Synthesis of Star-Shaped ε-Caprolactone Oligomers for use as Plasticizers of Poly(L-Lactide) Bioplastic films
Thanonchat Imsombut, Mangkorn Srisa-Ard and Yodthong Baimark
Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahasarakham University, Mahasarakham 44150, Thailand.
Corresponding Author. E-mail: yodthong.b@msu.ac.th
DOI : http://dx.doi.org/10.13005/ojc/330213
Article Received on : November 28, 2016
Article Accepted on : February 24, 2017
Liquid star-shaped e-caprolactone (CL) oligomers were synthesized by ring-opening reaction of CL using a six terminal hydroxyl group initiator, Boltorn® H2004, for use as new plasticizers of poly(L-lactide) (PLA) films. The Boltorn-CL oligomers containing two and four units of CL on each oligomer arm were prepared. The PLA/oligomer blend films were prepared by solution blending before film casting. A phase separation led to the formation of plasticizer droplets. The CL arms reduced the phase separation. The plasticizer blending decreased slightly the Tg, Tc and Tm of the PLA films. The crystallinities of the PLA films increased with the Boltorn-CL blend ratio but they did not with the Boltorn® H2004. The Boltorn-CL blending improved flexibility of the PLA films, but Boltorn® H2004 blending did not. The increasing of CL units decreased the flexibility of the PLA films. The Boltorn-CL oligomers could be used as plasticizers for PLA films.
KEYWORDS:Poly(L-lactide); Boltorn-CL; oligomer; plasticizer; mechanical properties
Download this article as:| Copy the following to cite this article: Imsombut T, Srisa-Ard M, Baimark Y. Synthesis of Star-Shaped ε-Caprolactone Oligomers for use as Plasticizers of Poly(L-Lactide) Bioplastic films. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Imsombut T, Srisa-Ard M, Baimark Y. Synthesis of Star-Shaped ε-Caprolactone Oligomers for use as Plasticizers of Poly(L-Lactide) Bioplastic films. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32426 |
Introduction
Poly(L-lactide) (PLA), one of the most important bioplastics, has attracted increasing attention as a candidate for use in many application fields, such as tissue scaffold, drug delivery systems, packaging films and so on, because of its renewability, biodegradability, biocompatibility, good processability and good mechanical properties.1-4 However, the low elongation at break and the high modulus of the PLA films have limited applications in packaging situations.
The flexibility of PLA films can be improved either by copolymerization5,6 or by plasticizer blending.7 The plasticizer blending is more convenient, more efficient, lower cost and faster compared to copolymerization. Low molecular weight plasticizers, such as citrate esters, significantly reduce the Tg and obviously improve the elongation at break of the PLA films.8,9 However, the migration of these plasticizers from the PLA film matrix to the film surface due to their high mobility increases the Tg and reduces the film drawability with aging, which is the main problem.6,10 High molecular weight plasticizers, such as poly(ethylene glycol) (PEG) and poly(propylene glycol) (PPG), were then investigated for plasticizing of PLA films to decrease the migration of plasticizers on aging.11-14 However, the high molecular weight plasticizers usually induce phase separation in the PLA film matrices. It has been reported that tiny pools of liquid plasticizers, such as PPGs, are dispersed in the PLA film matrices and may locally plasticize PLA during plastic flow and have better effects on drawability than the crystallizable plasticizers such as PEGs.13,14 The PPGs have also been shown to give efficient plasticization to improve the flexibility of the PLLA.
Semi-crystalline poly(e-caprolactone) (PCL) is a flexible biodegradable polyester due to its very low Tg (around -60°C). The five methylene units of the CL units induce high chain mobility and low Tg. PCL and CL oligomers have been investigated as biodegradable plasticizers.15-17 However, to the best of our knowledge, the plasticization effect of star-shaped CL oligomers on PLA films has not been reported so far.
Thus, this paper describes the synthesis of liquid CL oligomers with 6-arm star-shaped structures for plasticizing PLA film. Effects of the CL chain length (two and four units) on each arm and plasticizer blend ratios (5 – 20 wt%) on the phase separation, thermal properties and mechanical properties of the PLA blend films were evaluated. The PLA films blended with the 6-arm initiator, BoltornÒ H2004, were also prepared for comparison.
