Wool Functionalization by using Green Synthesized Silver Nanoparticles
Sakil Mahmud1, 2, Md. Nahid Pervez1, 3, Mst. Zakia Sultana1, 4, Md. Ahsan Habib1, 5 and Hui-Hong Liu1
1School of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan, P. R. China- 430200.
2Ningbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, Ningbo, P.R. China-315201.
3Research Institute of Flexible Materials, School of Textiles and Design, Heriot-Watt University, TD1 3HF, UK.
4NUS Graduate School for Integrative Science and Engineering, National University of Singapore, 28 Medical Drive, Singapore, Singapore-117456
5Institutes of Chemistry, Chinese Academy of Sciences, Beijing P. R. China- 100190.
Corresponding Author E-mail: huihongliu@126.com
DOI : http://dx.doi.org/10.13005/ojc/330507
In this study, for the first time wool fabric was functionalized through green based synthesized silver nanoparticles (AgNPs) employing sodium alginate as reducing agent. The diffusion of Ag-NPs into wool surface as well as wool polymer system seems to be dependent on both physical and chemical interactions. The resulting products were characterized with scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR) spectra, X-ray diffraction (XRD), Thermogravimetric analysis (TGA), and UV–vis absorbance. The results specified that Ag-NPs were successfully assembled on the wool surface when the liquor pH and temperature of the application medium was adjusted to 4 and 45oC, respectively for 2hr. This experiment also indicates that the wool fabric with Ag-NPs shows obvious multifunctional action because of the presence of the Ag-NPs. The treated wool fabrics exhibit optimistic colors due to the localized surface plasmon resonance (SPR) of Ag-NPs. The use of this technique to treat wool fabric may lead to new coloration technique and other functional improvement.
KEYWORDS:Green Synthesis; AgNPs; Sodium Alginate; Wool; Multifunctional
Download this article as:| Copy the following to cite this article: Mahmud S, Pervez M. N, Sultana M. Z, Habib M. A, Liu H. H. Wool Functionalization by using Green Synthesized Silver Nanoparticles. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Mahmud S, Pervez M. N, Sultana M. Z, Habib M. A, Liu H. H. Wool Functionalization by using Green Synthesized Silver Nanoparticles. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=39043 |
Introduction
With the advent of science and technology, a new era has emerged in the realm of textile processing by negotiating of metal nanoparticles due to nanoscale materials and structures, typically ranging from 1 to 100 nanometres (nm) [1]. A significant challenge in the textile industry is that conventional approaches to functionalize fabrics don’t prompt perpetual impacts [2]. For example, laundering decreases imparted functional effects. Hence, nanoparticles due to their diverse functions have been applied to impart flame retardant, UV-blocking, water repellent, self-cleanining, and antimicrobial properties to the textile fibres [3, 4]. The advantage of nanomaterials concerns developing function without altering the solace properties of the substrate [5]. Among several inorganic nanomaterials, silver is one of the most important and most studied due to their exceptional antibacterial, antifungal activity, less toxicity and low cost approach [6]. Researchers have devoted unique interest to diverse guidance techniques consisting of gamma irradiation, photo-catalytic reduction, chemical reduction, microwave processing, photo-chemical method, metallic wire explosion, sonochemical, polyols, electron irradiation, and biological methods for the synthesis of silver nanoparticles [7]. As of late expanding request must be joined by “green” synthesis methods [8, 9]. In the global efforts to reduce generated hazardous waste, “green” chemistry and chemical processes are steadily integrating with current developments in science and industry. Implementation of these sustainable processes should adopt the fundamental principles of green chemistry. These standards are outfitted to direct in limiting the utilization of unsafe products and maximizing the efficiency of chemical processes. Henceforth, any engineered course or substance process should address these standards by utilizing ecologically favourable solvents and nontoxic chemicals [10]. The green synthesis of AgNPs involves three main steps, which must be assessed in view of green chemistry perspectives, including (1) selection of solvent medium, (2) selection of environmentally benign reducing agent, and (3) selection of nontoxic substances for the Ag NPs stability [11]. Based on this approach, we have produced the green-chemistry type Ag NP synthesis processes in this research. Natural fibers are a decent media for developing diverse microorganism due to giving a good energy source of nutrients. The natural protein fiber wool is a superior natural textile material due to its resilience and comfort attributes. It is additionally an accommodating host for the era and engendering of microorganisms, bringing about fiber harms and even skin disturbances, especially for products such as garments and carpets. Because wool is mainly composed of keratin, the outermost part of the fiber is the cuticle cell, of which the surface is a fatty layer of 18-methyl Eicosanoic acid covalently bound to the protein layer of the wool cuticle via a thioester linkage. Due to the presence of this fatty layer, the surface of wool is hydrophobic [12]. The presence of this unique characteristic makes them ideal for the selective binding of metal ions. Therefore, the wool fiber with polyfunctional ligands can be a suitable template to grow metal nanoparticles. There are several reports in which the wool fabrics have been loaded with metal nanoparticles to impart antibacterial properties [13-16].
