Development of Antimicrobial Hybrid Materials from Polylactic Acid and Nano-Silver Coated Chitosan
Nollapan Nootsuwan1, Kankavee Sukthavorn2, Worawat Wattanathana1, Suchada Jongrungruangchok3, Chatchai Veranitisagul2, Nattamon Koonsaeng4 and Apirat Laobuthee1
1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Ladyao, Chatuchak, Bangkok 10900, Thailand.
2Department of Materials and Metallurgical Engineering, Faculty of Engineering, Rajamangala University of Technology Thanyaburi, Pathumthani 12110, Thailand.
3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Rangsit University, Pathumthani, 12000, Thailand.
4Department of Chemistry, Faculty of Science, Kasetsart University, Ladyao, Chatuchak, Bangkok 10900, Thailand.
Corresponding Author E-mail: fengapl@ku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/340210
Article Received on : December 12, 2017
Article Accepted on : January 20, 2018
This work aims to prepare chitosan (CS) coated with nano-silver as a filler for polylactic acid (PLA) to develop novel hybrid materials with antimicrobial property. Nano-silver coated chitosan with different silver contents was prepared by reduction of silver nitrate using benzoxazine as a reducing agent. The obtained nanosilver-coated chitosan exhibited the antimicrobial property against S. aureus, B. subtilis, M. luteus, E. coli, P. aeruginosa and C. albicans. Among all the prepared chitosan coated with nanosilver (CS-Ag), 10CS-Ag (the silver content of 8.4% by weight from thermogravimetric analysis) showed the best antimicrobial activity. Therefore, 10CS-Ag was selected to be a filler for making compound pellets with polylactic acid (PLA). According to melt flow index (MFI) study of CSAg-PLA compounding, the hybrid materials between CSAg and PLA were prepared by compression molding. The hybrid materials with 3% by weight of 10CS-Ag in PLA matrix (3CSAg-PLA) revealed the antibacterial property of 99.99% inhibition. This work showed the possibility of developing the hybrid materials between CSAg and PLA as antimicrobial products.
KEYWORDS:Chitosan; Nano-silver; Polylactic acid; Hybrid materials; Antimicrobial properties
Download this article as:| Copy the following to cite this article: Nootsuwan N, Sukthavorn K, Wattanathana W, Jongrungruangchok S, Veranitisagul C, Koonsaeng N, Laobuthee A. Development of Antimicrobial Hybrid Materials from Polylactic Acid and Nano-Silver Coated Chitosan. Orient J Chem 2018;34(2). |
| Copy the following to cite this URL: Nootsuwan N, Sukthavorn K, Wattanathana W, Jongrungruangchok S, Veranitisagul C, Koonsaeng N, Laobuthee A. Development of Antimicrobial Hybrid Materials from Polylactic Acid and Nano-Silver Coated Chitosan. Orient J Chem 2018;34(2). Available from: http://www.orientjchem.org/?p=44512 |
Introduction
Plastics play important role for our everyday life due to its cheap price, light weight and variety of applications. Plastics can be processed into different shapes so they are now replacing the usage of woods, metals and ceramics. Therefore, the demand for plastics is drastically increasing. However, a prevalence use of petroleum-based plastics leads to environmental problems as they take very long time to decompose [1-3]. Moreover, the shortage of petroleum and crude oil is also the issue [4]. To solve the problems mentioned, many researchers and industries develop the new products from biodegradable plastics, especially polylactic acid (PLA). Polylactic acid is one of the best choice of biodegradable plastics since it offers many advantages. The production of PLA is renewable and consumes low energy. With these reasons, PLA is the eco-friendly material that are now widely used as plastic bags, food containers as well as medical equipment. However, the use of neat PLA is not widespread because certain properties are needed to be improved. Hybrid materials are composite materials fabricated to have specific properties that are suitable for certain applications. Normally, hybrid materials are consisting of a polymer matrix and a filler. The addition of fillers commonly introduces the desired properties of the hybrid materials [5].
