Synthesis of ZnO Nanoparticles by Precipitation Method
Hamid Reza Ghorbani*, Fedos Parsa Mehr, Hossein Pazoki, Behrad Mosavar Rahmani
Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran
DOI : http://dx.doi.org/10.13005/ojc/310281
Article Received on :
Article Accepted on :
Article Published : 28 Apr 2015
In this work we develop a simple technique to synthesize ZnO nanoparticles using zinc nitrate and KOH in aqueous solution. The precipitated compound was calcined and characterized by UV – Vis spectroscopy, transmission electron microscopy (TEM), and dynamic light scattering (DLS). The ZnO nanoparticles displayed characteristic Surface Plasmon Resonance peak at around 372 nm. Particle size distribution by dynamic light scattering technique (DLS) showed that the particles are in the range of 30±15 nm.
KEYWORDS:ZnO; Nanoparticles; Precipitation; KOH
Download this article as:| Copy the following to cite this article: Ghorbani H. R, Mehr F. P, Pazoki H, Rahmani B. M. Synthesis of ZnO Nanoparticles by Precipitation Method. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ghorbani H. R, Mehr F. P, Pazoki H, Rahmani B. M. Synthesis of ZnO Nanoparticles by Precipitation Method. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8621 |
Introduction
Research in the field of synthesis methodology of nanomaterials is mainly oriented in controlling their shape, size and composition. Each of these factors is a key factor in determining the properties of materials that lead to different technological applications [1, 2].
Zinc oxide, with its unique physical and chemical properties, such as high chemical stability, high electrochemical coupling coefficient, broad range of radiation absorption and high photostability, is a multifunctional material [3, 4]. ZnO nanoparticles were synthesized by different methods. It is confirmed that the various applications of ZnO nanoparticles depend upon the control of both physical and chemical properties such as size, size dispersity, shape, surface state, crystal structure, organization onto a support, and dispensability [5]. This has led to the development of a great variety of techniques for synthesizing the compound. Hong et al. used a controlled precipitation method. The process of precipitating zinc oxide was carried out using zinc acetate (Zn(CH3COO)2·H2O) and ammonium carbonate (NH4)2CO3 [6]. A simple precipitation process for the synthesis of zinc oxide was carried out by Lanje et al. [7]. The single step process with the large scale production without unwanted impurities is desirable for the cost-effective preparation of ZnO nanparticles [7]. Wang et al. reported another process of controlled precipitation of zinc oxide. Nanometric zinc oxide was obtained by precipitation from aqueous solutions of NH4HCO3 and ZnSO4·7H2O [8]. Benhebal et al. prepared ZnO powder by sol-gel method from zinc acetate dihydrate, oxalic acid, using ethanol as solvent [9]. The technique of obtaining ZnO using microemulsion was also used by Yildirim and Durucan. They attempted to modify the microemulsion method so as to obtain monodisperse zinc oxide [10]. In 2014, Kang et al. reported the continuous synthesis of zinc oxide nanoparticles in a microfluidic system for photovoltaic application. Their work was carried out to investigate the synthesis and characterization of ZnO nanoparticles using numerical simulations and experimental methods [11].
This paper presents the synthesis of ZnO nanoparticles by simple method. In this work, we employed zinc nitrate as an initial reagent and KOH as a precipitating agent.
Materials and Methods
Materials
Zinc nitrate as the precursor, KOH as a precipitating agent to synthesize ZnO nanoparticles were purchased from Sigma-Aldrich.
Preparation
ZnO nanoparticles were synthesized by direct precipitation method using zinc nitrate and KOH as precursors. In this work, the aqueous solution (0.2 M) of zinc nitrate (Zn(NO3)2.6H2O) and the solution (0.4 M) of KOH were prepared with deionized water, respectively. The KOH solution was slowly added into zinc nitrate solution at room temperature under vigorous stirring, which resulted in the formation of a white suspension. The white product was centrifuged at 5000 rpm for 20 min and washed three times with distilled water, and washed with absolute alcohol at last. The obtained product was calcined at 500 °C in air atmosphere for 3 hr.
Characterization of Gold Nanoparticles
Uv-vis spectroscopy was used to prove the existence of nanoparticles. The morphology and size was determined by the transmission electron microscopy (TEM), and Dynamic light scattering (DLS) analysis.
Results
For analytical study of the prepared sample, the amount of absorption within wave length of 300–550 nm was observed by uv-vis spectroscopy. It is known that an absorption band at about 370 nm due to surface plasmon resonance in ZnO nanoparticles. Fig. 1 shows the UV-Vis spectra of ZnO nanoparticles recorded between 300 and 550 nm. As illustrated the SPR band cantered 372 nm confirms the formation of ZnO nanoparticles in the solution.
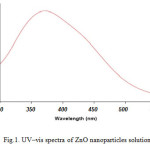 |
Figure1: UV–vis spectra of ZnO nanoparticles solution Click here to View figure |
Dynamic light scattering is a widely used technique for the determination of particle size in colloidal solution. As is seen in figure 2, average size of nanoparticle synthesized is 30 nm. The distribution of ZnO nanoparticles is about 20 nm which indicates moderate distribution of the nanoparticles.
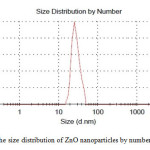 |
Figure2: The size distribution of ZnO nanoparticles by number Click here to View figure |
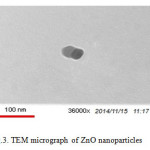 |
Figure3: TEM micrograph of ZnO nanoparticles Click here to View figure |
Also the ZnO nanoparticles synthesized were studied by transmission electron microscopy (TEM) and images show and confirm ZnO nanoparticles production at nano-size. TEM images of the produced nanoparticles are shown in figure 3.
Conclusions
In this study, the ZnO nanoparticles were successfully synthesized by direct precipitation method using zinc nitrate as zinc source and KOH as precipitating agent in aqueous solution. The size range of the generated ZnO powder was approximately 20–40 nm. In summary we have successfully designed a facile and fast synthesis route to produce ZnO nanoparticles and finally ZnO nanoparticles were characterized by UV-visible, TEM and DLS analysis.
References
- Chandross, E.A.; Miller, R.D. Chem. Rev., 1999, 99, 1641-1642.
- Djalali, R.; Samson, J.; Matsui, H. J. Am. Chem. Soc., 2004, 126, 7935-7939.
- Segets, D.; Gradl, J.; Taylor, R.K.; Vassilev, V.; Peukert, W. ACS Nano, 2009, 3, 1703–1710.
- Guo, R.; Lou, X. J. Sens. Trans. Technol., 1991, 3, 1‒5.
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Shin, H.S. Appl. Surf. Sci. 2007, 253, 7622‒7626.
- Hong, R.; Pan, T.; Qian, J.; Li, H. Chem. Eng. J. 2006, 119, 71–81.
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.S.; Pode, R.B. Adv. Powder Technol. 2013, 24, 331‒335.
- Wang, Y.; Zhang, C.; Bi, S.; Luo, G. Powder Technol. 2010, 202, 130‒136.
- Benhebal, H.; Chaib, M.; Salomon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Alex. Eng. J. 2013, 52, 517‒523.
- Yildirim, Ö.A.; Durucan, C. J. Alloy. Compd. 2010, 506, 944‒949.
- Kang, H.K.; Leem, J.; Yoona, S.Y.; Sung, H.J. Nanoscale., 2014, 6, 2840-2846.

This work is licensed under a Creative Commons Attribution 4.0 International License.









