Abstract
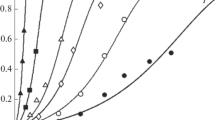
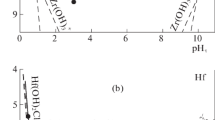
The dissolution of synthetic magnetite, maghemite, hematite, goethite, lepidocrocite, and akaganeite was faster in HCl than in HClO4. In the presence of H+, the Cl− ion increased the dissolution rate, but the ClO4− ion had no effect, suggesting that the formation of Fe-Cl surface complexes assists dissolution. The effect of temperature on the initial dissolution rate can be described by the Arrhenius equation, with dissolution rates in the order: lepidocrocite > magnetite > akaganeite > maghemite > hematite > goethite. Activation energies and frequency factors for these minerals are 20.0, 19.0, 16.0, 20.3, 20.9, 22.5 kcal/mole and 5.8 × 1011, 1.8 × 1010, 7.4 × 107, 5.1 × 1010, 2.1 × 1010, 3.0 × 1011 g Fe dissolved/m2/hr, respectively. The complete dissolution of magnetite, maghemite, hematite, and goethite is well described by the cube-root law, whereas that of lepidocrocite is not.
Резюме
Растворение синтетического магнетита, маггемита, гематита, гетита, лепидокрокита и акаганеита происходило быстрее в НСl, чем в НСlO4. В присутствии Н+, ион Сl− увеличивал скорость реакции, а ион СlO4− не имел никакого эффекта. Это указывает на то, что формирование поверхностных комплексов Fe-Cl содействует растворению. Эффект температуры на начальную скорость реакции может быть описан формулой Аррениуса, при порядке скоростей растворения: лепидокрокит > магнетит > акаганеит > маггемит > гетит. Энергии активации и факторы частот для этих минералов были соответственно: 20,0, 19,0, 16,0, 20,3, 20,9, 22,5 ккал/моль и 5,8 × 1011, 1,8 × 1010, 7,4 × 107, 5,1 × 1010, 2,1 × 1010, 3,0 × 1011 грамма Fe растворенного/м2/час. Полное растворение магнетита, маггемита, гематита и гетита хорошо описывается законом кубического корня, однако растворение лепидокрокита не совпадает с этим законом. [Е.С.]
Resümee
Synthetischer Magnetit, Maghemit, Haematit, Goethit, Lepidokrokit, und Akaganeit löste sich in HCl schneller als in HClO4. In Gegenwart von H+ vergrößerte Cl− die Löungsgeschwindigkeit, während ClO4− ohne Einfluß war. Dies deutet darauf hin, daß die Bildung von Fe-Cl-Oberfläichenkomplexen die Auflösung fördert. Der Temperatureffekt auf die anffängliche Lösungsgescbwindigkeiten kann durch die Arrhenius-Gleichung beschrieben werden, wobei sich für die Lösungsgeschwindigkeiten folgende Reihenfolge ergibt: Lepidokrokit > Magnetit > Akaganeit > Maghemit > Haematit > Goethit. Die Aktivierungsenergien bzw. Häufigkeitsfaktoren dieser Minerale sind 20,0, 19,0, 16,0, 20,3, 20,9, 22,5 kcal/Mol bzw 5.8 × 1011, 1,8 × 1010, 7,4 × 107, 5,1 × 1010, 2,1 × 1010, 3,0 × 1011 g Fe gelöst/m2/hr. Die vollständige Auflösung von Magnetit, Maghemit, Haematit, und Goethit wird durch das Kubikwurzelgesetz beschrieben, während es für die von Lepidokrokit nicht gilt. [U.W.]
Résumé
La dissolution de magnétite, maghémite, hématite, goethite, lépidocrocite, et d’akaganéite synthétiques était plus rapide dans HCl que dans HClO4. En présence d’H+, l’ion Cl− a augmenté l’allure de dissolution, mais l’ion ClO4− n’avait aucun effet, suggérant que la formation de complexes Fe-Cl de surface aide la dissolution. L’effet de la température sur l’allure de dissolution peut être décrite par l’équation d’Arrhenius avec les allures de dissolution dans l’ordre suivant: lépidocrocite > magnétite > akaganéite > maghémite > hématite > goethite. Les énergies d’activation et les facteurs de fréquence pour ces minéraux sont 20,0, 19,0, 16,0, 20,3, 20;9, 22,5, kcal/mole et 5,8 × 1011, 1,8 × 1010, 7,4 × l07, 5,1 × 1010, 2,1 × 1010, 3,0 × 1011 g Fe dissolu/m2/hr, respectivement. La dissolution complète de magnétite, maghémite, hématite, et de goethite est bien décrite par la loi de racine cubique, tandis que celle de la lépidocrocite ne l’est pas. [D.J.]