Chitosan (CS) is the product from deacetylation reaction of chitin, which comes from the peripheral parts of crustacean organisms such as shrimps and crabs [6-9]. Unlike many synthesized polymers, chitosan has notable properties, e.g. biodegradation and biocompatibility. Moreover, the compounds produced from the degradation of chitosan are non-toxic, non-allergic, and non-carcinogenic [10-12]. Furthermore, chitosan has various applications in several industries such as cosmetics and toiletries, pharmaceutical, agriculture, textiles, foods as well as chemical industries [13-17]. Due to the mentioned properties of chitosan, it would be possible to use as a biodegradable filler for PLA to produce novel hybrid materials for PLA.
Silver is renowned for its antimicrobial agent with a broad range of activity. It was ubiquitous that silver in any oxidation states (AgO, Ag+, Ag2+, and Ag3+) is commonly used as an antimicrobial agent against numerous bacterial strains and micro-organisms [18]. Especially in the nanoscale, silver nanoparticles (AgNPs), with the particle sizes smaller than 100 nm consisting of 20-15,000 silver atoms, shows incredible physical, chemical and biological properties, and antibacterial activity [19]. The reason for the prevailing use of the AgNPs antibacterial agent is due to low toxicity toward mammalian cells [20-21].
Recently, many efforts have been paid on hybrid of metallic salts and biopolymers on the basis of polymer characteristics with metal ion properties. Chitosan with high chelating strength for numerous metal ions is a promising candidate for the metallic cations and biopolymer hybrids. The chelation increases the number of metal ions on the polymer surface and therefore inhibits the particle growth [22]. It was proved that various nanoparticles in combination with chitosan are potential candidate in biomedical applications due to advantages of biodegradability, antibacterial and superb chelating characteristics. Numerous works have been done using chitosan with different nanoparticles. Farouk et al. integrated the ZnO nanoparticles with chitosan to show the effective antibacterial properties [23] while AbdElhady et al. used the similar materials for the induced antibacterial and UV protection in fabrics [24]. Interestingly, both silver and chitosan are antibacterial agents, so CS-AgNPs composite material is likely to be more antibacterial effective [25]. Therefore, many researches on this point have been carried out. For example, Arif and co-workers developed the cotton fabrics with chitosan-silver nanoparticles [26]. In our study, we therefore need to extend state-of-the-art to further investigate the novel antimicrobial materials from both the biodegradable matrix (PLA) and biomaterial filler (chitosan) with silver nanoparticles. To prepare the nanosilver coated chitosan for using as a filler for the desired hybrid materials, the silver nanoparticles was introduced into the surface of chitosan by chemical reduction of silver nitrate using benzoxazine which has been firstly reported by our group [27]. Furthermore, the detailed characterization on the structural, mechanical, and antimicrobial properties are examined.
Experimental
Materials
Chitosan with molecular weight of 150,000 Da and deacetylation degree of 0.95 was purchased from SEA FRESH CHITOSAN (Lab) Co., Ltd. (Thailand). Silver nitrate (AgNO3, Analytical reagent grade) with molecular weight of 108 Da was obtained from POCH company. Dichloromethane (CH2Cl2, commercial grade) with molecular weight of 84.93 Da was received from Lab Scan. 3,4-Dihydro-3,6-dimethyl-1,3-2H-benzoxazine was synthesized according to our previous literature [27] for using as a reducing agent for silver(I) ion from silver nitrate. Acetone (CH3COCH3, commercial grade) with molecular weight of 58 Da was purchased from Alcohol Paint Solvent, Thinner & Chemistry Supply Co., Ltd. Anhydrous sodium sulphate with molecular weight of 142.04 Da was purchased from Riedel-deHaen Co., Ltd. Polylactic acid used in the study was a commercial grade (PLA 2003D) and obtained from Nature works LLC, USA. All the chemicals are used as received.
