Aminomethylenephosphonic Acids Syntheses and Applications (A Review)
Didier Villemin1 and Mohamed Amine Didi2*
1Laboratoire de Chimie Moléculaire et Thioorganique, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ENSICAEN, 14050 Caen, France.
2Laboratory of Separation and Purification Technology, Tlemcen University,Faculty of Sciences, Department of Chemistry,Box 119, Algeria.
Corresponding Author Email: madidi13@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/31.Special-Issue1.01
Article Received on :
Article Accepted on :
Article Published : 11 Sep 2015
A review. In this paper, were reviewed the aminomethylenephosphonic acids which have several synthesis routes and many applications as separation of metals and rare earth by solvant extraction, N-C-P in medicinal chemistry, pesticides/herbicides and water-decontamination systems, organometallic complexes: MOF, gas absorption, proton conductivity and Magnetic shielding tensors / aminotris(methylenephosphonates).
KEYWORDS:aminomethylphosphonic; solvent extraction; chelate; rare earth metals; pesticides; herbicides
Download this article as:| Copy the following to cite this article: Villemin D, Didi M. A. Aminomethylenephosphonic Acids Syntheses and Applications (A Review). Orient J Chem 2015;31(Special Issue1). |
| Copy the following to cite this URL: Villemin D, Didi M. A. Aminomethylenephosphonic Acids Syntheses and Applications (A Review). Orient J Chem 2015;31(Special Issue1). Available from: http://www.orientjchem.org/?p=10779 |
Introduction
Aminophosphonic acid can be an attractive molecule because it can be used in a large range of field. The aim of this report is to summarize synthesis methods of aminomethylphosphonic acids and their applications. Most important characteristic of these compounds is their N-C-P molecular fragment that broadly defines them as analogues of amino acids, in which the carboxylic group is replaced by a phosphonic acid (Figure 1).
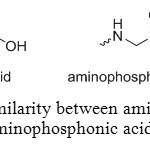 |
Figure 1: Similarity between amino acids and aminophosphonic acids. Click here to View figure |
This similarity gives to aminomethyl-phosphonic acid biological applications in healthcare and agriculture. Moreover, it is a weak organic acid with a pKa value of 0.5 and its toxicity is lower than glyphosate’s one, its main source.[[1]]
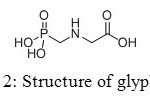 |
Figure 2: Structure of glyphosate. Click here to View figure |
First OH group in phosphonate acids is very acidic, with a pKa value of 0.5-1.5. However, second OH group has a significantly lower acidity with a pKa value of 5.0-6.5 (Figure 3a).
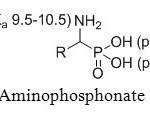 |
Figure 3a: Aminophosphonate acids pKa. Click here to View figure |
Like amino acids, aminophosphonic acids have a zwitterionic form (Figure 3b) due to internal hydrogen bonding between phosphonate and ammonium groups, a characteristic which is strongly demonstrated by NMR and X-ray studies.
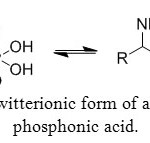 |
Figure 3b: Zwitterionic form of aminomethyl-phosphonic acid. Click here to View figure |
Aminomethylphosphonic acids and derivatives have several applications. They can reduce and stop corrosion and by this way, protect electroplating or chemical plating. Pesticides and herbicides are another field where aminomethylphosphonic acids are efficient. They are used as water decontaminator in order to remove some hazardous micropollutant molecules.
Its two hydroxyl functions linked to phosphorus atom can easily be connected to a metal and chelate it. This property allows to do gas absorption, notably CO2, to generate MOF and to extract selectively metal from a solid or a liquid phase, especially for lead. Magnetic shielding generated by phosphorus atom archives to create a perturbation in a metal-phosphoric acid crystal.
This report will be divided in two parts. First, several synthetic routes to aminomethylphosphonic acids will be detailed. On the other hand, applications of aminomethyphosphonic acids in various fields will be presented.
Preparation of N-substituted amino-methylphosphonic acid compounds
With the aim to get as efficiently as possible α-aminophosphonic acids, two main synthetic routes were developed. The most common one is in two distinct steps, synthesizing first the ester derivative, then hydrolyzed. Another method consists in a direct synthesis of the acid.
Preparation Aminomethylphosphonic Esters
Via Mannich Reaction
In this part will be detailed different synthetic routes to distinct α-aminophosphonic esters, whose hydrolyses will be itemized in next chapter.