In the present research, we report for the first time that green based AgNPs have been used to functionalize wool fibers employing sodium alginate (NaAlg) as a reducing agent. Synthesized AgNPs were applied to wool fabric by exhaustion which involves in three different steps i.e., initially wool sample was scoured well then impregnated with freshly prepared Ag-NPs colloids under optimized condition and finally rinsed out to remove unfix nanoparticles.
Materials and Methods
Materials
Wool fabrics were obtained from Jiangsu Shenzhou Woolen Co., Ltd, China. Materials used to synthesize silver nanoparticles were AgNO3 (Shanghai Zhanyun Chemical Co., Ltd), Sodium Alginate (Qingdao Yingfei Chemical Co., Ltd), Sodium Hydroxide (Sinopharm Chemical Co., Ltd). Nonionic commercial detergents were used to wash the silk sample. These materials were used without further purification. Deionized water was used throughout the experiment.
Synthesis of Silver Nanoparticles
The green synthesis of silver nanoparticles was accomplished using an indigenous protocol made in our laboratory. In a brief, 1mM of AgNO3 was prepared by silver nitrate salt. 1% w/v of sodium alginate was prepared with deionized water. NaOH solution was prepared by dissolving NaOH (0.399 g) in deionized water (100 ml). Typically, 1ml of sodium alginate (1% w/v) solution was dropped in 4ml of silver nitrate (0.001M) solution with addition of NaOH solution (approximately 1ml of 0.01M) to maintain pH 11 and to accelerate the reaction. These mixtures were stirred for 1 minute. Then the mixture is keep inside the heat bath (60oC) for 40 min. The transparent colorless solution was converted to pale yellow and then into brownish-red color indicated the formation of Ag-NPs. At this end the prepared solution was evaluated by using UV–visible spectra to affirm the production of silver nanoparticles.
Wool Functionalization
The wool fabrics were washed in a bath containing 2 g L-1 nonionic detergent with L: M = 50:1 (liquor to materials ratio) at 50oC for 15 min. Then, those were rinsed with distilled water and dried at 110oC for 5 min. Green based Ag-NPs were applied on wool surface by using exhaustion methods. The pH value of Ag-NPs solution was adjusted to 4.0 with acetic acid, and then the scoured wool fabric samples were immersed in an aqueous solution containing Ag-NPs (liquor ratio of 90:1) at 45oC for 2hr (hereafter referred as “optimized condition”) with different concentration of Ag-NPs. Afterward, the treated wool samples were rinsed with running deionized water.
Measurements and Characterization
The UV–vis spectra of wool fibers with silver nanoparticles were recorded using a Shimadzu 2600 UV spectrophotometer. The morphologies of samples were examined using a scanning electron microscope (SEM) (JEOL, Tokyo, Japan) after gold coating. During SEM test, an EDS spectrum was collected to analyze the chemical elements of the samples. Transmission electron microscopy (TEM) (Hitachi H-7600, Tokyo, Japan) was used to study the particle size distribution of silver nanoparticles. The crystal behavior of samples was obtained using an X-ray diffractometer (XRD) (Bruker, Germany). Fourier transform infrared (FT-IR) measurements were performed with a Bruker Tensor 27, Germany in a normal transmission mode. Thermal behavior of samples was performed on a thermogravimetric analyzer (TGA) (Mettler-Toledo Corp., Switzerland) at a heating rate of 10oC/min under nitrogen atmosphere. The color strength (K/S) and CIELAB color coordinates (under illuminate D65 and 10o standard observer) of samples were measured with the help of Macbeth Color Eye 7000A spectrophotometer. Wool fabrics were tested for the tensile properties before and after Ag-NPs treatment. The crease recovery angle of the samples was determined as per AATCC Test Method 66-2003 using Sasmira crease recovery tester (India). The stiffness in terms of bending length of untreated and Ag-NPs treated sample was tested as per AATCC Test Method 115-2005 using profile stiffness tester (India).