Synthesis of Nano-Silver Coated Chitosan
Chitosan with different masses, i.e. 20 g, 15 g, 10 g and 2.5 g, was subjected to coating with silver nanoparticles by the procedure reported in the literature [27]. The amounts of starting materials and nomenclatures are listed in Table 1.
Table 1 Amounts of starting materials for synthesis of nanosilver coated chitosans
| Starting material | Chitosan coated with silver nanoparticles | |||
| 2.5CS-Ag | 10CS-Ag | 15CS-Ag | 20CS-Ag | |
| Chitosan (g) | 2.5 | 10 | 15 | 20 |
| Silver nitrate (g) | 1 | 1 | 1 | 1 |
| The reducing agent (g) | 1 | 1 | 1 | 1 |
Preparation of Hybrid Materials
Polylactic acid was dried at 40°C 24 h in ambient air to remove adsorbed moisture. The obtained polylactic acid were mixed with nano-silver coated chitosan with the content of 1, 3, 5 and 7 phr. Hereinafter, they would be named 1AgCS-PLA, 3AgCS-PLA, 5AgCS-PLA and 7AgCS-PLA, respectively. The study on melt flow index (MFI) suggested that compression molding could be used to fabricate AgCS-PLA hybrid materials. The mixtures were compressed by using compression molding machine at 150°C with a pressing step of 10 min under the pressure of 100 bar and the cooling step of 10 min under the same temperature.
Material Characterization
Fourier Transform Infrared (FTIR) spectra were recorded in the range 4000-600 cm-1 by a Perkin-Elmer 2000-FTIR, using KBr pellet technique for identifying functional groups of the specimens. Morphologies of all the powders were investigated by scanning electron microscope (Philips, XL30) using acceleration voltage of 5 kV. The samples were sputter-coated with gold to increase electrical conductivity. Thermogravimetric analysis (TGA) of chitosan and nanosilver coated chitosan was conducted by TGA analyzer from Mettler Toledo. The analysis was performed with the heating rate of 5°C/minin N2 (20 psi) from 25 to 1000°C under static air flow of 20 psi. Elemental analysis was studied by X-ray fluorescent spectrometer (Horiba XGT-2000W) with the X-ray tube operated at 50 kV and 1 mA. Differential scanning calorimeter (DSC) was performed by DSC analyzer from Mettler Toledo with a heating rate of 10°C/min from 0 to 250°C. Impact test was performed using a Izod impact tester (CEAST: Model 6546). The samples for impact testing were prepared from compression molding sample according to the ASTM D256. Flexural test was performed using a Universal tensile testing machine (COMETECH: B1/E type). The specimens for flexural testing were prepared from compression molding sample according to the ASTM D790.
Test for Antimicrobial Property
Antimicrobial property of chitosan and nano-silver coated chitosan powders was studied by agar disc diffusion method. Gram positive bacteria (Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 6633), gram negative bacteria (Escherichia coli ATCC 25922, Micrococcus luteus ATCC 9341 and Pseudomonas aeruginosa ATCC 27853) and fungus (Candida albicans ATCC 10231) were used in this study. All bacteria were incubated at 37°C for 24 h while the fungus was incubated at 30°C for 72 h. Diameter of the inhibition zone was measured in order to evaluate the antimicrobial property.
Antimicrobial property of the hybrid materials of nanosilver coated chitosan and PLA was measured by quantifying the survival of bacterial cells according to JIS Z 2801. Firstly, 8 pieces of each specimen were put into the media with the microbe concentration of 1.5 × 105 cfu/ml. Next, the media was shaken with the speed of 120 rpm at 37°C for 24 h. 100 μL of the solution was transferred to nutrient agar (NA) for bacteria and potato dextrose agar (PDA) for fungus and then incubated at 37°C for 24 h. The antimicrobial activity is presented as percent reduction according to the formulation.