First synthesis of diethylaminomethyl-phosphonic diethylester using Mannich reaction is in two steps. Hydroxymethylamine intermediate is synthesized in excellent yield by causing to react together formaldehyde and diethylamine. Final product is achieved though phosphite diethylester addition (Figure 4).[[1]]
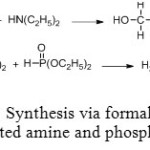 |
Figure 4: Synthesis via formaldehyde, disubstituted amine and phosphite ester. Click here to View figure |
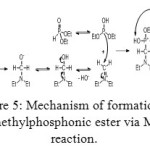 |
Figure 5: Mechanism of formation of aminomethylphosphonic ester via Mannich reaction. Click here to View figure |
Experimentally, 7.5 parts 40% aqueous formaldehyde is added to a cold mixture of 13.8 parts diethyl phosphite and 7.32 parts diethylamine. The resulting clear solution becomes hot. When it no longer evolves heat (10-15 minutes), it is distilled in vacuo. There are obtained 20.3 parts (84.2%) of diethylaminomethylphosphonic acid diethyl ester as a colorless oil boiling at 95°C at 3 mm pressure.
This reaction can be performed with other carbonyl compounds, other primary or secondary amines and other phosphite diesters. Water is always formed as a product in this reaction. In some cases, the carbonyl compound reacts relatively slowly and a side reaction may occur: some phosphite diester may be hydrolysed by the gradually formed water.
To face this problem, an alternative method for preparing aminomethyl-phosphonic ester was discovered. Using a tetra-substituted alkylidenediamine with a phosphite diester allows to avoid formation of water (Figure 6).[1]
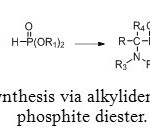 |
Figure 6: Synthesis via alkylidenediamine and phosphite diester. Click here to View figure |
Mannich mechanism is exactly the same with the exception of formation of water which is replaced by a secondary amine (Figure 7).
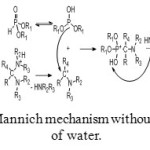 |
Figure 7: Mannich mechanism without formation of water. Click here to View figure |
In this case, 13.8 parts of diethyl phosphite are added to 10.32 parts of methyloldiethylamine. The mixture is kept below 40°C by cooling till heat is no longer evolved, then distilled. There are obtained 23.6 parts (98%) of diethylaminomethyl-phosphonic acid diethyl ester boiling at 95°C at 3 mm pressure.
Via enantioselective Synthesis
Aminomethylphosphonic ester are also accessible via reaction between imine and phosphite diethylester (Figure 8). In this case, the use of an enantiopure catalyst (Figure 9) allows to getting one major compound, with distinct enantiomeric excess, depending on the catalyst and R1 and R2 groups (Table 1).[[1]]
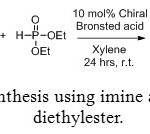 |
Figure 8: Synthesis using imine and phosphite diethylester. Click here to View figure |
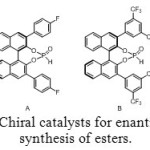 |
Figure 9: Chiral catalysts for enantioselective synthesis of esters. |
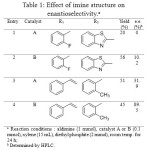 |
Table 1: Effect of imine structure on enantioselectivity.a Click here to View table |
In line with our earlier observation that catalyst B was more suited to cinnamaldehyde-derived imines (entry 4) compared to the one derived from benzaldehyde and heterocyclic amine (entry 2), the catalyst A showed too a similar trend with cinnamaldehyde in enhancing the enantioselectivity (entry 1 vs. entry 3).
Via Kabachnik-Fields Reaction
Mechanism of this reaction called Kabachnik-Fields was discovered in 1952 by eponymous researchers and depends on the nature of the substrates. It played a relevant role in drug discovery research for generating peptidomimetic compounds.[[1]]
The amine and phosphonate form a complex, in which either one of the partners may react with the carbonyl compound. Usually, the basicity of the amine determines the reaction pathway. In the Kabachnik-Fields reaction mixture, two nucleophiles (dialkyl phosphite and the amine) compete for the electrophilic carbonyl compound.
Experimental results revealed that the first stage of the Kabachnik–Fields reaction, namely, the formation of a dialkyl phosphite–amine complex is of critical importance for further reaction pathway. Depending on the acidity–basicity relationship between the dialkyl phosphite and the amine, the readily polarizable complex can have a structure like.
