Abstract
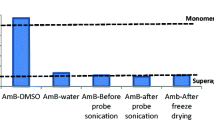
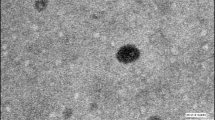
The oral administration of amphotericin B (AmB) has the major drawback of poor bioavailability. The aim of this work was to evaluate the potential of AmB-loaded cubosomes as an oral formulation with improved bioavailability. This manuscript firstly developed AmB-loaded cubosomes by using the SolEmuls technology. The encapsulation efficiency, the in vitro release, and stability studies in simulated gastrointestinal fluid were used to evaluate AmB-loaded cubosomes. The acute nephrotoxicity, bioavailability, and tissue distribution study of AmB-loaded cubosomes were assayed upon oral administration to rats. SAXS and cryo-TEM exhibited AmB-loaded cubosomes as a bicontinuous cubic liquid crystalline phase with Pn3m geometry. The encapsulation efficiency and the results of in vitro release and stability studies in simulated gastrointestinal fluid further demonstrated that AmB was successfully encapsulated in cubosomes. AmB-loaded cubosomal formulation orally administrated in rats did not show nephrotoxicity and its relative bioavailability was approximately 285% as compared to Fungizone®. The AmB-loaded cubosomal formulation presented an effective potential approach for enhancing the oral bioavailability of AmB.






Similar content being viewed by others
References
Kleinberg M. What is the current and future status of conventional amphotericin B? Int J Antimicrob Agents. 2006;27:12–6.
Barrett JP, Vardulaki KA, Conlon C, Cooke J, Daza-Ramirez P, Evans EG, et al. A systematic review of the antifungal effectiveness and tolerability of amphotericin B formulations. Clin Ther. 2003;25:1295–320.
Meis JF, Verweij PE. Current management of fungal infections. Drugs. 2001;61:13–25.
Sachs-Barrable K, Lee SD, Wasan EK, Thornton SJ, Wasan KM. Enhancing drug absorption using lipids: a case study presenting the development and pharmacological evaluation of a novel lipid-based oral amphotericin B formulation for the treatment of systemic fungal infections. Adv Drug Deliv Rev. 2008;60:692–701.
Thornton SJ, Wasan KM. The reformulation of amphotericin B for oral administration to treat systemic fungal infections and visceral leishmaniasis. Expert Opin Drug Deliv. 2009;6:271–84.
Wasan EK, Bartlett K, Gershkovich P, Sivak O, Banno B, Wong Z, et al. Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans. Int J Pharm. 2009;372:76–84.
Gershkovich P, Wasan EK, Lin M, Sivak O, Leon CG, Clement JG, et al. Pharmacokinetics and biodistribution of amphotericin B in rats following oral administration in a novel lipid-based formulation. J Antimicrob Chemother. 2009;64:101–8.
Lynch ML, Ofori-Boateng A, Hippe A, Kochvar K, Spicer PT. Enhanced loading of water-soluble actives into bicontinuous cubic phase liquid crystals using cationic surfactants. J Colloid Interface Sci. 2003;260:404–13.
Chung H, Kim J, Um JY, Kwon IC, Jeong SY. Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia. 2002;45:448–51.
Yang ZW, Peng XS, Tan YH, Chen MW, Zhu XQ, Feng M, et al. Optimization of the preparation process for an oral phytantriol-based amphotericin B cubosomes. J Nanomater. 2011;2011:1–10.
Espada R, Josa JM, Valdespina S, Dea MA, Ballesteros MP, Alunda JM, Torrado JJ. HPLC assay for determination of amphotericin B in biological samples. Biomed Chromatogr. 2008;22:402–7.
Robbie G, Chiou WL. Elucidation of human amphotericin B pharmacokinetics: identification of a new potential factor affecting interspecies pharmacokinetic scaling. Pharm Res. 1998;15:1630–6.
Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22:2163–73.