Reduction (%) = (B-A)/A * 100
where
A : Number of bacteria recovered after dynamic contact of to the sample (at time 24 h)
B : Number of bacteria before the addition of the sample (at time 0 h)
Results and discussion
Synthesis, Characterization and Antimicrobial Test of Nanosilver Coated Chitosan
Physical appearances of chitosan and nanosilver coated chitosan are shown in Figure 1(a) and 1(b), respectively. It was observed that the nanosilver coated chitosan had darker color than uncoated chitosan. SEM image of chitosan (Figure 1(c)) illustrates the smooth surface of the chitosan. For nanosilver-coated chitosan (Figure 1(d)), there were small particles dispersing on the surface of the chitosan. This supported that the silver nanoparticles were successfully introduced to the surface of chitosan. Figure 1(e) is the low-magnification SEM image for silver coated chitosan. Again, it revealed that silver nanoparticles were distributed evenly on the surface of chitosan. The result agreed with the XRF mapping (Figure 1(f)) and XRF spectra (Figure 1(g)).
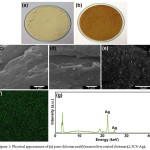 |
Figure 1: Physical appearances of (a) pure chitosan and (b) nanosilver coated chitosan (2.5CS-Ag). Click here to View figure |
SEM images of (c) pure chitosan, (d) and (e) nanosilver coated chitosan (2.5CS-Ag). (f) XRF mapping of Ag element for nanosilver coated chitosan (2.5CS-Ag). (g) XRF spectra for nanosilver coated chitosan (2.5CS-Ag)
FTIR spectra of the chitosan and nano-silver coated chitosans are shown in Figure 2(a). FTIR spectrum of chitosan shows the peaks at 3600-3000, 2885, 1590, 1310 and 1149 cm-1 which were assigned to amino, hydroxyl, amide I, II and III respectively, consistent with the previous work from Tze-Wen Chung et al. [28]. However, the FTIR spectrum of nano-silver coated chitosan with various nano-silver contents were like pure chitosan. It can be concluded that the coating nano-silver on the surface of chitosan would not change the chemical structure of chitosan.
TGA thermograms of chitosan and nano-silver coated chitosan were shown in Figure 2(b). On heating, the chitosan exhibited weight loss in three steps. The first weight loss around 26-107°C was corresponding to the moisture evaporation. The second weight loss from 107-323°C was caused by the hydrocarbon decomposition. The final weight loss from 323-537°C was ascribed to the decomposition of the residual ash from chitosan. Nano-silver coated chitosan showed weight loss in three steps like chitosan. However, the TGA thermograms of the nano-silver coated chitosan were found to have weight remaining at 1000 °C. The final weight residues of 2.5CS-Ag, 10CS-Ag, 15CS-Ag, and 20CS-Ag was 16.6, 8.4, 5.7 and 3.9 wt% respectively. This was because the metallic silver was left after the heat treatment. Moreover, the decomposition of the nanosilver coated chitosan occurred at the lower temperature than neat chitosan because the metallic silver acted as a catalyst for decomposition reaction.
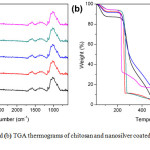 |
Figure 2a: FTIR spectra and (b) TGA thermograms of chitosan and nanosilver coated chitosan with different silver contents Click here to View figure |
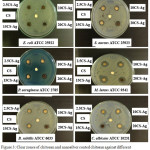 |
Figure 3: Clear zones of chitosan and nanosilver coated chitosan against different types of microbes Click here to View figure |
Agar disc diffusion was used for antimicrobial testing of chitosan and nanosilver coated chitosan. Clear zones showing antimicrobial inhibitions are illustrated in Figure 3 as well as Table 2. The result showed that pure chitosan did not exhibit the antimicrobial activity. This might be due to the chitosan was in the crystalline form. However, the nanosilver coated chitosan revealed antimicrobial property against all the microbes tested. From the table, the antimicrobial activities were similar. Therefore, only 10CS-Ag was used for further study.