Results and Discussion
Characterization of Ag-NPs
In the present observe, silver nanoparticles (Ag NPs) had been synthesized in a ‘green’ method using natural biopolymer alginate with the aid of certainly heating an aqueous combination of sodium alginate and AgNO3. The preliminary affirmation of nanoparticles formation turned into ascertained via recording the absorbance of the colloidal suspension the use of UV-visible spectrophotometry in the range of 200-700 nm and color change observation. The solution colour turned into reddish brown that indicating reduction of silver ions and appropriate the acknowledged synthesis of AgNPs is apparent in (Fig. 1A inset). The distinctive colors of silver nanoparticles are due to a phenomenon known as plasmon absorbance. Incident light creates oscillations in conduction electrons on the surface of the nanoparticles and electromagnetic radiation is enamored [17]. Fig 1A shows the UV-vis spectra of pure silver nitrate (AgNO3), sodium alginate (Na-Alg) and AgNPs. In the attendance of Na-Alg, the band at ∼301 nm exhibited by AgNO3 in aqueous solution disappear indicating possible chelation of Ag+ by way of OH and COOH groups of alginate. The Ag+ chelate produces Ag0 on heating. The intensity and role of the shoulder of the alginate spectrum at approximately 290 nm turned into barely shifted, confirming that there have been interactions among the polymer and the metallic precursor. The UV-Vis spectrum of the Ag-NP showed a characteristic peak at about 400nm, assigned to a strong surface plasmon resonance has been properly-documented for various metal nanoparticles with sizes ranging from 2 to 100 nm [18]. For the synthesis of AgNPs, the typically accepted mechanism shows a two-step process, i.e. atom formation and then polymerization of the atoms. In the first step, a portion of metal ions in the solution is reduced by the available reducing groups. The atoms for that reason produced act as nucleation centers and catalyze the discount of the ultimate metal ions in the bulk solution. Compared with other water-soluble polymers, alginate is an anionic polymer with high charge density; the negatively charged alginate allows the appeal of the definitely charged silver ions to the polymeric chains, which have been then decreased by way of the present reducing corporations. The resulting surface negative charge of alginate fragments containing carboxylic groups stabilizes nanoparticles against coalescing with the following one due to electrostatic repulsion and steric outcomes [19].
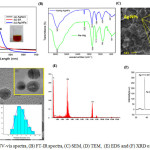 |
Figure 1a: UV-vis spectra, (B) FT-IR spectra, (C) SEM, (D) TEM, (E) EDS and (F) XRD of AgNPs Click here to View figure |
The FT-IR measurements were executed out to analyze the functional group of the material. Fig 1B shows the pure Na-Alg spectra presents two primary peaks at about 3435 cm−1 (corresponding to the absorption of stretching of the OH groups)and at 2025 cm−1 (corresponding to the C H stretching of the CH2groups). The CO asymmetrical stretching at max 1609 cm−1 along with a weaker symmetrical stretching band at 1414 cm−1 [20] were empiric due to salt nature of carboxylic acid groups at pure Na-Alg. An accelerated band at 1083 cm−1 is apery the stretching of C–O–C group; the peaks at 601 cm−1 belong to C–O–C glycosidic linkage (ring breathing) [21]. They are accompanying to its saccharide structure [22]. In the case of AgNPs, the band of CO2− is shifted to 1613 cm−1 in addition the peaks of 3435 and 1414 cm−1 shift to 3422 and 1384 cm−1 due to ring stretching of metal groups and indicating that Na-Alg was doped by NO3− because of the stabilization of Ag-NPs. The comparison of FTIR spectra between the Na-Alg and Na-Alg/Ag-NPs showed only minor changes in the position as well as the absorption bands. Thereby, FT-IR spectrum confirms that Ag-NPs have been capped with the aid of the lone pair electrons around the oxygen atoms in organic compound in Na-Alg with van der waals interaction forces [23]. Fig 1C presents SEM image of Na-Alg loaded Ag-NPs. It is manifest that silver nanoparticles synthesized by greenly can cautiously be admired as nanoparticles with spherical nature. The agglomeration of silver nanoparticles was due to interactions of hydrogen bond and electrostatic interactions between the bioorganic capping molecules apprenticed to the AgNPs [24]. TEM image with the corresponding particle size distribution (PSD) of the prepared Ag-NPs is shown in Fig 1D. It demonstrates the formation of Ag-NPs and offers us clean view of shape, length and distribution of the particles in nanoscale. From the image, it could be seen that the Ag NPs located are round in form and nicely separated in aqueous medium which are covered via layer. The layer can be the phytoconstituents of sodium alginate. In addition, the aggregation is lower because of less collision of silver