temperature and aerated with CO2 at atmospheric concentration. For almost all cultures, the lack of an adaptation phase or a very short one was observed: the exponential phase started before completing the first day of culture. However, for photoperiod of 10:14 and low irradiance values, both microalgal species showed longer adaptation phases. Generally, almost all cultures presented an exponential growth phase with the same duration, reaching the stationary phase on the seventh day of culture. Similar behavior was observed by Jacob-Lopes et al. [31] when the effect of light cycles on the cultures of the cyanobacterium Aphanothece microscopica Nägeli was analyzed. However, for cultures where light supply was higher (longer light periods and higher irradiance values), stationary phase was not reached at all before the end of the experiment. This results indicate that nutrients can be a growth-limiting factor. Two possible limiting nutrients are nitrogen and phosphorus. According to the study performed by Bhola et al. [32], C. vulgaris reached its maximum concentration for nitrogen and phosphorus supplied at 5 and 0.04 g L−1, respectively. In this study, nitrogen and phosphorus were supplied at 0.03 and 0.0032 g L−1, respectively. As these values are lower than those used in the study performed by Bhola et al. [32], the possibility of nutrient limitation is very high.
Regarding the maximum biomass concentration achieved in each culture, the data presented in Figure 1 show that this value was higher for the microalga P. subcapitata for the photoperiod of 10:14 and light irradiance of 96 μE m−2 s−1 (0.808 ± 0.021 g L−1, representing ten times more than the initial concentration of the culture). This kinetic parameter did not present statistical differences varying the light irradiance (p > 0.05), but it was statistically different for other photoperiods (p < 0.05). The achievement of the highest value in a discontinuous light supply may be related with possible photooxidation [33, 34]. The oxygen generated by photosynthesis may accumulate in culture medium, reaching values that in combination with intense light can damage microalgal cells.
Lipid analysis
The method described in this study enables the simple and rapid quantification of lipids from small amounts of microalgal biomass during cell growth experiments. Long-chain fatty acids used in this study showed linear responses for concentrations between 0 and 190.5 mg L−1, as shown in Figure 2. One-sample t test showed that the extinction coefficients, in liters per milligram per centimeter, for all fatty acids were not significantly different (p > 0.05), indicating a similar behavior between fatty acids of different chain lengths. For this reason, lipid quantification was assessed by using an average of extinction coefficients for all fatty acids (Figure 3): y = 1.327 × 10- 2 x + 2.911 × 10- 2 (R2 = 0.999), where y is the absorbance measured at 440 nm and x is the fatty acid concentration in milligrams per liter.
Figure 4 shows the lipid yields of C. vulgaris and P. subcapitata determined for different light supplies. Lipid yields achieved range from 4.611 ± 0.939 to 28.234 ± 0.581 mg g−1 cell dry weight (CDW) for C. vulgaris and from 8.562 ± 1.204 to 39.287 ± 1.879 mg g−1 CDW for P. subcapitata. Similar values were reported by different authors [35, 36]. Ranjan et al. [36] achieved lipid yields between 6.0 and 60 mg g−1 CDW when applying organic solvent extraction to recover lipids from the microalga Scenedesmus sp. Using the same extraction procedure, Lee et al. [35] achieved lipid yields of 49.0 mg g−1 CDW for C. vulgaris. The effect of light supply on lipid yields was similar for both microalgal strains (p > 0.05). Lipid yields were significantly lower (p < 0.05) for the lowest irradiance (36 μE m−2 s−1). Concerning the other light supplies, lipid yields were not statistically different (p > 0.05). Similar results were obtained by Solovchenko et al. [37] who showed that the accumulation of total fatty acids by the microalga Parietochloris incisa increased with irradiance (35, 200, and 400 μE m−2 s−1). Renaud et al. [38] also demonstrated that lipid yields from Isochrysis sp. grown under light irradiance values ranging from 107 to 140 μE m−2 s−1 increased from 7.9 to 10.5 pg cell−1. The same study has revealed that for higher irradiance values, lipid yields did not significantly increase [38]. Weldy and Huesemann [12] also showed that lipid concentration in Dunaliella salina cells increased with higher irradiance value. Different photoperiods did not influence lipid yields achieved for both microalgae; however, for 96 μE m−2 s−1, continuous light supply led to a significant increase in lipid yields. To our knowledge, this is the first study demonstrating the effects of different photoperiods on lipid yields from microalgae. Higher lipid yields observed when irradiance value is increased may be due to the storage of excessive light energy into chemical energy in the form of FA, thus avoiding photooxidative cell damage [39]. Non-increasing lipid yields under the highest irradiance values studied, 96 and 126 μE m−2 s−1, and for longer light periods are a consequence of the photooxidative mechanism: when exposed to high irradiance values, chlorophyll molecules are excited forming a very unstable form, which, in turn, reacts with dissolved oxygen and transfers energy to it. The excited oxygen then reacts with fatty acids to form lipid peroxides, resulting in the reduction of FA concentrations [40]. Another possible reason is that cells can store excessive light energy into chemical energy in the form of other compounds, such as polysaccharides [41].