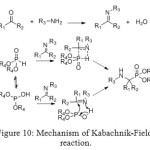 |
Figure 10: Mechanism of Kabachnik-Fields reaction. Click here to View figure |
If additional catalysts are used, both acids and bases can have a positive influence on the reaction rate. Sometimes, the chemical yield and the diastereoselectivity of the formation of aminomethylphosphonates are higher in two-component systems using preformed imines. In this case, due to the phosphonate–phosphite tautomerism, the addition to the imine could occur by either a four- or five-membered transition state.[[1]]
Under Microwave Conditions
Ester derivatives of aminomethyl-phosphonic acids can also be obtained using microwave heating. The lanthanide triflates (Yb(OTf)3 for example), catalyzing the Kabachnik-Fields reaction, gave excellent yields in ionic liquids under MW irradiation. However, new microwave-assisted solvent-free and catalyst-free method was recently developed for the synthesis of α-aminomethylphosphonates.[[1]]
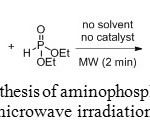 |
Figure 11: Synthesis of aminophosphonates under microwave irradiation. Click here to View figure |
Microwave heating was introduced to reduce the reaction time. Indeed, after the reaction mixture was irradiated in a multimood microwave reactor at 180 W for only 2 minutes, the reaction was completed and the yield was increased to 98%.
The challenge to get α-amino-phosphonates was widely achieved, scientists faced another difficulty in preparation of aminomethylphosphonic acids. High stability of phosphonic esters makes them hardly hydrolysable.
Preparation of aminomethylphosphonic acids from aminomethylphosphonic acids esters
This part will deal with strategic approaches of researchers to hydrolyze ester derivatives to get aminomethylphosphonic acids.
Hydrolysis with Silicium
Different ways exist to generate the target molecule from aminomethylphosphonic esters. One of these ways uses a reagent which contains silica. The affinity between silica and oxygen is well-known. Therefore, double bond between oxygen and phosphorus atoms reacts with silica and generates cationic phosphorus atom. To recover neutrality of this atom, the O-R’ bond is broken. This mechanism is repeated another time to give a phosphorus with a double O-Si-R’ substitution.
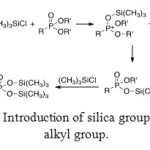 |
Figure 12: Introduction of silica group instead of alkyl group. Click here to View figure |
Last step of hydrolysis of phosphonic ester is the reaction between previous molecule and an alcohol. There are three different kinds of alcohol attack. The first one is on phosphorus atom and provides access to phosphonic ester (Figure 13a).
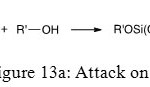 |
Figure 13a: Attack on P Click here to View figure |
The second one is on silicon atom and directly generates the phosphonic acid (Figure 13b).
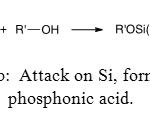 |
Figure 13b: Attack on Si, formation of phosphonic acid. Click here to View figure |
The last one is a blend of these two attacks and gives one OH and one OR substitution on phosphorus atom (Figure 13c).
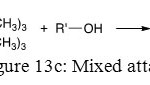 |
Figure 13c: Mixed attack |
Enzymatic way
In this method, the first step of this synthesis uses a basic reagent to convert one ester into one acid. Secondly, phosphodiesterase is employed at a pH value of 9 to convert the second O-Ph function.[[1]]
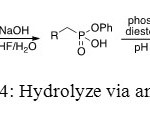 |
Figure 14: Hydrolyze via an enzyme. Click here to View figure |
Reaction in Harness Conditions
*Hydrolyses by concentrated acid milieu
Another way to hydrolyze a phosphonic ester in phosphonic acid uses concentrated hydrochloride acid conditions:
 |
Figure 15: Hydrolyze of phosphonic acid. Click here to View figure |
Experimentally, starting material was treated with 500 mL of concentrated HCl and the mixture was stirred at room temperature overnight. Toluene was then added, and phases were separated. The aqueous layer was evaporated in vacuo with a bath temperature of 35-40°C to leave a residue which was dissolved in 400 mL of MeOH and filtered. The filtrate was treated with a solution of 110 mL of propylene oxide in 60 mL of MeOH, drop wise at 30°C. The mixture was stirred at 0°C overnight and the solid was filtered and washed with 200 mL of MeOH to give the final product.[[1]]
Hydrolyses with a basis
The strategy of synthesis is to convert O-Ph substituent into O-Bn group because the mechanism which gives OH function from O-Bn group is well known. It is the same mechanism as the deprotection of an alcohol function. It is the reason why palladium on coal is used to obtain phosphonic acid.