Experimental Section
Materials
The poly(L-lactic acid) (PLA) was synthesized in our research unit at Mahasarakham University by ring-opening polymerization of a L-lactide monomer in bulk at 165°C for 2.5 h under a nitrogen atmosphere using 0.01 mol% stannous octoate (95%, Sigma) and 0.14 mol% 1-dodecanol (98%, Fluka) as the initiating system. The obtained PLA was granulated before drying in a vacuum at 110°C for 2 h to remove any un-reacted lactide. The intrinsic viscosity ([η]) and viscosity-average molecular weight (Mv) of the PLA were determined in chloroform at 25°C, and they were 2.53 dL/g and 104,700 g/mol, respectively. The e-caprolactone (CL, 99%, Acros Organics) monomer was purified by distillation under reduced pressure before use. A liquid fatty acid modified dendritic polyol with six terminal hydroxyl groups, trade name Boltorn® H2004, with a molecular weight of 3,100 g/mol (Perstrop) was used without further purification. All reagents used were analytical grade.
Synthesis of CL Oligomers
The CL oligomers were synthesized by ring-opening polymerization of the CL monomer in bulk at 145°C for 24 h under a nitrogen atmosphere using 0.04 mol% stannous octoate and Boltorn® H2004 as the initiating system, as shown in Scheme 1. The resulting oligomers were purified by heating at 110°C under a vacuum for 6 h to remove un-reacted CL monomer. The CL monomers with two and four units were reacted at the hydroxyl end-groups of the Boltorn® H2004, giving Boltorn-2CL and Boltorn-4CL, respectively.
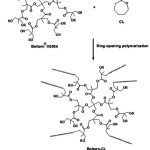 |
Scheme 1: Reaction of Boltorn-CL formation. |
Characterization of CL Oligomers
The molecular weight characteristics, including number-average molecular weight (Mn) and molecular weight distribution (MWD), of the CL oligomers were determined by Gel Permeation Chromatography (GPC) using a Waters e2695 separation module equipped with PLgel 10 mm mixed B 2 columns operating at 40°C at a flow rate of 1.0 mL/min and employing a refractive index detector. Tetrahydrofuran was used as the solvent.
The chemical structures of the CL oligomers were investigated by 1H-NMR spectrometry using a Varian Mercury Plus 400 MHz 1H-NMR spectrometer at 25°C with CDCl3 as the solvent. Tetrametylsilane was used as the internal reference.
The thermal transition properties of the CL oligomers were determined by Differential Scanning Calorimetry (DSC) under a nitrogen flow using a Perkin-Elmer Pyris Diamond DSC to detect the glass transition temperature (Tg) and melting temperature (Tm). For DSC, samples (3 – 5 mg) were heated at 10oC/min over a temperature range of 0 to 200°C for the 1st heating scan. Then, the samples were quenched to 0°C according to the DSC instrument’s own default cooling mode before heating from 0 to 200°C for the 2nd heating scan. The Tg was taken as the midpoint of the heat capacity increment associated with the glass-to-rubber transition. The Tm was measured as the peak value of the endothermal phenomena in the DSC curve.
The thermal stability (or thermal decomposition) of the CL oligomers was determined by thermogravimetric analysis (TGA) in a non-isothermal mode using a TA-Instrument SDT Q600 TGA. For the TGA analysis, samples of 5 – 10 mg were heated at 20°C/min under a nitrogen atmosphere over the temperature range of 50 to 800°C. The TG thermogram was obtained as a weight loss profile. The temperature of the maximum decomposition rate (Td, max) was derived from a derivative TG (DTG) thermogram.