Conclusions
The use of colorimetric/spectrophotometric methods for rapid screening of microalgal lipids constitutes a promising and accurate technique to determine lipid productivities from microalgae. P. subcapitata presented higher lipid productivity than C. vulgaris. High light supplies (irradiance values greater than 72 μE m−2 s−1) led to the achievement of higher lipid accumulation for both microalgae. However, no statistically significant differences in lipid accumulation were observed for the studied photoperiods. Under the highest light irradiance value with continuous light supply, a slight decrease in lipid productivity was observed that can be associated with photooxidation. Another possible reason is the storage of excessive light energy into chemical energy in the form of other compounds, such as polysaccharides.
References
Oyedepo SO: Efficient energy utilization as a tool for sustainable development in Nigeria. Int. J. Energy Env. Eng. 2012, 3: 11–22. 10.1186/2251-6832-3-11
Betiku E, Adepoju TF: Methanolysis optimization of sesame ( Sesamum indicum ) oil to biodiesel and fuel quality characterization. Int. J. Energy Env. Eng. 2013, 4: 9–16. 10.1186/2251-6832-4-9
Demirbas A, Demirbas MF: Importance of algae oil as a source of biodiesel. Energy Conv. Manag. 2011, 52: 163–170. 10.1016/j.enconman.2010.06.055
Raj MT, Kandasamy MKK: Tamanu oil - an alternative fuel for variable compression ratio engine. Int. J. Energy Env. Eng. 2012, 3: 18–25. 10.1186/2251-6832-3-18
Gonçalves AL, Pires JCM, Simões M: Biodiesel from microalgal oil extraction. In Environmental Chemistry for a Sustainable World. Volume 3. Edited by: Lichtfouse E, Schwarzbauer J, Robert D. Heidelberg: Springer; 2013.
Kim MH, Song HB, Song Y, Jeong IT, Kim JW: Evaluation of food waste disposal options in terms of global warming and energy recovery: Korea. Int. J. Energy Env. Eng. 2013, 4: 1–12. 10.1186/2251-6832-4-1
Eddine BT, Salah MM: Solid waste as renewable source of energy: current and future possibility in Algeria. Int. J. Energy Env. Eng. 2012, 3: 17–28.
Chisti Y: Biodiesel from microalgae. Biotechnol. Adv. 2007, 25: 294–306. 10.1016/j.biotechadv.2007.02.001
Mercer P, Armenta RE: Developments in oil extraction from microalgae. Eur. J. Lipid. Sci. Technol. 2011, 113: 539–547. 10.1002/ejlt.201000455
Brennan L, Owende P: Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energ. Rev. 2010, 14: 557–577. 10.1016/j.rser.2009.10.009
Liu Z-Y, Wang G-C, Zhou B-C: Effect of iron on growth and lipid accumulation in Chlorella vulgaris . Bioresour. Technol. 2008, 99: 4717–4722. 10.1016/j.biortech.2007.09.073
Weldy CS, Huesemann MH: Lipid production by Dunaliella salina in batch culture: effects of nitrogen limitation and light intensity. J. Undergrad. Res. 2007, 7: 115–122.
Bligh EG, Dyer WM: A rapid method of lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37: 911–917.
Goutx M, Gerin C, Bertrand JC: An application of Iatroscan thin-layer chromatography with flame ionization detection—lipid classes of microorganisms as biomarkers in the marine environment. Org. Geochem. 1990, 16: 1231–1237. 10.1016/0146-6380(90)90158-V
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD: Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128: 219–240. 10.1016/0022-0981(89)90029-4
Molina Grima E, Belarbi EH, Fernández FGA, Medina AR, Chisti Y: Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 2003, 20: 491–515. 10.1016/S0734-9750(02)00050-2
Capasso JM, Cossıío BR, Berl T, Rivard CJ, Jiménez C: A colorimetric assay for determination of cell viability in algal cultures. Biomol. Eng. 2003, 20: 133–138. 10.1016/S1389-0344(03)00037-6
Chen Y, Vaidyanathan S: A simple, reproducible and sensitive spectrophotometric method to estimate microalgal lipids. Anal. Chim. Acta. 2012, 724: 67–72.