nanoparticles. From the scale distribution picture, it could be ascribed that the maximum number of nanoparticles are inside the length among 6 to 10 nm. The reductive properties of sodium alginate are notably more desirable owing to the base hydrolysis with the formation of low molecular weight reducing fragments, and consequently, reflecting the twin function of sodium alginate as stabilizing and reducing agent in alkaline medium [25]. The additional support of reduction of Ag+ ions to elemental silver was confirmed by EDS analysis is shown in Fig 1E. The optical absorption peak is observed approximately at 3 keV, which is typical for the absorption of metallic silver nanocrystalline due to surface plasmon resonance [26], which confirms the presence of nanocrystalline elemental silver. An XRD pattern of Na-AgNPs become completed to verify the crystal phase of the prepared AgNPs within the range of 30–80o. Fig 1F suggests typical XRD pattern of the alginate–AgNPs prepared with several distinct diffraction peaks at approximately 38.1o, 44.2o, 64.3o and 77.4o are assigned to reflections from the (111), (200), (220), and (311) planes of the silver crystal, respectively, which confirms the existence of silver and further on the basis that they can be indexed as face-centered-cubic (FCC) structure of silver. These peaks are due to the crystalline and amorphous natural stages, accompanying crystallized AgNPs. In addition to the Bragg peaks adumbrative of silver nanocrystals, added peaks were aswell observed, although they were not assigned to the spectrum and may accept been due to amoebic compounds, responsible for silver ion reduction and the stabilization of the resultant nanoparticles [27].
Optimization of Treatment Conditions
Fig. 2 (a) represents the effect of pH on color strength (K/S) values of wool treated by 35 and 70 ppm Ag-NPs conc. with respect to controlled conditions. When the pH of Ag-NPs solution was 2, the solution was approximately colorless after treatment of wool fabrics and the assembling trend of Ag-NPs was the same as that at pH 4. But still better result observed when pH of the application medium 4 than pH 2 in terms of K/S value. From the Fig. 2 (a), it can be depicted that maximum color strength (K/S) value obtained at pH 4, a significant proportion of internal amino groups are protonated, leading to a neutralization of this surface charge; however, the carboxylate anions are not substantially protonated until the pH approaches high acidic medium. This is the reason for the use of acid when dyeing wool with highly hydrophilic leveling acid dyes and it is clear from Fig. 2 (a) that Ag-NPs behave in a similar manner to those dyes [28]. However, the Ag-NPs deposition did not occur when the application medium was approximately neutral condition like pH 7 as there is no significant change in K/S value. Similar phenomenon has been observed when the solution was in highly alkaline medium like pH 11. In addition, the pH value played a vital contribution in the Ag-NPs treatment process because the different surface characteristics of wool polymer under different pH values. So it could be concluded that the Ag-NPs on wool surface is applicable may be due to the electrostatic interaction between carboxylate groups of Ag-NPs and amino groups of wool polymer.
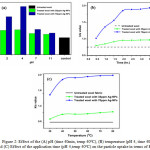 |
Figure 2: Effect of the (A) pH (time 40min, temp 40oC), (B) temperature (pH 4, time 40min) and (C) Effect of the application time (pH 4,temp 40oC) on the particle uptake in terms of K/S value Click here to View figure |
Optimization of treatment time can be an important step in sustainable textile functionalization with respect to the energy savings. Fig 2 (b) shows the progress of wool treatments with different concentration of Ag-NPs at times ranging from 30min to 4hr in terms of K/S value. The result revealed that, the K/S value increases from 30min to 2hr time interval. This result can be attributed due to the changing pattern of the wool materials and the zeta potential of the Ag-NPs. Because the wool surface having positive charge and Ag-NPs surface having negative charge resulting a strong attraction between them [29]. Therefore, up to 2hr of exhaustion time, the rate of Ag-NPs uptake by wool was very high; however, there was no significant change in curve but asymptotic behaviour with more exhaustion time beyond 2hr. The application of Ag-NPs on wool fabrics can be controlled by the temperature of application medium. The Fig 2 (c) evident that the rate of Ag-NPs uptake by wool samples were increased up to 40oC temperature and thus completed in 2hr. An interesting correlation between temperature and time was observed in this experiment. When the temperature was increased to 85oC, the deposition was finished in about 1hr at same concentration because of higher kinetic energy of the system at higher temperature [30].