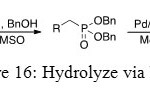 |
Figure 16: Hydrolyze via NaH. Click here to View figure |
Direct preparation of aminomethylphosphonic acids
Grafting of phosphonic acid on polymer
Phosphonic acid function can form a polymer in order to give some extraction and purification properties. To synthesize phosphonated polyethylenimines (PEIP), first step is formation of polyethylenimines (PEI) (Figure 17).
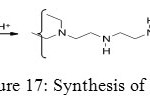 |
Figure 17: Synthesis of PEI. Click here to View figure |
Secondly, phosphonation aims to add phosphonic acid functions by a Moedritzer-Irani reaction to give final product (Figure 18).[[1]]
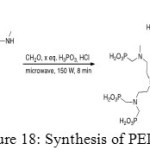 |
Figure 18: Synthesis of PEIP. |
Engelmann and Pick’s procedure
In this method, three compounds react together to prepare aminophosphonic acid: an amide, formaldehyde and a phosphorus trichloride. The proposed mechanism gets through a N-(hydroxyl-methyl)amide. Reaction with phosphorus trichloride then results in conversion of the hydroxyl group into an easily leaving group, which undergoes phosphate-phosphonate rearrangement either intermolecular or via nucleophilic substitution by a second molecule of phosphorus trichloride.[[1]]
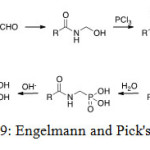 |
Figure 19: Engelmann and Pick’s reaction. Click here to View figure |
Applications
Aminomethylphosphonic acids are found in several fields, from applications in materials, builders and cobuilders in detergents, to agriculture compounds. Firstly used as corrosion inhibitors, they also showed interesting properties to lubricate surfaces. However, new studies appeared in the eighties concerning toxicity of aminomethyl-phosphonic acid, main product of decomposition of glyphosate (Figure 20).[[1]]
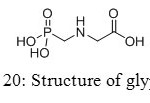 |
Figure 20: Structure of glyphosate. Click here to View figure |
In this chapter will be specified applications of aminomethylphosphonic acids in biological fields as herbicides, pesticides but also as medicines. The other important utilization of these compounds is as organometallic complexes to protect materials against corrosion or as Metal Organic Frameworks (MOF) to fix or trap various kinds of molecules.
Biologic applications
N-C-P in medicinal chemistry
Aminomethylphosphonic acids are broadly defined as analogues of amino acids in which the carboxyl group is replaced by a phosphonic acid. The biological activity and natural occurrence of these compounds were discovered half a century ago[[1]] with applications ranging from agrochemistry to medicine.[[2]]
The mode of action of aminomethylphosphonates primarily involves inhibition of enzymes. The N-C-P molecular fragment and its chemistry offer many possibilities for structural modifications (Figure 21).[[1]] Even if carboxylic and phosphonic acid groups differ in shape, acidity and steric bulk, they exhibit similar properties, with the phosphonic acid being recognized by enzymes or receptors as false substrates or inhibitors.
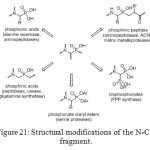 |
Figure 21: Structural modifications of the N-C-P fragment. Click here to View figure |
Moreover, aminomethylphosphonic acids are medicinally important compounds because they are hydraulically stable analogues of pyrophosphate characterized by a common P-C-P fragment. Their primary medical application is in combating osteoporosis (Figure 22), hindering the formation and dissolution of calcium phosphate crystals in vitro.[[1]]
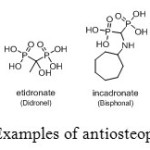 |
Figure 22: Examples of antiosteoporotic drugs. Click here to View figure |
Phosphonic pseudopeptides are used for medical applications that target such important diseases as cancer (development and metathesis), hypertension and malaria. Replacing carboxylic acids by phosphonic acids often creates mimes of amino acids used for the preparation of neuroactive analogues. One example of this approach is R-R-amino-5-chloro-1-(phosphonomethyl)-1H-benzimidazole-2-propanoic acid (Figure 7), a potent noncompetitive antagonist of synaptic N-methyl-D-aspartate (NMDA) receptors.[[1]] It is expected to be developed a drug to prevent strokes.