Similar content being viewed by others
References
Atkinson, R. J., Posner, A. M., and Quirk, J. P. (1968) Crystal nucleation in Fe(III) solutions and hydroxide gels: J. Inorg. Nucl. Chem. 30, 2371–2381.
Atkinson, R. J., Posner, A. M., and Quirk, J. P. (1977) Crystal nucleation and growth in hydrolyzing iron(III) chloride solutions: Clays & Clay Minerals 25, 49–56.
Cornell, R. M., Posner, A. M., and Quirk, J. P. (1974) Crystal morphology and the dissolution of goethite: J. Inorg. Nucl. Chem. 36, 1937–1946.
Cornell, R. M., Posner, A. M., and Quirk, J. P. (1975) The complete dissolution of goethite: J. Appl. Chem. Biotech. 25, 701–706.
Cornell, R. M., Posner, A. M., and Quirk, J. P. (1976) Kinetics and mechanisms of the acid dissolution of goethite (α-FeOOH): J. Inorg. Nucl. Chem. 38, 563–567.
Deer, W. A., Howie, R. A., and Zussman, J. (1962) Rock-Forming Minerals, Vol. 5. Non-Silicates: Longmans, London, 371 pp.
Fischer, W. R. (1973) Die Wirkung von zweiwertigen Eisen auf Auflosung und Umwandlung von Eisen(III)-hydroxiden: in Pseudogley and Gley: Genesis and Use of Hydromorphic Soils, E. Schlichting and V. Schwertmann, eds. Comm. Vard VI. Verlag Chemie, Weinheim/Bergstraase, Germany, 37–44.
Glasstone, S., Laidler, K. J., and Eyring, H. (1941) The Theory of Rate Processes: McGraw Hill, New York, 661 pp.
Hixson, A. W. and Crowell, J. H. (1931) Dependence of reaction velocity upon surface agitation. Ind. Eng. Chem. 23, 923–981.
Klug, H. P. and Alexander, L. E. (1954) X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials: Wiley, New York, 716 pp.
Krestov, G. A., Shormanov, V. A., and Pimenova, N. I. (1973) Kinetic study of the dissolution of α-iron(III) oxide in aqueous solutions of inorganic acids, Izv. Vyssh. Ucheb. Zaved. Khim. Khim. Tekhnol. 16, 377–381.
Kullerud, G., Donnay, G., and Donnay, J. D. H. (1969) Omission solid solution in magnetite: Kenotetrahedral magnetite: Z. Kristallog. 128, 1–17.
Mackay, A. L. (1962) β-Ferric oxyhydroxide-akaganeite: Mineral Mag. 33, 270–280.
McKeague, J. A., Brydon, J. E., and Miles, N. M. (1971) Differentiation of forms of extractable iron and aluminum in soils: Soil Sci. Soc. Amer. Proc. 35, 33–38.
Mitchell, B. D., Farmer, V. C., and McHardy, W. J. (1964) Amorphous inorganic materials in soils: Adv. Agron. 16, 327–383.
Murphy, P. J., Posner, A. M., and Quirk, J. P. (1976) Characterization of partially neutralized ferric Perchlorate solutions: J. Colloid Interface Sci. 56, 298–311.
Schwertmann, V. and Taylor, R. M. (1977) Iron oxides: in Minerals in Soil Environments, J. B. Dixon and S. B. Weed, eds., Soil Science Society of America, Madison, Wisconsin, 145–176.
Sidhu, P. S., Gilkes, R. J., and Posner, A. M. (1978) The synthesis and some properties of Co, Ni, Zn, Cu, Mn, and Cd substituted magnetites: J. Inorg. Nucl. Chem. 40, 429–435.
Vogel, A. I. (1961) A Textbook of Quantitative Inorganic Analyses, Longmans, London, 1216 pp.
Watson, J. H. L., Cardell, R. R., and Heller, W. (1962) The internal structure of colloidal crystals of β-FeOOH and remarks on their assemblies in Schiller layer: J. Phys. Chem. 66, 1757–1763.
Author information
Authors and Affiliations
Additional information
Deceased August 1980.
Rights and permissions
About this article
Cite this article
Sidhu, P.S., Gilkes, R.J., Cornell, R.M. et al. Dissolution of Iron Oxides and Oxyhydroxides in Hydrochloric and Perchloric Acids. Clays Clay Miner. 29, 269–276 (1981). https://doi.org/10.1346/CCMN.1981.0290404
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1981.0290404