Wawrik B, Harriman BH: Rapid, colorimetric quantification of lipid from algal cultures. J. Microbiol. Methods 2010, 80: 262–266. 10.1016/j.mimet.2010.01.016
Lee S, Yoon B-D, Oh H-M: Rapid method for the determination of lipid from the green alga Botryococcus braunii . Biotechnol. Techniques. 1998, 12: 553–556. 10.1023/A:1008811716448
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q: A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 2009, 77: 41–47. 10.1016/j.mimet.2009.01.001
Genicot G, Leroy JLMR, Soom AV, Donnay I: The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 2005, 63: 1181–1194. 10.1016/j.theriogenology.2004.06.006
Izard J, Limberger RJ: Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55: 411–418. 10.1016/S0167-7012(03)00193-3
Elsey D, Jameson D, Raleigh B, Cooney MJ: Fluorescent measurement of microalgal neutral lipids. J. Microbiol. Methods 2007, 68: 639–642. 10.1016/j.mimet.2006.11.008
Bertozzini E, Galluzzi L, Penna A, Magnani M: Application of the standard addition method for the absolute quantification of neutral lipids in microalgae using Nile red. J. Microbiol. Methods 2011, 87: 17–23. 10.1016/j.mimet.2011.06.018
Duncombe WG: The colorimetric micro-determination of long-chain fatty acids. Biochem. J. 1963, 88: 7–10.
Ayers CW: Estimation of the higher fatty acids C7-C18. Anal. Chim. Acta 1956, 15: 77–83.
Iwayama Y: New colorimetric determination of higher fatty acids. J. Pharmaceut. Soc. Japan 1959, 79: 552–554.
OECD: Test no. 201: freshwater alga and cyanobacteria, growth inhibition test. OECD Guidelines for the Testing of Chemicals, Section 2. Paris: OECD Publishing; 2011.
Kwon B, Park N, Cho J: Effect of algae on fouling and efficiency of UF membranes. Desalination 2005, 179: 203–214. 10.1016/j.desal.2004.11.068
Jacob-Lopes E, Scoparo CHG, Lacerda LMCF, Franco TT: Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem. Eng. Process. 2009, 48: 306–310. 10.1016/j.cep.2008.04.007
Bhola V, Desikan R, Santosh SK, Subburamu K, Sanniyasi E, Bux F: Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris . J. Biosci. Bioeng. 2011, 111: 377–382. 10.1016/j.jbiosc.2010.11.006
Molina Grima E, Fernández J, Acién FG, Chisti Y: Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92: 113–131. 10.1016/S0168-1656(01)00353-4
Chisti Y: Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26: 126–131. 10.1016/j.tibtech.2007.12.002
Lee J-Y, Yoo C, Jun S-Y, Ahn C-Y, Oh H-M: Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101: S75-S77. 10.1016/j.biortech.2009.03.058
Ranjan A, Patil C, Moholkar VS: Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010, 49: 2979–2985. 10.1021/ie9016557
Solovchenko A, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak M: Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa . J. Appl. Phycol. 2008, 20: 245–251. 10.1007/s10811-007-9233-0
Renaud S, Parry D, Thinh LV, Kuo C, Padovan A, Sammy N: Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. J. Appl. Phycol 1991, 3: 43–53. 10.1007/BF00003918
Asada K: Production and action of active oxygen species in photosynthetic tissues. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Edited by: Foyer CH, Mullineaux PM. Boca Raton: CRC; 1994:77–104.
Carvalho A, Silva S, Baptista J, Malcata F: Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89: 1275–1288. 10.1007/s00253-010-3047-8
Falkouski PG, Raven JA: Aquatic Photosynthesis. Princeton: Princeton University Press; 2007.
Acknowledgements
AL Gonçalves and JCM Pires are grateful to the Foundation for Science and Technology, POPH-QREN, and FSE for their fellowships, SFRH/BD/88799/2012 and SFRH/BPD/66721/2009, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ALG carried out the experimental assays and statistical analysis and drafted the document. JCMP participated in the design of the study and coordination. MS conceived the study and participated in its coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gonçalves, A.L., Pires, J.C. & Simões, M. Lipid production of Chlorella vulgaris and Pseudokirchneriella subcapitata. Int J Energy Environ Eng 4, 14 (2013). https://doi.org/10.1186/2251-6832-4-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-4-14