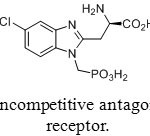 |
Figure 23: Noncompetitive antagonist of NMDA receptor. Click here to View figure |
Other short peptides phosphonate esters are potential drug candidates for the treatment of cancer,[[1]] type 2 diabetes,[[2]] hematopoetic disorders and some viral infections. Finally, potential applications of specific enzymatic activities among hydrolases and ligases could provide new therapies for tuberculosis, bacterial and protozoan infections and HIV.[[3]]
Pesticides/herbicides and water-decontamination systems
Modern agrochemicals should have a combination of properties, including high levels of herbicidal or pesticidal activity, low application rates, crop tolerance, and low levels of toxicity to mammals.[[1]] Pesticide pollution of the aquatic environment is also a major concern for our society because of the potential adverse effects of these compounds to ecosystems.[[2]] Organophosphorous pesticides are usually preferred rather than organochlorine ones because they show a lower persistence and bioaccumulation and a higher biodegradability.[[3]]
Glyphosate, first marketed by Monsanto as Roundup®,[[1]] has become the world’s leading agrochemical,[[2]] especially in recent years with the widespread of genetically modified glyphosate-tolerant crops,[[3]] and its physical, chemical, environmental, and toxicological properties have been well-documented.[[4]] It is rapidly degraded by cations[[5]] or soil microorganisms, yielding glyoxylate and aminomethyl-phosphonic acid as primary intermediates. Contrary to glyoxylate, aminomethyl-phosphonic acid is more toxic than the parent molecule and more persistent in water and soil.[[6]]
Decomposition of glyphosate and aminomethylphosphonic acid was studied because of its toxicity to birds, fish, aquatic invertebrates and humans.[[7]] Several methods were developed to detect low concentrations in soils and water. Decomposition kinetics of these compounds under natural conditions was investigated.[[8]] Photo-Fenton process[[9]] and photocatalysis with TiO2[[10]]allow to remove glyphosate from aqueous solutions whereas photodegradation in the presence of ferrioxalate,[[11]] oxidation by manganese oxide,[[12]] or electrooxidation with DSA anodes[9] can be used for the removal of aminomethylphosphonic acid.
PEIP is already used in France to purify sea water. Hydroxyl groups on oxidized steel react with phosphonic acids in water to form a layer of ferric phosphonate, which self-associates in lamellar assemblage (MOF).[9] PEIP have multiple applications, as anticorrosive agent, but also as micropollutant purifiers. It can be grafted on cyclodextrin via an addition of cyanuric chloride on them in order to remove hazardous molecules like ethinylestradiol which is present in oral contraceptives.
Organometallic complexes: MOF, Gas Absorption, Proton conductivity, extraction
Gas absorption
Metal organic frameworks (MOF) are inorganic-organic compounds whose organic part can be composed of aminomethylphosphonic ligands. Recent studies on MgH6ODTMP ∙ 2 H2O (DMF)0.5 synthesized using octamethylenediamine-N,N,N’,N’-tetrakis(methylenephosphonic acid) demonstrate useful gas absorbing properties with a high selectivity toward CO2 absorption in CO2/CH4 mixtures and low heat of absorption for CO2 uptake.[[1]]
Extraction
Magnesium was an excellent candidate as a light metal ion with coordinative properties similar to those of various divalent metal ions of the first transition series. Other cases of magnesium-containing metal organic frameworks were reported.[[1]] Polyphosphonic ligands like aminomethylenephosphonic acid form a set of new compounds with different typologies and interesting properties.[[2]]
Synthesis of fused heterocyclic nitrogen systems containing phosphorus atom, has led at from selective systems of metals extraction.[38]
The liquid-solid extraction of Cu(II) from sulfate media at 25°C by sodium homoionic montmorillonites impregnated either with di-2,4,4-trimethylpentyl phosphonic acid gave a capacity of extraction of 200 mmol/kg.[39]
Unfortunately, relatively low absorption capacity of MgH6ODTMP ∙ 2 H2O(DMF)0.5 makes this material inappropriate for absorption processes and gas storage. Nevertheless, this metal organic framework shows high proton conductivity, attributed to a Grotthuss transfer mechanism via water molecules.[40]
On the other hand, other organometallic complexes made of aromatic tetraphosphonic acids were studied for their proton conductivity.[41]
Concerning M[(HO3PCH2)2N(H)CH2 C6H4CH2N(H)(CH2PO3H)2(H2O)2] · 2 H2O (M = Mg or Zn) luminescence properties were observed with a slight change in λmax in the emission spectra depending on metallic atom considered. The electronic structure of the phosphonate ligand is also perturbed.
Aminomethylphosphonic acid can be used to extract metals by sorption. With the aim of extracting uranium (VI), ethylenediaminotris(methylenephosphonic) acid was grafted on a polystyrene resin. This compound was synthesized from a Merrifield resin (Figure 24).[42]
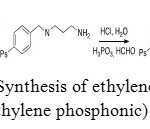 |
Figure 24: Synthesis of ethylenediaminetris (methylene phosphonic) acid. Click here to View figure |
The experimental capacity obtained is 41.76 mg/g in optimal conditions of time, pH, uranium concentration and electrolytic effects. In addition, negative value of Gibbs free energy indicates spontaneous nature of sorption.