Preparation of PLA/Oligomer Blend films
The PLA/oligomer blend films were prepared by solution blending before film casting. Chloroform was used as a blending solvent. The blend solution (0.4 g/20 ml) was poured on to a glass petri dish and evaporated at 40°C for 24 h before drying in a vacuum at 70°C for 24 h. The PLA blend films with PLA/oligomer blend ratios of 95/5, 90/10 and 80/20 %wt were investigated. The neat PLA and PLA/Boltorn® H2004 blend films were also prepared by the same method for comparison. The film thicknesses were approximate 50 mm.
Characterization of PLA/Oligomer Blend films
The morphology of the blend films was determined by scanning electron microscopy (SEM) using a JEOL JSM-6460LV SEM. The film samples were coated with gold to enhance conductivity before scanning.
The thermal transition properties of the blend films were investigated by the DSC method as described above to observe the Tg, Tm, crystallizing temperature (Tc), heat of crystallization (ΔHc) and heat of melting (ΔHm). The Tc was measured as the peak value of the exothermal phenomena in the DSC curve. The ΔHc and ΔHm were calculated from the total areas of the Tc and Tm peaks, respectively. The degree of crystallinity (Xc) of the PLA phase was calculated from equation (1).
Xc (%) = [(ΔHm – ΔHc)/(wPLA Χ ΔHm,100%)] Χ 100% (1)
Where wPLA is the weight fraction of PLA in the blend films. ΔHm and ΔHc are the heat of melting and heat of crystallization, respectively, that were obtained from the DSC method. The heat of melting for 100% crystallinity (ΔHm,100%) of PLA is 93.7 J/g.18-20The mechanical properties, including stress at break, elongation at break and initial Young’s modulus, of the blend films were determined at 25°C and 65% relative humidity with a Lloyds LRX+ Universal Mechanical Testing Machine. The film samples (80 Χ 10 mm) were tested with a gauge length of 25 mm and a crosshead speed of 10 mm/min. The mechanical properties were averaged from five measurements for each sample.
Results and Discussion
Characterization of CL Oligomers
The yields of the CL oligomers measured by the evaporation of un-reacted CL were higher than 90%. Both the Boltorn-2CL and the Boltorn-4CL were liquid at room temperature, similar to the initiator, Boltorn® H2004. The Mn and MWD of the Boltorn® H2004 obtained from GPC were 2,600 g/mol and 1.5, respectively. The GPC curves of both the Boltorn-2CL and the Boltorn-4CL were of the unimodal type with the MWDs being 1.6 and 1.8, respectively. The Mns of the CL oligomers from the GPC method were higher than the Boltorn® H2004 and increased with the CL units. The Mns of the Boltorn-2CL and the Boltorn-4CL were 3,000 and 3,500 g/mol, respectively. The GPC results indicated that the CL monomers were connected to the Boltorn® H2004 molecules. CL oligomers with different CL chain lengths can be prepared.
The chemical structures of the CL oligomers were determined from 1H-NMR. Figure 1 shows the 1H-NMR spectra of the CL oligomers including the peak assignments. The peaks of a – e were assigned to the methylene protons (-CH2-) of the CL units.21 The peaks of f and g were assigned to the methylene (-CH2-) and methyl (-CH3) protons at the outer structure of the Boltorn units, respectively.22 Meanwhile the peaks of f’ and g’ were assigned to the methylene (-CH2-) and methyl (-CH3) protons at the inner structure of the Boltorn units, respectively.22 The 1H-NMR results confirm that the Boltorn-CL oligomers consisted of both the Boltorn and the CL characters. It should be noted that the peak area ratio of the CL/Boltorn units such as area ratio of peak a/peak f + f’, increased as the CL units increased from two to four units in each arm. The 1H-NMR results support that the CL units increased with the initial CL feed ratio.
 |
Figure 1: 1H-NMR spectra of (above) Boltorn-2CL and (below) Boltorn-4CL in CDCl3 (peak assigments as shown). |
From the DSC analysis (DSC thermograms not shown), both the 1st and the 2nd heating scans did not exhibit the Tm of the CL crystalline. The CL sequences with two and four units of the Boltorn-2CL and the Boltorn-4CL, respectively, could not be crystallized. They are liquid at room temperature.
