Abstract
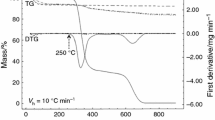
This study investigated the potential use of mesoporous silica nanoparticles (MSNs) as a carrier for duloxetine hydrochloride (DX), which is prone to acid degradation. Sol–gel and solvothermal methods were used to synthesize the MSNs, which, after calcination and drug loading, were then characterized using X-ray diffraction (XRD), Brunauer-Emmett-Teller (BET) technique, thermogravimetric analysis (TGA), Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and diffuse reflectance ultraviolet-visible (DRS-UV-Vis) spectroscopy. Releases of DX from the MSNs were good in pH 7.4 (90%) phosphate buffer but poor in acidic pH (40%). In a comparative release study between the MSNs in phosphate buffer, TW60-3DX showed sustained release for 140 h, which was higher than the other nanoparticles. The mechanism of DX release from the MSNs was studied using Peppas kinetics model. The “n” value of all three MSNs ranged from 0.45 to 1 with a correlation coefficient (r 2) >0.9, which indicated that the release of the drug from the system follows the anomalous transport or non-Fickian diffusion. The results supported the efficacy of mesoporous silica nanoparticles synthesized here as a promising carrier for duloxetine hydrochloride with higher drug loading and greater pH-sensitive release.





Similar content being viewed by others
References
Vallet-Regi M, Ra’mila A, del Real RP, Pe’rez-Pariente JA. New property of MCM-41: drug delivery system. Chem Mater. 2001;13:308–11.
Salonen J, Kaukonen AM, Hirvonen J, Lehto VP. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97:632–53.
Colilla M, González B, Vallet-Regí M. Mesoporous silica nanoparticles for the design of smart delivery nanodevices. Biomater Sci. 2013;1:114–34.
Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–34.
Hirvonen J, Laaksonen T, Peltonen L, Santos H, Lehto V-P, Heikkilä T, et al. Feasibility of silicon-based mesoporous materials for oral drug delivery applications. Dosis. 2008;24:129–49.
Horcajada P, Ramila A, Perez-Pariente J, Vallet-Regi M. Influence of poresize of MCM-41 matrices on drug delivery rate. Micro Meso Mater. 2004;68:105–9.
Friedrich H, Fussnegger B, Kolter K, Bodmeier R. Dissolution rate improvement of poorly water-soluble drugs obtained by adsorbing solutions of drugs inhydrophilic solvents onto high surface area carriers. Eur J Pharm Biopharm. 2006;62:171–7.
Chen C-Y, Li H-X, Davis ME. Studies on mesoporous materials. I. Synthesis and characterization of MCM-41. Micro Meso Mater. 1993;2:17–26.
Tanev PT, Pinnavaia TJ. A neutral templating route to mesoporous molecular sieves. Science. 1995;267:865–7.
Shahbazi M-A, Herranz B, Santos HA. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter. 2012;2:296–312.
Ukmar T, Planinek O. Ordered mesoporous silicates as matrices for controlled release of drugs. Acta Pharm. 2010;60:373–85.
Mehrdad M. Synthesis and characterization of pH-sensitive silica nanoparticles for oral-insulin delivery. Current Drug Deliv. 2011;8:607–11.
Kumar D, SailajaChirravuri SV, Shastri NR. Impact of surface area of silica particles on dissolution rate and oralbioavailability of poorly water soluble drugs: a case study with aceclofenac. Int J Pharm. 2014;461:459–68.
Charnay C, Begu, Tourn-Pateilh C, Nicole L, Lerner DA, Devoisselle JM. Inclusion of ibuprofen in mesoporous templated silica: drug loading and release property. Eur J Pharm Biopharm. 2004;57:533–40.
Ambrogi V, Perioli L, Marmottini F, Accorsi O, Pagano C, Ricci M, et al. Role of mesoporous silicates on carbamazepine dissolution rate enhancement. Micro Meso Mater. 2008;113:445–52.
Zhu Y, Shi J, Shen W, Dong X, Feng J, Ruan M, et al. Uniform magnetic hollow spheres with a magnetic core/mesoporous silica shell structure. Angew Chem Int Ed Engl. 2005;4:5083–7.
Mal N, Fujiwara M, Tanaka Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature. 2003;421:350–3.
He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32:7711–20.
Van SM, Mellaerts R, Thi TD, Martens JA, Van Humbeeck J, Annaert P, et al. Preventing release in the acidic environment of the stomach via occlusion in ordered mesoporous silica enhances the absorption of poorly soluble weakly acidic drugs. J Pharm Sci. 2011;100:4864–76.