A polymer including aminomethyl-phosphonic acid function can be used to extract lead from a solid phase. The polyethyleneimine methylphosphonic acid (PEIMPA) shows good efficiency in capture of lead from acetate aqueous solutions. Currently, it is the most powerful chelator of lead with 609 mg/g. Selectivity of separation of Pb(II) from Zn(II) is good at low pH.[43]
Coating against corrosion
Metallic surface coatings comprising organic phosphorus are copolymer dispersions formed of aminomethyl-phosphonic acid, the corrosion inhibitor, and an alcohol which react to form the phosphoric ester.[[1]] Metallic pieces are then contacting with this aqueous copolymer solution to be protected against corrosion. The phosphoric functions are considered to be the most effective chemical group against corrosion process.[44]
One domestic application concerns a novel inhibitor composition using to protect the metal, removing of scale deposits in boilers, in cooling water systems or in closed water loop fountains.[45] Polyphosphate solutions are preferably used and ideal formulations and concentrations were precisely set out. Relatively highly concentrated polyphosphate solutions provide very good corrosion protection, but so do solutions of more diluted, which provides a degree of flexibility.
Studies also demonstrate that for open-air installations with plate storage, polyphosphate concentration may be different in the whole system.[46] Aminomethyl-phosphonic acids are excellent sequestering agents for electroplating, chemical plating, degreasing and cleaning.
It was shown that piperidin-1-yl-phosphonic acids (PPA) and (4-phosphono-piperazin-1-yl) phosphonic acid (PPPA) are used to reduce corrosion of iron in a NaCl medium, even if PPPA is more efficient than PPA. Moreover, inhibition efficiency value and corrosion rate increase with inhibitor concentration until a maximum. Phosphonic acids reduce accessibility of chloride ions to the metal and form a protective coating on the surface of iron in order to retard the destruction action.
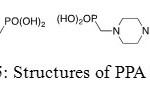 |
Figure 25: Structures of PPA and PPPA. Click here to View figure |
Magnetic shielding tensors / aminotris (methylenephosphonates)
This section will deal with aminotris(methylenephosphonates) (AMP) and specificity of its coordination with distinct metals.
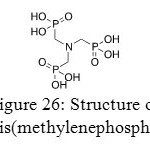 |
Figure 26: Structure of animotris(methylenephosphonates). Click here to View figure |
Aminotris(methylenephosphonates) could be coordinated with a large range of metals such as Zn, Mg, Ca or Ba. In solution or in solid form, they are zwitterionic. This correlation between a metal (II) and an aminotris(methylenephosphonates) is disturbed by phosphorus atom of aminotris(methylenephosphonates) especially in Ca(AMP). 4.5(H2O). Actually, in any phosphonates, the phosphorus atom is at the center of a shielding tensor. Basis vectors are deviated in a systematic way from the nearest neighbor and a dynamical disorder in the crystal structure is generated.[47]
Conclusion
This report could be summarized highlighting that aminomethylenephosphonic acids have several synthesis routes and many applications. There are three main approaches to synthesize them:
Direct methods, either by grafting on a polymer or following the Engelmann and Pick’s procedure;
Indirect routes, consisting in synthesizing aminophosphonate ester (via Kabachnik-Fields or Mannich reaction) and then converting aminophosphonate ester into aminophosphonate acid with the enzymatic way or the hydrolysis with silicon.
The second part of this report aimed to reference most important applications of aminomethylenephosphonic acid.
First, biologic applications were studied. The similarity between amino acid and aminomethylenephosphonic acid is the origin of many applications. Indeed, the C-NP bond is present in many biologic molecules.
Secondly, aminomethylenephosphonic acid can be complexed with several metals. These molecules are then used to extract micropolluant from water or to absorb gases.
References
- Kukhar, V. P.; Romanenko, V. D. Amino Acids, Peptides and Proteins in Organic Chemistry, vol 2: 191-262 (2009).
