Abstract
Interlayer K in muscovite, biotite, phlogopite, illite and vermiculite-hydrobiotite samples was replaced by cation exchange with Na. The rate and amount of exchange varied with the mineral and the level of K in solution.
Essentially, all the K in muscovite, biotite, phlogopite and vermiculite was exchangeable when the mass-action effect of the replaced KT was reduced by maintaining a very low level of K in solution. The time required for this exchange varied from < 10 hr with vermiculite to > 45 weeks with muscovite. Only 66% of the K in the illite was exchangeable under these conditions. When the replaced K was allowed to accumulate in the solution, the amount of exchange was determined by the level of K in solution required for equilibrium. These levels decreased with the degree of K-depletion and with the selectivity of the mica for K. The order of selectivity was muscovite > illite > biotite > phlogopite > vermiculite. Decreasing the K in solution from 10 to 7 ppm increased the exchangeable K in biotite from 30 to 100%. A K level of only 0.1 ppm restricted the exchange of K in muscovite to 17%.
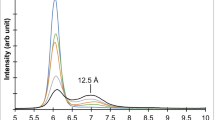
A decrease in layer charge was not required for K exchange, but a decrease did occur in K-depleted biotite and vermiculite. Muscovite with the highest layer charge (247 meq/100 g), least expansion with Na (12.3Å), and least sensitivity to solution pH had the highest selectivity for K and the slowest rate of exchange. The K in vermiculite was the most readily exchangeable.
Similar content being viewed by others
References
Arnold, P. W. (1960) Nature and mode of weathering of soil-potassium reserves, J. Sci. Food Agr. 11, 285–92.
Babshad, I. (1954) Cation exchange in micaceous minerals, II. Replaceability of ammonium and potassium from vermiculite, biotite and montmorillonite, Soil Sci. 78, 67–76.
Bassett, W. A. (1960) Role of hydroxyl orientation in mica alteration, Bull. Geol. Soc. Am. 71, 449–66.
Bolt, G. H., Sumner, M. E., and Kamphobst, A. (1963) A study of the equilibria between three categories of potassium in an illitic soil, Proc. Soil Sci. Soe. Amer. 27, 294–99.
Flaschka, H., and Babnard, A. J., Jr. (1960) Tetraphenylboron (TPB) as an analytical reagent, Advan. Anal. Chem. Instr. 1, 1–117.
Hanway, J. J., Scott, A. D., and Stanfobd, G. (1957) Replaceability of ammonium fixed in clay minerals as influenced by ammonium or potassium in the extracting solution, Proc. Soil Sci. Soc. Amer. 21, 29–34.
Helfferich, F. (1962) Ion Exchange, McGraw-Hill, New York.
Jackson, M. L., and Sherman, G. D. (1953) Chemical weathering of minerals in soils, Advan. Agron. 5, 219–318.
Mabshall, S. E. (1964) The Physical Chemistry and Mineralogy of Soils, Vol. I., Soil Materials, John Wiley, New York.
Mabshall, C. E., and Mcdowell, L. L. (1965) The surface reactivity of micas, Soil Sci. 99, 115–31.
Mobtland, M. M., and Lawton, K. (1961) Relationships between particle size and K release from biotite and its analogues, Proc. Soil Sci. Soc. Amer. 25, 473–6.
Reed, M. G., and Scott, A. D. (1962) Kinetics of potassium release from biotite and muscovite in sodium tetraphenylboron solutions, Proc. Soil Sci. Amer. 26, 437–40.
Reed, M. G., and Scott, A. D. (1966) Chemical extraction of potassium from soils and micaceous minerals with solutions containing sodium tetraphenylboron, IV, Muscovite, Proc. Soil Sci. Soc. Amer. (in press).
Rich, S I., and Black, W. R. (1964) Potassium exchange as affected by cation size, pH, and mineral structure. Soil Sci. 97, 384–90.
Scott, A. D., Hanway, J. J., and Edwards, A. P. (1958) Replaceability of ammonium in vermiculite with acid solutions, Proc. Soil Sci. Soc. Amer. 22, 388–92.
Scott, A. D., Hunsziker, R. R., and Hanway, J. J. (1960) Chemical extraction of potassium from soils and micaceous minerals with solutions containing sodium tetraphenylboron, I, Preliminary experiments, Proc. Soil Sci. Soc. Amer. 24, 191–4.
Scott, A. D., and Reed, M. G. (1962) Chemical extraction of potassium from soils and micaceous minerals with solutions containing sodium tetraphenylboron, II, Biotite, Proc. Soil Sci. Soc. Amer. 26, 41–5.
Scott, A. D., and Reed, M. G. (1965) Expansion of potassium-depleted muscovite, Clays and Clay Minerals, Proc. 13th Conf. Pergamon Press, New York (in press).
Smith, S. J., and Scott, A. D. (1966) Extractable potassium in Grundite illite, I, Method of extraction, Soil Sci. (in press).
Tucker, B. M. (1964) The solubility of potassium from soil illites, II, Mechanisms of potassium release, Australian Jour. Soil Research. 2, 67–75.
Author information
Authors and Affiliations
Additional information
Journal Paper No. J-5216 of the Iowa Agricultural and Home Economics Experiment Station, Ames, Iowa, Project No. 1234.
Rights and permissions
About this article
Cite this article
Scott, A.D., Smith, S.J. Susceptibility of Interlayer Potassium in Micas to Exchange with Sodium. Clays Clay Miner. 14, 69–81 (1966). https://doi.org/10.1346/CCMN.1966.0140106
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1966.0140106