Figure 2 shows the thermogravimertic (TG) and derivative TG (DTG) thermograms of the Boltorn® H2004 and the CL oligomers from the TGA analysis. From the TG thermograms, the initial decomposition temperatures (T0s) of the Boltorn® H2004 and the CL oligomers were approximate 300°C and 250°C, respectively. This may be due to the CL chains reducing the intermolecular forces of the Boltorn® H2004 molecules. From the DTG thermograms, the hyperbranched Boltorn® H2004 showed two Td, max values at 371°C and 415°C that may be attributed to the decomposition of its outer and inner structures. The thermal stability changes of the CL oligomers can be clearly observed from the DTG thermograms. Both the Td, max values of the Boltorn cores slightly decreased as the CL units were added and increased. Then the thermal stability of the CL oligomers was a little lower than the Boltorn® H2004.
 |
Figure 2: TG (above) and DTG (below) thermograms of (a) Boltorn® H2004, (b) Boltorn-2CL and (c) Boltorn-4CL. |
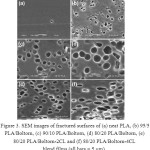 |
Figure 3: SEM images of fractured surfaces of (a) neat PLA, (b) 95/5 PLA/Boltorn, (c) 90/10 PLA/Boltorn, (d) 80/20 PLA/Boltorn, (e) 80/20 PLA/Boltorn-2CL and (f) 80/20 PLA/Boltorn-4CL blend films (all bars = 5 mm). |
Characterization of PLA Blend films
The film morphology was determined from SEM images. The film surfaces were smooth for all the PLA blend films (SEM images not shown). Figures 4(a) – 4(d) illustrate the fractured surfaces of the neat PLA and the PLA/Boltorn® H2004 blend films. The fractured surface of the neat PLA film was continuous. Meanwhile, the PLA/Boltorn® H2004 blend films revealed emptied voids on the fractured surfaces where the liquid Boltorn® H2004 may have been accumulated during film drying similar to the PLA/PPG blend films.13,14 This suggests that phase separation between the continuous PLA and the dispersed Boltorn® H2004 phases had occurred. This may be due to the hydrophilicity of the six hydroxyl end-groups of the Boltorn® H2004 that induced the phase separation. The void sizes increased significantly as the Boltorn® H2004 ratio increased [see Figures 4(b) – 4(d)]. Similar features were found on the fractured surfaces of the PLA/Boltorn-2CL and the PLA/Boltorn-4CL blend films. Emptied voids dispersed throughout the PLA/Boltorn-CL film matrices were also detected.
However, the void sizes of the PLA/Boltorn-CL blend films were smaller than those in the PLA/Boltorn® H2004 blend films for the same blend ratio, and examples of these are shown in Figures 4(e) and 4(f) for the 20 wt% Boltorn-2CL and the 20 wt% Boltorn-4CL, respectively. This suggests that the hydrophobic CL chains of the oligomers enhanced the phase compatibility by decreasing the hydrophilicity of the Boltorn® H2004 cores.
The thermal transition properties of the PLA blend films were investigated from the 2nd heating scan DSC thermograms, an example of which is shown in Figure 4 for the PLA/ Boltorn® H2004 blend films. The Tg, Tc and Tm of the PLA phase were detected. The DSC results are summarized in Table 1, including the Xc values. The Tg, Tc and Tm of the PLA blend films were a little lower than for the neat PLA film. This indicates that the Boltorn® H2004 and the Boltorn-CL acted as plasticizers to decrease the Tg, Tc and Tm of the PLA by enhancing the segmental mobility of the PLA in an amorphous phase. The Tg slightly decreased as the plasticizer ratio increased.