Absorption Characteristics
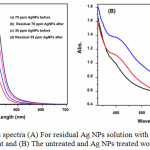 |
Figure 3: UV–vis spectra (A) For residual Ag NPs solution with before and after treatment and (B) The untreated and Ag NPs treated wool fabrics. Click here to View figure |
The UV–Vis absorption spectrum of the solutions of silver nanoparticle before and after coating was presented in Fig 3(A). It can be acknowledge that, 70 ppm AgNPs solution absorption intensity is higher than 35 ppm AgNPs solution after coating. This could be due to the high affinity of wool material that’s enough sufficient to adsorb nearly all Ag+ and Ag NPs. The emerged colours of the treated fabrics had been associated with the surface plasmon resonance (SPR) properties of green synthesized AgNPs on wool fabrics. In order to analyze the SPR of AgNPs on dealt with fabric, the absorption traits of the 35 and 70 ppm AgNPs treated fabrics had been evaluated using a spectrophotometer within the variety of 200-800 nm is shown in Fig 3(B) and illustrate that, with the increasing of Ag-NPs concentration on the wool surface, the absorbency increased and the main plasmon peak was observed around 425 nm. Also, the intensity of the observed plasmon peaks was increased with raising the Ag-NPs concentration. Another prominent peak of untreated wool at 460 nm gradually disappears with the increasing of Ag-NPs concentration. It is assumed that, this because of the interaction of the very small Ag-NPs with the wool polymer system.
Color Measurements
The treated wool with Ag-NPs exhibits higher K/S value than the untreated wool sample due to the adsorption of Ag-NPs (Table 1). It was described that K/S value can be a measure of deposited Ag-NPs concentration on fabric. The K/S is the ratio between the absorption (K) and scattering coefficient (S) of the treated materials. However, the K/S value of the treated wool fabric has different trend comparing to the Ag content. Ag-NPs absorption considerably depends on the shape, size and application media [31] and varying synthesizing condition can influence these characteristics [32, 33]. Therefore, increasing the concentration of Ag-NPs can effect on the absorption features of the loaded Ag-NPs but may not ensure any correlation between the color strength of the treated silk and Ag content.
Table 1: Color coordinates data of untreated and Ag-NPs treated wool fabrics
|
Sample |
CIE DE | CIE L* | CIE a* | CIE b* | K/S value |
| Untreated wool | 0.76 | 82.97 | 0.70 | 14.55 | 0.7556 |
| Treated wool with 35ppm Ag-NPs | 7.47 | 76.45 | 3.36 | 15.05 | 0.9577 |
| Treated wool with 70ppm Ag-NPs | 17.81 | 67.52 | 4.80 | 21.05 | 1.8529 |
As shown in Fig 4, the wool fabrics appeared in vivid colors derived from the LSPR of silver NPs. However, the colors of these treated wool fabrics were slightly different from that of original silver NP solutions, which is due to the environment changes around silver NPs from water to the interface of air and wool fiber [34].
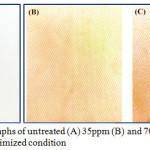 |
Figure 4: Photographs of untreated (A) 35ppm (B) and 70ppm (C) Ag-NPs under optimized condition Click here to View figure |
FT-IR Spectra
Fig 5 shows the FT-IR transmission spectra of the untreated wool and AgNPs treated wool fabrics. Wool is composed of a cuticle and cortex, whereas the cortex makes up the main portion of the wool. It is made up of more than 18 amino acids, which can be alienated into four distinct groups: cationic, anionic, nonpolar, and polar. The main functional groups include carboxyl (-COOH), amino (-NH2), and hydroxyl (-OH) groups [35]. Overall, as shown in Fig. 5, all wool fibers exhibited similar absorption at the following wavelengths: 3260 cm-1 (N-H and O-H), 2881 cm-1 (-CH2), 1670 cm-1 (amide I), 1540 cm-1 (amide II), and 1270 cm-1 (amide III). When spectra of untreated Fig 5 (a) and treated Fig 5 (b and c) were compared, new peaks at 1260 cm−1 and 1100 cm−1 were observed. The intensity of these peaks is stronger than the peaks for the spectrum of untreated wool, due to interaction between the OH of sodium alginate and the amide bond of the wool. This suggests that the Ag-NPs were successfully adsorbed on wool and confirms that the two new peaks in spectra are derivative from the interaction of the wool polymer system and Ag-NPs [36].