Table 2: Clear zones of chitosan and nanosilver coated chitosan against different types of microbes.
| Sample | Microbial strains (clear zone, cm) | |||||
| E. coliATCC 25922 | S. aureusATCC 25923 | P. aeruginosaATCC2785 | M. luteusATCC 9341 | B. subtilis ATCC 6633 | C. albicansATCC 10231 | |
| CS | – | – | – | – | – | – |
| 2.5CS-Ag | 1.33 | 1.31 | 1.71 | 2.23 | 1.30 | 1.72 |
| 10CS-Ag | 1.43 | 1.56 | 1.62 | 1.83 | 1.32 | 1.81 |
| 15CS-Ag | 1.32 | 1.57 | 1.86 | 2.19 | 1.50 | 1.83 |
| 20Cs-Ag | 1.26 | 1.65 | 1.80 | 2.07 | 1.65 | 1.94 |
Fabrication, characterization, mechanical properties, and antimicrobial test of nanosilver coated chitosan and PLA composites
All the chitosan or nanosilver coated chitosan and PLA composites were fabricated using 10CS-Ag by compression molding method. The obtained composites were 1 phr, 3 phr, 5 phr and 7 phr of the 10CS-Ag filler in PLA matrix. The color of the obtained samples got darker with the higher content of the 10CS-Ag. XRF technique showed the silver peaks for all the composites with the 10CS-Ag filler. Moreover, the XRF mapping confirmed the good distribution of the silver in the polymer matrix.
TGA thermograms of the hybrid materials were shown in Figure 4(a).
Patterns of decomposition temperature of pure PLA and all of hybrid materials are similar. The initial decomposition temperature of all the samples are 50-100°C. It was responsible for the loss of moisture. The second decomposition temperature of all the samples was 350-450°C. For pure PLA, there was no residue left but the hybrid materials (1, 3, 5 and 7 phr) revealed the remaining product which was metallic silver with the content of 0.1, 0.15, 0.2 and 0.3 wt%, respectively. Moreover, the 7AgCS-PLA composite decomposed more rapidly than those of other PLA composites as well as neat PLA. The decomposition started at 320°C. This would be due to the metallic silver behaving as a catalyst for thermal decomposition.
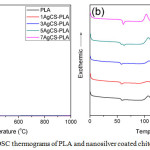 |
Figure 4a: TGA and (b) DSC thermograms of PLA and nanosilver coated chitosan and PLA composites Click here to View figure |
DSC thermograms of neat PLA and the prepared hybrid materials are shown in Figure 4(b). Tg of the PLA was found to be about 59°C while Tg of 1 phr (1AgCs-PLA) and 3 phr (3AgCs-PLA) was 57 °C. When the content of nanosilver coated chitosan rose 5 phr (5AgCs-PLA), two Tg peaks of at 57 and 61°C were observed. Moreover, the hybrid material with the content of nano-silver coated chitosan of 7 phr (7AgCs-PLA) showed one peak of Tg at 61°C. This might be due to the nano-silver coated chitosan inserted in chain molecular of PLA. It can be caused space and free volume within chain molecular of PLA which chain molecular of PLA could improve to easy movement and lower Tg. But the PLA could difficult move with increasing nano-silver coated on the surface of chitosan. As a result, the Tg values of PLA increased. Tc peak of nanosilver coated PLA composites was decreased with the content of the filler. This was because the nanosilver coated chitosan filler behaved like a nucleating agent. It was observed that the Tc temperature was changed from 114°C to 105°C. Moreover, the area under the Tc peak was also increased with the nanosilver coated chitosan content. This revealed that the crystallinity of the hybrid materials got higher. The result was in line with the Tm temperature. For the hybrid material with high nanosilver coated chitosan, Tm signal was split to two peaks at 147 and 154°C.
Impact strength of the prepared materials is illustrated in Figure 5(a). Neat PLA had the impact strength (absorbed energy) of 1.75 kJ/m2 which was higher than all its composite materials. It was observed that nanosilver coated chitosan increased the brittleness of the composite materials. Impact strength was slightly increased with the amount of the filler. The impact strength for 1 phr, 3 phr and 5 phr samples were 1.26, 1.30 and 1.33 kJ/m2, respectively.