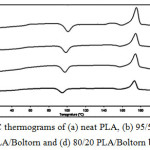 |
Figure 4: DSC thermograms of (a) neat PLA, (b) 95/5 PLA/Boltorn, (c) 90/10 PLA/Boltorn and (d) 80/20 PLA/Boltorn blend films. |
The Xc of the PLA are also summarized in Table 1, and they decreased slightly when the PLA was blended with the Boltorn® H2004 and the blend ratio was increased. The Xc of the PLA also decreased when the Boltorn-2CL and the Boltorn-4CL were blended for the 5 wt% blend ratio. However, the Xc of the PLA increased as the Boltorn-CL blend ratio increased from 5 wt% to 10 and 20 wt%. This demonstrates that the Boltorn® H2004 inhibited crystallization of the PLA, although the Boltorn® H2004 and the Boltorn-CL had practically the same effect on the Tg. This result is similar to the plasticizing and nucleating effects on PLA films of poly(propylene glycol) (PPG) and poly(ethylene glycol) (PEG).13,14 The Tg of the PLA films was depressed by blending with both the PPG and the PEG. However, the PPG affected the Xc of the PLA less. In addition, the Tg, Tc and Tm of the PLA/Boltorn-2CL and PLA/Boltorn-4CL blend films were similar for the same blend ratio. The Tg, Tc and Tm slightly decreased as the Boltorn-CL ratio increased. The results suggested that the number of CL units did not affect on the thermal transition properties of the PLA in significant.
Table 1: Thermal transition properties of neat PLA and PLA blend films from the 2nd heating scan DSC thermograms.
|
PLA/plasticizer ratio (w/w) |
Tg (°C) |
Tc (°C) |
Tm (°C) |
Xc (%) |
|
Neat PLA film PLA/Boltorn blend films 95/5 90/10 80/20 PLA/Boltorn-2CL blend films 95/5 90/10 80/20 PLA/Boltorn-4CL blend films 95/5 90/10 80/20 |
55 51 50 47 51 50 49 53 49 48 |
100 98 97 94 99 98 95 99 97 94 |
175 174 174 173 174 173 173 173 173 172 |
28.6 27.6 26.6 21.2 19.3 23.5 42.9 17.6 23.9 49.8 |
The thermal stabilities of the PLA blend films were determined from the TG thermograms, as shown in Figure 5. It can be seen that the thermal stabilities of all the plasticizers was better than those of the neat PLA and the PLA blend films. The neat PLA and the 95/5 wt% PLA/plasticizer blend films showed similar single decomposition profiles in the temperature range of 300 – 400°C, with the initial decomposition temperature about 300°C. The weight losses of the 10 wt% and the 20 wt% plasticizer blend ratios were faster than the neat PLA film. The weight loss changes of the PLA films blended with Boltorn, Boltorn-2CL and Boltorn-4CL, were similar for the same blend ratios.
 |
Figure 5: TG thermograms of (a) neat PLA and PLA blend films prepared with PLA/plasticizer blend ratios of (b) 95/5, (c) 90/10 and (d) 80/20 (w/w) and (e) plasticizer. |
Figure 6 shows the DTG thermograms of the PLA blend films compared with their plasticizers. The PLA blend films exhibited single Td, max peaks. Table 2 reports the Td, max of the PLA blend films. The Td, max of the 95/5 wt% PLA/plasticizer blend films were higher than the neat PLA films for all the plasticizers. However, increasing the plasticizer ratios from 5% to 10% and 20% significantly depressed the Td, max.
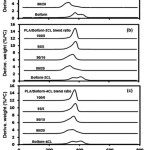 |
Figure 6: DTG thermograms of (a) PLA/Boltorn, (b) PLA/Boltorn-2CL and (c) PLA/Boltorn-4CL blend films preprared with different blend ratios.
|
Table 2: Td, max values of neat PLA and PLA blend films from DTG thermograms.