 |
Figure 5: FT-IR spectra of samples Click here to View figure |
Thermogravimetric Analysis (TGA)
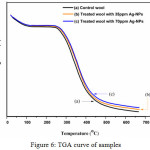 |
Figure 6: TGA curve of samples Click here to View figure |
Thermogram of weight loss in percentage of wool fabric by heating was recorded and compared with treaded wool with different concentration of Ag-NPs are illustrated in Fig 6. The untreated (blank) wool fabric after the loss of regains water (occurring from 30oC to 160oC and is accompanied by a decrease of 7% in wool fibers mass and is ascribed to the loss of water), the initial thermocracking reaction begins at approximately 280oC and the weight became reduced. This is because of destruction of disulphide linkages and the elimination of H2S, followed by the thermal pyrolysis of the chain linkages, peptide bridges and some other lateral chains, which finally leads to backbone degradation [37, 38]. On the other hand, wool fabrics treated with Ag-NPs were found to have a delayed thermocracking reaction. So, the Ag-NPs treating of wool fabrics lead to increase its thermal stability, which is assured the consequence of Ag-NPs in advanced treating effectiveness. This is because sodium alginate & silver nano composite may be act as cross linker or filler of wool polymer system; therefore, heat has significant effect on the treated fabrics. So, we can conclude the untreated wool having a higher weight loss, while the highest Ag-NPs concentration offerings the lowest weight loss. The results are in consensus with the results of silver content.
SEM Analysis
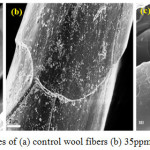 |
Figure 7: SEM images of (a) control wool fibers (b) 35ppm and (c) 70ppm Ag-NPs Click here to View figure |
SEM analysis were done to observe the surfaces morphologies of untreated and treated wool fabrics with different concentration of Ag-NPs. Fig 7 display the SEM characterization of wool fabrics before and after treatment with Ag-NPs. A clear and distinctive scaled assembly of a wool fiber can be detected in the case of control sample. It comprises of cuticle cells covering to form a pattern like that of tiles on a roof [39]. The result shows a high level of heterogeneity of the surface covering; namely, certain areas are tightly covered, whereas in others, a thinly covered pattern can be detected. The presence of Ag-NPs on the surface of treated wool fibers indicate that the Ag-NPs were successfully adsorbed on the wool fibers. There were silver clusters on the surface of the wool fibers. Some of them were well separated when the concentration of Ag-NPs was 35ppm due to the protection by Na-Alg, but larger aggregates can also be observed in the case of 70ppm. These aggregations create a smooth outer layer of wool fibers which suggest a possible layer by layer nano-coating technique for multifunctional application.
Functional Properties
The significance of Ag-NPs on tensile features, crease recovery performance and bending properties has been examined. It was noticed from the Table 2 that the treatment of Ag-NPs in to the wool polymer results in progression in the load bearing capacity of the fiber. According to this consequence, it can be determined that the Ag-NPs treated wool sample amplifies its mechanical behavior. Because of the particle size in nano range, Ag-NPs having enough capability to enter in between the wool polymer system and possibly act as a filler or crosslinking agent which also guarantee to the load shearing ability during the load application to the material. There is little improvement in crease recovery angle of Ag-NPs treated wool fabric with a little increase in bending length. This phenomenon ensures that the particle entered in between the polymer molecules do not interface much to the polymer flexibility of the system. The behavior is thus devoid of harshness to the materials. No distinct change can be felt in hand feeling between untreated and Ag-NPs treated wool fabrics; all the wool fabrics feel soft and smooth equally. To further observe the influences of the assembly of Ag-NPs on handle of the wool fabrics, bending lengths were measured (Table 2).