However, the impact strength was decreased to 1.26 kJ/m2 for the 7 phr sample. This was because the filler strengthened the hybrid material but the impact force might be localized at only the certain position. Therefore, the impact strength of PLA was higher than all its composites. For the hybrid materials with nanosilver coated chitosan of 1 phr, 3 phr and 5 phr, the filler helped the PLA to receive the impact force leading to the slight increase in impact strength. However, the amount of nanosilver coated chitosan in 7 phr sample was too high so the agglomeration of the filler made the hybrid material (7AgCS-PLA) more brittle. Moreover, the distribution of the impact force was very poor. Therefore, the impact strength of the 7 phr composite was the lowest. Flexural strength and percentage elongation of the hybrid materials are displayed in Figure 5 (b) and (c), respectively. The testing procedure was followed the standard ISO14125 using the speed of 10 mm2/min. The flexural strength and % elongation of pure PLA were 178.8 MPa and 6.17, respectively. Flexural strengths were 129.7, 109.1, 97.9, 97.4 MPa while the percentages of elongation were 3.7, 3.5, 3.4 and 3.4 for 1 phr, 3 phr, 5 phr and 7 phr hybrid materials, respectively. The study on mechanical properties showed that the addition of the CS-Ag filler worsened the mechanical properties of PLA. However, the CS-Ag filler was still needed to improve the antimicrobial property of the hybrid materials.
 |
Figure 5a: Impact strength, (b) Flexural strength and (c) percentage elongation of the prepared hybrid materials together with neat PLA Click here to View figure |
The antimicrobial property of neat PLA and the nanosilver coated chitosan and PLA composites was investigated according to the JIS Z 2801 standard. In this study, only neat PLA, 3 phr and 7 phr hybrid materials were subjected to the antimicrobial testing. The testing results are shown in Figure 6 and Tables 3 – 7. According to the test, PLA did not exhibit antimicrobial property. However, the 3AgCS-PLA and 7AgCS-PLA composites showed the inhibition against all types of the microbes being studied.
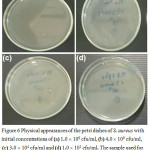 |
Figure 6: Physical appearances of the petri dishes of S. aureus with initial concentrations of (a) 1.0 × 109 cfu/ml, (b) 4.0 × 109 cfu/ml, (c) 3.0×104 cfu/ml and (d) 1.0 × 101 cfu/ml. The sample used for (a), (b) and (c) is 3AgCS-PLA while the sample used in (d) is 7AgCS-PLA. |
Table 3: Antimicrobial property of the prepared hybrid materials against S.aureus
| Initial concentration of S. aureus (cfu/ml) | Sample | No. of sample (pieces) | No. of colonies dishes (cfu/ml) | Percentage inhibition |
| 1.5 ´ 105 | S.aureus | – | 1.0 ´109 | – |
| 1.5 ´ 105 | PLA | 8 | 4.0 ´ 109 | neg |
| 1.5 ´ 105 | 3AgCS-PLA | 8 | 3.0 ´ 104 | 99.99 |
| 1.5 ´ 105 | 7AgCS-PLA | 8 | 1.0 ´ 105 | 99.99 |
Table 4: Antimicrobial property of the prepared hybrid materials against P. aeruginosa
| Initial concentration of P. aeruginosa (cfu/ml) | Sample | No. of sample (pieces) | No. of colonies dishes (cfu/ml) | Percentage inhibition |
| 1.5 ´ 105 | P. aeruginosa | – | 4.0 ´ 107 | – |
| 1.5 ´ 105 | PLA | 8 | 8.0 ´ 107 | neg |
| 1.5 ´ 105 | 3AgCS-PLA | 8 | 1.0 ´ 104 | 99.99 |
| 1.5 ´ 105 | 7AgCS-PLA | 8 | 1.0 ´ 104 | 99.99 |