|
PLA/plasticizer blend ratio (w/w) |
Td, max (°C) |
|
Neat PLA blend film PLA/Boltorn blend films 95/5 90/10 80/20 PLA/Boltorn-2CL blend films 95/5 90/10 80/20 PLA/Boltorn-4CL blend films 95/5 90/10 80/20 |
360 366 352 334 365 358 336 367 360 358 |
The mechanical properties, including stress at break, elongation at break and initial Young’s modulus, of the film samples were determined by tensile testing. Figure 7 shows the tensile properties of the films as a function of the plasticizer type and the blend ratio. It can be seen that the elongation at break of the PLA films was not changed by the Boltorn® H2004 blending. While the Boltorn-2CL and the Boltorn-4CL blending can improve the elongation at break of the PLA films. In addition, the PLA/Boltorn-2CL and the PLA/Boltorn-4CL blend films exhibited the yield or plasticizing effects for all the blend ratios, but the PLA/ Boltorn® H2004 blend films did not. The results of the mechanical properties are summarized in Table 3. It was found that the stress at break and the initial Young’s modulus decreased and the elongation at break increased as the Boltorn-2CL and the Boltorn-4CL were blended and the blend ratio increased, except for the Boltorn® H2004 blending. The results of the mechanical properties indicate that the Boltorn-2CL and the Boltorn-4CL improved the flexibility of the PLA films.
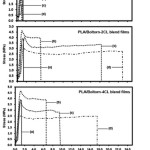 |
Figure 7: Tensile curves of (a) neat PLA and PLA blend films prepared with PLA/plasticizer ratios of (b) 95/5, (c) 90/10 and (d) 80/20 wt%. |
Table 3. Mechanical properties of PLA blend films.
|
PLA/plasticizer blend ratio (w/w) |
Stress at break (MPa) |
Young’s modulus (MPa) |
Elongation at break (%) |
|
Neat PLA film PLA/Boltorn blend films 95/5 90/10 80/20 PLA/Boltorn-2CL blend films 95/5 90/10 80/20 PLA/Boltorn-4CL blend films 95/5 90/10 80/20 |
3.8 ± 0.2 2.6 ± 0.2 2.2 ± 0.4 1.6 ± 0.4 3.9 ± 0.4 3.3 ± 0.2 2.8 ± 0.4 3.8 ± 0.4 2.8 ± 0.5 2.4 ± 0.2 |
213.0 ± 17.0 158.6 ± 20.9 147.6 ± 17.0 87.6 ± 8.8 193.5 ± 24.6 167.2 ± 27.9 155.1 ± 38.6 204.5 ± 10.7 182.8 ± 27.8 148.8 ± 13.1 |
5.4 ± 0.8 4.4 ± 0.7 4.2 ± 0.2 4.8 ± 0.5 28.5 ± 2.1 73.3 ± 7.8 91.8 ± 8.7 26.6 ± 4.2 38.4 ± 5.9 69.9 ± 10.8 |
Kulinski et al. reported the plasticization of PLA films by tiny liquid pools of PPG during plastic flow, and this had positive effects on the film drawability.13 Thus, in this work, the tiny liquid pools of the Boltorn-CL enhanced the film drawability. The phase separation occurred on the PLA/Boltorn-CL blend films and induced a further slight decrease of the Tg but largely enhanced the drawability of the PLA films, while the PLA/ Boltorn® H2004 blend films did not have this for all the blend ratios. This may be explained by the hydrophilicity between the continuous PLA and the dispersed Boltorn® H2004 phases being very different.
Conclusions
Two liquid Boltorn-CL oligomeric plasticizers were prepared by a ring-opening reaction of the CL monomer using the liquid star-shaped BoltornÒ H2004 containing six hydroxyl end-groups as the initiator. The different CL units of oligomers (two and four units on each arm for the Boltorn-2CL and the Boltorn-4CL, respectively) were supported by 1H-NMR and TGA analyses.
The phase separation between the PLA and plasticizer phases can be clearly observed as the formation of emptied voids in the SEM images of their fractured surfaces. These emptied voids were the tiny pools of liquid plasticizer. The phase separation increased (sizes of tiny pools increased) with the plasticizer blend ratio. However, both the PLA/Boltorn-CL blend films showed less phase separation than the PLA/Boltorn® H2004 blend film. The Boltorn® H2004 and the Boltorn-CL blending slightly depressed the Tg of the PLA. The tiny pools of the liquid Boltorn-CL oligomers obviously enhanced the plastic deformation to improve the drawability of the PLA films, but the liquid Boltorn® H2004 did not. In conclusion, the flexibility of the PLA blend films can be tailored by controlling the Boltorn-CL blend ratio and the CL chain length for potential use as flexible bioplastic films in packaging applications.