Table 2: Physical and mechanical properties of samples
| Properties | Untreated wool | 35ppm Ag-NPs | % change | 70ppm Ag-NPs | % change | |
| BKL | 6.32 | 6.42 | +1.58 | 6.49 | +2.69 | |
| CRA | 140 | 147 | +5.00 | 150 | +7.14 | |
| BL | 2.08 | 2.18 | +4.81 | 2.22 |
+5.71 |
BKL= Breaking load (kg), CRA= Crease recovery angle (o), BL= Bending length (cm)
Since fabric bending length is related to fabric stiffness or handle, these results propose that the assembly of Ag-NPs did not change the handle of wool fabrics, due to the size of Ag-NPs with in nano range. Wool fabrics are hydrophobic in nature. The surface assembling and modification can change the wettability of fabrics, which can ensure better comfortableness of textile. In this study, the adsorption ability of the wool fabrics to Ag-NPs colloid was measured by increasing weight ratios of Ag-NPs colloid to wool. All the Ag-NPs in the colloid were nearly assembled on the wool when the ratio was changed to 500 from 100, which can be inferred from extinction spectrum of Ag-NPs colloid after treatment (data not shown). Furthermore, to evaluate the stability of the Ag-NPs on the wool with respect to K/S value of the wool fabric before and after placement for a period of time were recorded and compared. These results exhibit that the wool fabrics treated with Ag-NPs possess good stability, which is substantial for industrial application of treatment with Ag-NPs. It is supposed that the oxygen in air did not oxide the Ag-NPs assembled on wool fabric though the morphologies of the Ag-NPs in colloid can be converted by assistance of dissolved oxygen in solution under heating [40], which is possibly attributed to the change of neighboring medium around Ag-NPs.
Conclusion
In this research, we have demonstrated the Ag-NPs could be successfully bonded to the wool fibre surface with appropriate surface modification by physical & chemical interactions. In particular, it is maximal at low pH in which there are very few anionic groups on the wool fiber; the NPs also need to have sufficient charge to maintain their stability as dispersion. FT-IR characterizations demonstrated that the Ag-NPs were effectively assembled on the wool fibres. SEM analysis confirmed that the industrial process to obtain fabrics did not alter the strong adhesion of Ag-NPs to the fibers and their uniform distribution. The produced silver-treated wool fabrics maintained the high flexibility and elasticity typical of wool. The stability of the Ag-NPs treatment is satisfactory: even after several cycles of thermal treatments. Ag-NPs endowed the wool fabrics with different colors which suggest eliminate the limitation of traditional wool dyeing process. We also suggest that this product can serve to functionalize fabric with the high performance. It has been shown that nanotechnology and textiles can be combined to develop novel fabrics with technical multi-functions, which can facilitate extensive applications of nanoparticles in textiles.
Acknowledgement
The authors wish to thanks the Runhe chemical industry, china & Color root (Hubei) technology limited, china, for providing the technical support and the school of chemistry & chemical engineering, Wuhan Textile University, china, for providing chemicals and all measurements.
References
- Whitesides, G. M., Small, 2005,1 172-179
CrossRef - Yetisen, A. K., Qu, H., Manbachi, A., Butt, H., Dokmeci, M. R., Hinestroza, J. P., Skorobogatiy, M., Khademhosseini, A., and Yun, S. H., ACS nano, 2016,10 3042-3068
CrossRef - Qian, L., AATCC rev, 2004,4 14-16
- Bozzi, A., Yuranova, T., and Kiwi, J., J. of Photochem. and Photobio. A: Chem., 2005,172 27-34
CrossRef - Avila, A. G. and Hinestroza, J. P., Nat. nanotech., 2008,3 458-459
- Zhao, G. and Stevens, S. E., Biometals, 1998,11 27-32
CrossRef - Iravani, S., Korbekandi, H., Mirmohammadi, S., and Zolfaghari, B., Res. in phar. sci., 2014,9 385
- Bonilla, J. J. A., Guerrero, D. J. P., Sáez, R. G. T., Ishida, K., Fonseca, B. B., Rozental, S., and López, C. C. O., J. of Nanosci. and Nanotech., 2017,17 1729-1739
CrossRef - Sarsar, V., Selwal, K. K., and Selwal, M. K., J. of Microbio. and Biotech. Res., 2017,3 27-32
- Poliakoff, M., Fitzpatrick, J. M., Farren, T. R., and Anastas, P. T., Science, 2002,297 807-810
CrossRef - Raveendran, P., Fu, J., and Wallen, S. L., J. of the Ame.. Chem. Soci., 2003,125 13940-13941
CrossRef - Maclaren, J. A. and Milligan, B. Vol. 122. 1981, Marrickville, Australia: Science Press.