Ganesh M, Hemalatha P, Mei Mei P, Palanichamy M, Ubaidulla U, Jang HT. Synthesis and characterization of pharmaceutical surfactant templated mesoporous silica: its application to controlled delivery of duloxetine. Mater Res Bull. 2014;51:228–35.
Roggers R, Kanvinde S, Boonsith S, Oupick D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS PharmSciTech. 2014;15:1163–71.
Ganesh M, Lee SG. Synthesis, characterization and drug release capability of new cost effective mesoporous silica nano particles for ibuprofen drug delivery. Int J Cont Auto. 2013;6:207–16.
Salonen J, Laitinen L, Kaukonen AM, Tuura J, Bjorkqvist M, Heikkila T, et al. Mesoporous silicon microparticles for oral drug delivery: loading and release of five model drugs. J Contol Release. 2005;108:362–74.
Popovici RF, Seftel EM, Mihai GD, Popovici E, Voicu AV. Controlled drug delivery system based on ordered mesoporous silica matrices of captopril as angiotensin-converting enzyme inhibitor drug. J Pharm Sci. 2011;100:704–14.
Ng JBS, Kamali-Zare P, Brismar H, Bergstrom L. Release and molecular transport of cationic and anionic fluorescent molecules in mesoporous silica spheres. Langmuir. 2008;24:11096–102.
Vinita V, Nagesh U, Mittapalli N, Kumar P, DR. Reddy’s Laboratories, assignee. Pharmaceutical formulations comprising duloxetine, United States patent, 20090226517A1, 2009
Zhao R-K, Cheng G, Tang J, Song J, Peng W-X. Pharmacokinetics of duloxetine hydrochloride enteric-coated tablets in healthy Chinese volunteers: a randomized, open-label, single- and multiple-dose study. Clin Ther. 2009;31:1022–36.
Gopferich A, Tessmar J. Polyanhydride degradation and erosion. Adv Drug Deliv Rev. 2002;54:911–31.
Patel K, Padhye S, Nagarsenker M. Duloxetine HCl lipid nanoparticles: preparation, characterization, and dosage form design. AAPS PharmSciTech. 2012;13:125–33.
Ganesh M, Hemalatha P, Mei Mei P, Rajasekar K, Jang HT. A new fluoride mediated synthesis of mesoporous silica and their usefulness in controlled delivery of duloxetine hydrochloride a serotonin re-uptake inhibitor. J Ind Eng Chem. 2012;18:684–9.
Yunoos M, Sankar DG, Kumar BP, Hameed S, Hussain A. Simple UV spectrophotometric determination of duloxetine hydrochloride in bulk and in pharmaceutical formulations. E-J Chem. 2010;7:785–8.
Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Marjo CE, Bhadbhade M, Hook JM, Rich AM. Polymorphism and a metastable Solvate of duloxetine hydrochloride. Mol Pharm. 2011;8:2454–64.
Newa M, Bhandari KH, Oh DH, Kim YR, Sung JH, Kim JO, et al. Enhanced dissolution of ibuprofen using solid dispersion with poloxamer. 407. Arch Pharm Res. 2008;31:1497–507.
Labudzinska A, Gorczynska K. The UV difference spectra as a characteristic feature of phenols and aromatic amines. J Mol Str. 1995;349:469–72.
Sugimoto Y, Nishimura S, Imoto E. The ultraviolet spectra of the thiophene derivatives. Opera 1959;71–81
Landau MV, Varkey SP, Herskowitz M, Regev O, Pevzner S, Sen T, et al. Wetting stability of Si-MCM-41 mesoporous material in neutral, acidic and basic aqueous solutions. Micro Meso Mater. 1999;33:149–63.
Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–5.
Kuang Y, Zhao L, Zhang S, Zhang F, Dong M, Xu S. Morphologies, preparations and applications of layered double hydroxide micro-/nanostructures. Materials. 2010;3:5220–35.
Wei M, Pu M, Guo J, Han J, Li F, He J, et al. Intercalation of l-dopa into layered double hydroxides: enhancement of both chemical and stereochemical stabilities of a drug through host-guest interactions. Chem Mater. 2008;20:5169–80.
Acknowledgments
This work was financially supported by grants from the Korea CCS R&D Center, funded by the Ministry of Education, Science and Technology of the Korean Government. The authors extend their thanks to National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (Grant No. 2014-004694) for their partial financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 7874 kb)
Rights and permissions
About this article
Cite this article
Ganesh, M., Ubaidulla, U., Hemalatha, P. et al. Development of Duloxetine Hydrochloride Loaded Mesoporous Silica Nanoparticles: Characterizations and In Vitro Evaluation. AAPS PharmSciTech 16, 944–951 (2015). https://doi.org/10.1208/s12249-014-0273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0273-x