Acknowledgements
This work was supported by the Division of Research Facilitation and Dissemination, Mahasarakham University (2015). The Center of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand is also acknowledged. The authors are very grateful to Dr. Jolyon Dodgson, Department of Biology, Faculty of Science, Mahasarakham University for his improvement of the English language in this manuscript.
References
- Garlotta D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63-84.
CrossRef - Lim, L. T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820-852.
CrossRef - Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2003, 4, 835-864.
CrossRef - Zhang, Z.; Chen, L. -B.; Gao, J.; Bao, F.; Yin, J.; Chen, B.; Wang, H.; Chen, Y.; Shang, L. Preparation of poly(sebacic anhydride) and polylactic acid pills used as drug carriers for levofloxacin controlled release. J. Polym. Eng. 2013, 33, 659-664.
CrossRef - Hassouna, F.; Raquez, J. -M.; Addiego, F.; Toniazzo, V.; Dubois, P.; Ruch, D. New development on plasticized poly(lactide): chemical grafting of citrate on PLA by reactive extrusion. Euro. Polym. J. 2012, 48, 404-415.
CrossRef - Choi, K.; Choi, M. -C.; Han, D. -H.; Park, T. -S.; Ha, C. S. Plasticization of poly(lactic acid) (PLA) through chemical grafting of poly(ethylene glycol) (PEG) via in situ reactive blending. Euro. Polym. J. 2013, 49, 2356-2364.
CrossRef - Liu, H,; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B: Polym. Phys. 2011, 49, 1051-1083.
CrossRef - Ljungberg, N.; Andersson, T.; Wesslen, B. Film extrusion and film weldability of poly(lactic acid) plasticized with triacetine and tributyl citrate. J. Appl. Polym. Sci. 2003, 88, 3239-3247.
CrossRef - Maiza, M., Benaniba, M. T.; Massardier-Nageotte, V. Plasticizing effects of citrate esters on properties of poly(lactic acid). J. Polym. Eng. 2015, 36, 371-380.
- Ljungberg N.; Wesslen, B. Tributyl citrate oligomers as plasticizers for poly(lactic acid): thermo-mechanical film properties and aging. Polymer 2003, 44, 7679-7688.
CrossRef - Hu, Y.; Hu, Y. S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol). Polymer 2003, 44, 5681-5689.
CrossRef - Hu, Y.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 1. Poly(lactide) with low stereoregularity. Polymer 2003, 44, 5701-5710.
CrossRef - Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(L-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128-2135.
CrossRef - Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(L-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178-7188.
CrossRef - Middle, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335-2346.
CrossRef - Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762-798.
CrossRef - Shi, G.; Cooper, D. G.; Maric, M. Poly(e-caprolactone)-based ‘green’ plasticizers for poly(vinyl chloride). Polym. Degrad. Stab. 2011, 96, 1639-1647.
CrossRef - Baimark, Y.; Srihanam, P. Influence of chain extension on thermal properties and melt flow index of stereocomplex PLA. Polym. Test. 2015, 45, 52-57.
CrossRef - Baimark, Y.; Cheerarot, O. Effect of chain extension on thermal stability behaviors of polylactide bioplastics. Orient. J. Chem. 2015, 31, 635-641.
CrossRef - Pasee, S., Cheerarot, O., Baimark, Y. Preparation of stereocomplex polylactide bioplastics from stae-shaped/linear polylactide blending. Orient. J. Chem. 2015, 31, 1551-1558.
CrossRef - Niamsa, N.; Baimark, Y. Synthesis and characterization of poly(L-lactide-co-e-caprolactone)-b-poly(L-lactide) biodegradable diblock copolyesters: effect of block lengths on their thermal properties. J. Appl. Polym. Sci. 2007, 106, 3315-3320.
CrossRef - Zhang, W.; Zheng, S. Synthesis and characterization of dendritic star poly(L-lactide)s. Polym. Bull. 2007, 58, 767-775.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