- Pollini, M., Paladini, F., Licciulli, A., Maffezzoli, A., Nicolais, L., and Sannino, A., J. of App. Poly. Sci., 2012,125 2239-2244
CrossRef - Sepahi Rad, P., Montazer, M., and Karim Rahimi, M., J. of App. Poly. Sci., 2011,122 1405-1411
CrossRef - Ki, H. Y., Kim, J. H., Kwon, S. C., and Jeong, S. H., J. of Mat. Sci., 2007,42 8020-8024
CrossRef - Montazer, M., Behzadnia, A., Pakdel, E., Rahimi, M. K., and Moghadam, M. B., J. of Photochem. and Photobio. B: Biology, 2011,103 207-214
CrossRef - Slistan-Grijalva, A., Herrera-Urbina, R., Rivas-Silva, J., Ávalos-Borja, M., Castillón-Barraza, F., and Posada-Amarillas, A., Phy. E: Low-dim. Sys.and Nanostru., 2005,27 104-112
- Stamplecoskie, K. G. and Scaiano, J. C., J. of the Ame. Chem. Soci., 2010,132 1825-1827
CrossRef - Abdel-Halim, E. and Al-Deyab, S. S., Carbo. Poly., 2011,86 1615-1622
CrossRef - Manuja, A., Kumar, S., Dilbaghi, N., Bhanjana, G., Chopra, M., Kaur, H., Kumar, R., Manuja, B. K., Singh, S. K., and Yadav, S. C., Nanomedicine, 2014,9 1625-1634
CrossRef - Cardenas‐Jiron, G., Leal, D., Matsuhiro, B., and Osorio‐Roman, I., J. of R. Spectro., 2011,42 870-878
CrossRef - Sartori, C., Finch, D. S., Ralph, B., and Gilding, K., Polymer, 1997,38 43-51
CrossRef - Mandal, A., Sekar, S., Chandrasekaran, N., Mukherjee, A., and Sastry, T. P., RSC Adv., 2015,5 15763-15771
- Devaraj, P., Kumari, P., Aarti, C., and Renganathan, A., J. of nanotech., 2013,2013
- El-Rafie, M., El-Naggar, M., Ramadan, M., Fouda, M. M., Al-Deyab, S. S., and Hebeish, A., Carbo. Poly., 2011,86 630-635
CrossRef - Mohammed Fayaz, A., Balaji, K., Girilal, M., Kalaichelvan, P., and Venkatesan, R., J. of Agr. and F. Chem., 2009,57 6246-6252
CrossRef - Roopan, S. M., Madhumitha, G., Rahuman, A. A., Kamaraj, C., Bharathi, A., and Surendra, T., Ind.Crop. and Prod., 2013,43 631-635
CrossRef - Briggs, T. and Bull, A., The J. of Phy. Chem., 1922,26 845-875
- Tang, B., Wang, J., Xu, S., Afrin, T., Xu, W., Sun, L., and Wang, X., J. of coll. and int. sci., 2011,356 513-518
- King, D. G. and Pierlot, A. P., Color. Tech., 2009,125 111-116
CrossRef - Cobley, C. M., Skrabalak, S. E., Campbell, D. J., and Xia, Y., Plasmonics, 2009,4 171-179
CrossRef - Mulvaney, P., Langmuir, 1996,12 788-800
CrossRef - Shin, H. S., Yang, H. J., Kim, S. B., and Lee, M. S., J. of coll. and int. sci., 2004,274 89-94
- Hu, M., Chen, J., Marquez, M., Xia, Y., and Hartland, G. V., The J. of Phy. Chem. C, 2007,111 12558-12565
- Hsieh, S. H., Huang, Z., Huang, Z., and Tseng, Z., J. of App. Poly. Sci., 2004,94 1999-2007
CrossRef - Bose, P. P., Drew, M. G., and Banerjee, A., Org. lett., 2007,9 2489-2492
CrossRef - Davies, P. J., Horrocks, A. R., and Miraftab, M., Poly. int., 2000,49 1125-1132
- Forouharshad, M., Montazer, M., Moghadam, M. B., and Saligheh, O., Ther. Acta, 2011,516 29-34
CrossRef - Gallico, L. L., Vigliano Biellese, Italy, 2000,
- Tang, B., Xu, S., Hou, X., Li, J., Sun, L., Xu, W., and Wang, X., ACS app. mat. & int., 2013,5 646-653

This work is licensed under a Creative Commons Attribution 4.0 International License.