Table 5: Antimicrobial property of the prepared hybrid materials against E. coli
| Initial concentration of E. coli (cfu/ml) | Sample | No. of sample (pieces) | No. of colonies dishes (cfu/ml) | Percentage inhibition |
| 1.5 ´ 105 | E. coli | – | 41.0 ´ 107 | – |
| 1.5 ´ 105 | PLA | 8 | 1.0 ´ 107 | neg |
| 1.5 ´ 105 | 3AgCS-PLA | 8 | 2.0 ´ 104 | 99.98 |
| 1.5 ´ 105 | 7AgCS-PLA | 8 | 2.0 ´ 104 | 99.98 |
Table 6: Antimicrobial property of the prepared hybrid materials against B. subtilis
| Initial concentration of B. subtilis (cfu/ml) | Sample | No. of sample (pieces) | No. of colonies dishes (cfu/ml) | Percentage inhibition |
| 1.5 ´ 105 | B. subtilis | – | 2.0 ´ 108 | – |
| 1.5 ´ 105 | PLA | 8 | 65.0 ´ 108 | neg |
| 1.5 ´ 105 | 3AgCS-PLA | 8 | 1.0 ´ 104 | 99.99 |
| 1.5 ´ 105 | 7AgCS-PLA | 8 | 1.0 ´ 103 | 99.99 |
Table 7: Antimicrobial property of the prepared hybrid materials against C.albicans
| Initial concentration of C. albicans (cfu/ml) | Sample | No. of sample (pieces) | No. of colonies dishes (cfu/ml) | Percentage inhibition |
| 1.5 ´ 105 | C.albicans | – | 40.0 ´ 105 | – |
| 1.5 ´ 105 | PLA | 8 | 2.0 ´ 106 | neg |
| 1.5 ´ 105 | 3AgCS-PLA | 8 | 1.0 ´ 105 | 95.00 |
| 1.5 ´ 105 | 7AgCS-PLA | 8 | 2.0 ´ 105 | 90.00 |
Conclusion
The nano-silver coated chitosan (CS-Ag) was successfully synthesized under mild condition by mixing silver nitrate and benzoxazine, the reducing agent, with chitosan powder. SEM images showed that the nanosilver particles were spherical shape with a uniform size and well dispersed and coated on chitosan surface. Different CS-Ag powders were prepared using the fixed amount of silver nitrate and the reducing agent but various masses of chitosan. The CS-Ag powders prepared from 2.5, 10, 15 and 20 g of chitosan were called 2.5CB-Ag, 10CB-Ag, 15CB-Ag and 20CB-Ag, respectively. TGA study revealed that the masses of silver residual after thermal decomposition of chitosan for 2.5CB-Ag, 10CB-Ag, 15CB-Ag and 20CB-were 16.6, 8.4, 5.7 and 3.9 wt% respectively. From the antimicrobial test, 10CS-Ag was the best one as it showed relatively good activity and it had relatively low silver content compared to other CS-Ag powders. Therefore, 10CS-Ag was selected to use for fabrication of AgCS-PLA hybrid materials. The compound pellets of CS-Ag and PLA were prepared, and the melt flow index (MFI) of the pellets was investigated. The MFI result suggested that compression molding method was used to make AgCS-PLA hybrid materials.
Thermal analysis of AgCS-PLA hybrid materials showed that the nanosilver acted as a catalyst for thermal decomposition and lower the decomposition temperature of the hybrid materials. Moreover, it behaved like a nucleating agent for polylactic acid so that the crystallization peak decreased but its area increased. Even though, the CS-Ag filler lowered the mechanical properties of hybrid materials, it was still required to improve the antimicrobial property of the hybrid materials. According to this work, 3AgCS-PLA hybrid materials showed microbial inhibition up to 99.99% when tested by the method of the JIS Z 2801 standard. Therefore, our work reported the possibility of developing the AgCS-PLA hybrid materials as novel biodegradable and antimicrobial materials for certain applications.