- Kirby Fields, E. Process for producing aminomethylphosphonic acid compounds 1953, US 2635112 A
- Xu, W. ; Zhang, S. ;Yang, S. ; Jin L.-H. ; Bhadury, P. S.; Hu, D.-Y. ; Zhang, Y. Molecules 2010, 15, 5782-5796
- Naydenova, E. D. ; Todorov, P. T., Troev, K. D. Amino acids, 2010, 38, 23-30
- Cherkasov R Russ. Chem. Rev. 1998, 67, 857–882
- Mu XJ, Lei MY, Zou JP, Zhang W Tetrahedron Lett. 2006, 47, 1125–1127.
- Hwang, K-J.; Kim, B-T.; Kim, B-O. Bull. Korean. Chem. Soc. 2009, 30 (6), 1391-1393
- Lambert, R. W.; Atherton, F. R.; Hassall, C. H. J. Med. Chem. 1986, 29, 29-40
- Villemin, D.; Monteil, C.; Bar, N.; Didi, M. A.; Phosph Sulfur. Silicon Relat. Elem. 2015, in press. DOI: 10.1080/10426507.2014. 981633
- Todorov, P. T.; Naydenova, E. D.; Troev K. D. Amino. Acid. 2010, 38, 23-30
- Coupe, R. H.; Kalkhoff, S. J.; Capel, P. D.; Gregoire, C. Pest Management Science. 2012, 68(1), 16-30
- (a) Hiroguchi, M.; Kandatsu, M. Nature 1959, 184, 901–902. (b) Mastalerz, P. Arch. Immunol. Ter. Dosw. 1959, 7, 201–210
- (a) Kafarski, P.; Lejczak, B. Phosphorus, Sulfur Silicon Relat. Elem.1991, 63, 193–215. (b) Collinsová, M.; Jiráček, J. Curr. Med. Chem. 2000, 7, 629–647. (c) Kafarski, P.; Lejczak, B. Curr. Med. Chem.: Anti-Cancer Agents 2001, 1, 301–312. (d) Berlicki, L.; Kafarski, P. Curr. Org. Chem. 2005, 9, 1829–1850. (e) Wardle, N. J.; Bligh, S. W. A.; Hudson, H. R. Curr. Org. Chem. 2007, 11, 1635–1651. (f) Ecker, S. J.; Erion, M. D. J. Med. Chem. 2008, 51, 2328–2345. (g) Lejczak, B.; Kafarski, P. Top. Heterocycl. Chem. 2009, 20, 31–63. (h) Orsini, F.; Sello, G.; Sisti, M. Curr. Med. Chem. 2010, 17, 264–289. (i) Azema, L.; Baron, R.; Ladame, S. Curr. Enzyme Inhib. 2006, 2, 61–72
- Mucha, A. ; Kafarski, P. ; Berlicki, L. J. Med. Chem. 2011, 54, 5955-5980
- Fleisch, H.; Russel, R. G. G.; Staruman, F. Nature 1966, 212, 901–903.
- Sun, L.; Chiu, D.; Kowal, D.; Simon, R.; Smeyne, M.; Zukin, R. S.; Olney, J.; Baudy, R.; Lin, S. J. Pharmacol. Exp. Ther. 2004, 310, 563–570
- Vassiliou, S. ; Węglarz-Tomczak, E. ; Berlicki, L. ; Pawelczak, M. ; Nocek, B. ; Mulligan, R. ; Joachimiak, A. ; Mucha, A. J. Med. Chem. 2014, 57, 8140-8151
- (a) Doupis, J.; Veves, A. Adv. Ther. 2008, 25, 627–643. (b) Gupta, R.; Walunj, S. S.; Tokala, R. K.; Parsa, K. V.; Singh, S. K.; Pal, M. Curr. Drug Targets 2009, 10, 71–87. (c) Augustyns, K.; Van der Veken, P.; Senten, K.; Haemers, A. Curr. Med. Chem. 2005, 12, 971–998
- Peyman, A. Aminophosphonic and Aminophosphinic Acids. Chemistry and Biological Activity 2000, 559-577
- Forlani, G. ; Berlicki, L. ; Duò, M. ; Dziędziola, G. ; Giberti, S. ; Bertazzini, M. ; Kafarski, P. J. Agric. Food Chem. 2013, 61, 6792-6798
- Rice, P. J.; Rice, P. J.; Arthur, E. L.; Barefoot, A. C. Advances in pesticide environmental fate and exposure assessments. J. Agric. Food Chem. 2007, 55, 5367–5376
- Diagne, M.; Oturan, N.; Oturan, M. A.; Sires, I. Environ. Chem. Lett. 2009, 7 (3), 261-265
- Barrett, K. A.; Mcbride, M. B. Environ. Sci. Technol. 2005, 39, 9223–9228Baylis, A. D. Pest Manage. Sci. 2000, 56, 299–308