Siekmann B, Bunjes H, Koch MH, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. Int J Pharm. 2002;244:33–43.
Rizwan SB, Dong YD, Boyd BJ, Rades T, Hook S. Characterisation of bicontinuous cubic liquid crystalline systems of phytantriol and water using cryo field emission scanning electron microscopy (cryo FESEM). Micron. 2007;38:478–85.
Italia JL, Yahya MM, Singh D, Ravi Kumar MN. Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone. Pharm Res. 2009;26:1324–31.
Risovic V, Boyd M, Choo E, Wasan KM. Effects of lipid-based oral formulations on plasma and tissue amphotericin B concentrations and renal toxicity in male rats. Antimicrob Agents Chemother. 2003;47:3339–42.
Müller RH, Schmidt S, Buttle I, Akkar A, Schmitt J, Brömer S. SolEmuls-novel technology for the formulation of i.v. emulsions with poorly soluble drugs. Int J Pharm. 2004;269:293–302.
Akkar A, Müller RH. Formulation of intravenous carbamazepine emulsions by SolEmuls technology. Eur J Pharm Biopharm. 2003;55:305–12.
Akkar A, Muller RH. Intravenous itraconazole emulsions produced by SolEmuls technology. Eur J Pharm Biopharm. 2003;56:29–36.
Akkar A, Namsolleck P, Blaut M, Müller RH. Solubilizing poorly soluble antimycotic agents by emulsification via a solvent-free process. AAPS PharmSciTech. 2004;5:1–6.
Shadkhan Y, Segal E, Bor A, Gov Y, Rubin M, Lichtenberg D. The use of commercially available lipid emulsions for the preparation of amphotericin B-lipid admixtures. J Antimicrob Chemother. 1997;39:655–8.
Chang CM, Bodmeier R. Swelling of and drug release from monoglyceride-based drug delivery systems. J Pharm Sci. 1997;86:747–52.
Rizwan SB, Boyd BJ, Rades T, Hook S. Bicontinuous cubic liquid crystals as sustained delivery systems for peptides and proteins. Expert Opin Drug Deliv. 2010;7:1133–44.
Yaghmur A, Glatter O. Characterization and potential applications of nanostructured aqueous dispersions. Adv Colloid Interface Sci. 2009;147–148:333–42.
Korjamo T, Heikkinen AT, Mönkkönen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98:4469–79.
Sallam AS, Khalil E, Ibrahim H, Freij I. Formulation of an oral dosage form utilizing the properties of cubic liquid crystalline phases of glyceryl monooleate. Eur J Pharm Biopharm. 2002;53:343–52.
Um JY, Chung H, Kim KS, Kwon IC, Jeong SY. In vitro cellular interaction and absorption of dispersed cubic particles. Int J Pharm. 2003;253:71–80.
Katneni K, Charman SA, Porter CJ. An evaluation of the relative roles of the unstirred water layer and receptor sink in limiting the in-vitro intestinal permeability of drug compounds of varying lipophilicity. J Pharm Pharmacol. 2008;60:1311–9.
Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother. 2007;59:941–51.
Ellis M. New dosing strategies for liposomal amphotericin B in high-risk patients. Clin Microbiol Infect. 2008;14:55–64.
Acknowledgements
The authors are grateful to BASF company, Germany, for generous gift samples of Poloxamer 407. The authors are grateful to NCPC Company, China, for generous gift samples of Fungizone®. We thank National Basic Research Program of China (No 81173002) for the financial support. This work was also supported in part by National Key Technology R&D Program (NO:2012BAI35B02) and by Ministry of Science and Technology of China (Projects of International Cooperation and Exchanges; NO2008DFA31080).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Tan, Y., Chen, M. et al. Development of Amphotericin B-Loaded Cubosomes Through the SolEmuls Technology for Enhancing the Oral Bioavailability. AAPS PharmSciTech 13, 1483–1491 (2012). https://doi.org/10.1208/s12249-012-9876-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9876-2