Acknowledgements
The authors would like to thanks Kasetsart University Research and Development Institute (KURDI), Kasetsart University and Rajamangala University of Technology Thanyaburi for their financial supports.
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Science 2015, 347, 768-771.
CrossRef - Derraik, J.G.B. Mar. Pollut. Bull. 2002, 44, 842-852.
CrossRef - Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Waste Manag. 2009, 29, 2625-2643.
CrossRef - Ren, X. J. Clean. Prod. 2002, 11, 27-40.
CrossRef - González-García, D.M., Téllez Jurado, L., Jiménez-Gallegos, R., Rodríguez-Lorenzo, L.M. Mater. Sci. Eng. C 2017, 75, 375-384.
CrossRef - Şenel, S.; McClure, S.J. Adv. Drug. Deliv. Rev. 2004, 56, 1467-1480.
CrossRef - Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Carbohydr. Polym. 2010, 82, 227-232.
CrossRef - Sadeghi-Kiakhani, M.; Gharanjig, K.; Arami, M. J. Ind. Eng. Chem. 2015, 28, 78-85.
CrossRef - Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Prog. Polym. Sci. 2011, 36, 981-1014.
CrossRef - Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Int. J. Food. Microbiol. 2010, 144, 51-63.
CrossRef - Lee, S.-H.; Kim, M.-J.; Park, H. J. Appl. Polym. Sci. 2010, 117, 623-628.
CrossRef - Alonso, D.; Gimeno, M.; Olayo, R.; Vázquez-Torres, H.; Sepúlveda-Sánchez, J.D.; Shirai, K. Carbohydr. Polym. 2009, 77, 536-543.
CrossRef - Dev, V.R.G.; Venugopal, J.; Sudha, S.; Deepika, G.; Ramakrishna, S. Carbohydr. Polym. 2009, 75, 646-650.
CrossRef - Crini, G.; Badot, P.-M. Prog. Polym. Sci. 2008, 33, 399-447.
CrossRef - Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Carbohydr. Polym. 2011, 83, 1446-1456.
CrossRef - Sadeghi-Kiakhani, M.; Safapour, S. Clean Technol. Environ. Policy 2015, 17, 1019-1027.
CrossRef - Sadeghi-Kiakhani, M.; Safapour, S. Fiber. Polym. 2015, 16, 1075-1081.
CrossRef - Clement, J. L.; Jarrett, P. S. Met. Based Drugs 1994, 1, 467-482.
CrossRef - Chen, X.; Schluesener, H.J. Toxicol. Lett. 2008, 176, 1-12.
CrossRef - Galeano, B.; Korff, E.; Nicholson, W.L. Appl. Environ. Microbiol. 2003, 69, 4329-4331.
CrossRef - Thomas, S.; McCubbin, P. J. Wound Care 2003, 12, 305-308.
CrossRef - Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Biomacromolecules 2003, 4, 1457-1465.
CrossRef - Farouk, A.; Moussa, S.; Ulbricht, M.; Textor, T. Int. J. Carbohydr. Chem. 2012, 2012, e693629.
- AbdElhady, M. M. Int. J. Carbohydr. Chem. 2012, 2012, e840591
- Akmaz, S.; Dilaver Adgüzel, E.; Yasar, M.; Erguven, O. Adv. Mater. Sci. Eng. 2013, 2013, e690918.
- Arif, D.; Niazi, M. B. K.; Ul-Haq, N.; Anwar, M. N.; Hashmi, E. Fiber. Polym. 2015, 16, 1519-1526.
CrossRef - Kaewvilai, A.; Wattanathana, W.; Jongrungruangchok, S.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. Mater. Chem. Phys. 2015, 167, 9-13.
CrossRef - Wattanathana, W.; Nonthaglin, S.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. J. Mol. Struct. 2014, 1074, 118-125.
CrossRef - Chung, T.-W.; Chang, C.-H.; Ho, C.-W. J. Taiwan Inst. Chem. Eng. 2011, 42, 592-597.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