- Duke, S. O.; Powles, S. B. Pest Manage. Sci. 2008, 64, 319–325
- (a) Duke, S. D. In Herbicides; Chemistry, Degradation and Mode of Action Kearney, P. C., Kaufman, D. D., Eds.; Marcel Dekker: New York, 1988. (b) Cox, C. J. Pest. Reform 2004, 24, 10–15. (c) Kolpin, D. W.; Thurman, E. M.; Lee, E. A.; Meyer, M. T.; Furlong, E. T.; Glassmeyer, S. T. Sci. Total Environ. 2006, 354, 191–197
- Shifu, C.; Yunzhang, L. Chemosphere 2007, 67, 1010–1017
- Balci, B.; Oturan, M. A.; Oturan, N.; Sires, I. Journal of Agricultural and Food Chemistry 2009, 57(11), 4888-4894
- Battaglin, W. A.; Kolpin, D. W.; Scribner, E. A.; Kuivila, K. M.; Sandstrom, M. W. J. Am. Water Works Assoc. 2005, 323-332
- Grunewald, K.; Schmidt, W.; Unger, C.; Hanschmann, G. J. Plant Nutr. Soil Sci. 2001, 164, 65-70
- Huston, P. L.; Pignatello, J. J. Water Res. 1999, 5, 1238–1246
- Shifu, C.; Yunzhang, L. Chemosphere 2007, 67, 1010–1017
- Chen, Y.; Wu, F.; Lin, Y.; Deng, N.; Bazhin, N.; Glebov, E. J. Hazard. Mater. 2007, 148, 360–365
- (a) Barrett, K. A.; Mcbride, M. B. Environ. Sci. Technol. 2005, 39, 9223–9228. (b) Nowack, B.; Stone, A. T. Environ. Chem. Lett. 2003, 1, 24–31
- Aquino Neto, S.; de Andrade, A. R. Electrochim. Acta 2008, 54, 2039–2045
- Colodrero, R. M. P. ; Olivera-Pastor, P. ; Losilla, E. R. ; Hernández-Alonso, D. ; Aranda, M. A. G. ; Leon-Reina, L. ; Rius, J. ; Demadis, K. D. ; Moreau, B. ; Villemin, D. ; Palomino, M. ; Rey, F. ; Cabeza, A. Inorg. Chem. 2012, 51, 7689-7698
- Kickelbick, G. Hybrid Materials: Strategies, Syntheses, Characterization and Applications Ed. Wiley-VCH: Weinheim, (2007).
- Plabst, M.; McCusker, L. B; Bein, T. J. Am. Chem. Soc. 2009, 131, 18112.
- Dina A. Bakhotmah and Reda Mohammadi Abdel-Rahman, Orient. J. Chem. 2015, 31(1), 01-15
- Bouazza D., Tayeb A., Sassi M. , Bengueddach A., Boos A. Orient. J. Chem. 2013, 29(3), 991-1000
- Colodrero, R. M. P. ; Angeli, G. K. ; Bazaga-Garcia, M. ; Olivera-Pastor, P. ; Villemin, D. ; Losilla, E. R. ; Martos, E. Q. ; Hix, G. B. ; Aranda, M. A. G. ; Demadis, K. D. ; Cabeza, A. Inorg. Chem. 2013, 52, 8770-8783
- Kadous, A.; Didi, M. A.; Villemin, D. J. Radioanal. Nucl. Chem. 2010, 284, 431-438
- Chaikin, Y. ; Kedem, O. ; Raz, J. ; Vaskevich, A. ; Rubinstein, I. Anal. Chem. 2013, 85, 10022-10027
- Schade, C.; Maeder, C.; Witteler, H. Mixtures for coating metal surfaces comprising organic corrosion inhibitors. U.S.Pat. Appl. Publ. 2014, US 20140141269 A1 20140522.
- Amar, H.; Benzakour, J.; Derja, A.; Villemin, D.; Moreau, B. J. Electroanal. Chem. 2003, 558, 131-139
- Clark, H. D. Protecting metal during acid contact. U.S. 1973, US 3778377 A 19731211.
- Brown, D. Methods and solutions for improvement of offset printing U.S. 1977, US 4013008 A 19770322.
- Weber, J. ; Grossmann, G. ; Demadis, K. D. ; Daskalakis, N. ; Brendler, E. ; Mangstl, M. ; Schmedt auf der Günne, J. Inorg. Chem. 2012, 51, 11466-11477.

This work is licensed under a Creative Commons Attribution 4.0 International License.









