Switching Colors with Electricity
By Roger J. Mortimer
Electrochromic materials can be used in glare reduction, energy conservation and chameleonic fabrics
Electrochromic materials can be used in glare reduction, energy conservation and chameleonic fabrics

DOI: 10.1511/2013.100.38
In response to a small electrical voltage (typically around 1 volt), electrochromic materials will change, evoke or bleach their color. The electricity induces in the material a process of either reduction (gain of electrons) or oxidation (loss of electrons). A chemical has a characteristic range of energies over which it will interact with wavelengths in the electromagnetic spectrum, but these reduction or oxidation processes (collectively called redox reactions) alter the energy bands the chemical will absorb. In electrochromic materials, the change corresponds to the visible region of the electromagnetic spectrum.

Image reprinted from A. L. Dyer et al., ACS Applied Materials and Interfaces 3:1787, with permission of the American Chemical Society.
When a thin film of an electrochromic material is incorporated into a circuit, it forms a color-switchable electrochemical cell. The material is deposited at one electrode, which is combined with a counter-electrode. Alternately, the electrochromic material can be dissolved in an electrolyte solution between the electrodes. Electrically charging and discharging the circuit induces redox and hence color changes (see Figure 2).
Commercial forms of electrochromic devices already exist. They include mirrors on several million cars that automatically dim to eliminate glare, and adjustably darkening “smart” airplane windows to reduce cabin brightness. Other proposed applications include multicolor displays, protective eyewear, camouflage materials and chameleonic fabrics. The materials also have great potential for energy conservation. A building could conserve power use with, say, an electrochromic coating on its roof that switches from a heat-absorbing dark color in winter to a reflective light color in summer, or an electrochromic glazing on its windows that could darken to keep out sunlight during the brightest part of the day.
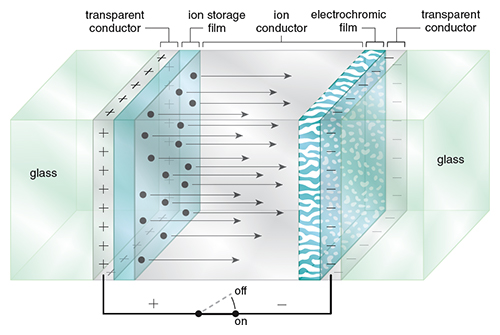
Illustration by Tom Dunne.
One advantage of electrochromic devices is their low power use. Once the color change has been effected, the new redox state exists with little or no input of power in what is called a “memory effect.” In addition, the materials can often be switched between several shades or colors, potentially simplifying devices that might otherwise need to use color filters. With recent advances in electrode technology, the switching times in some cases have been reduced to around 200 milliseconds, making them responsive enough for practical use.
Although the electrochromic effect was observed as far back as the early 19th century, it was not until the 1960s that the mechanism was understood and the materials became an active topic of research. Since then, five major classes of electrochromic materials have been developed.
The addition of oxygen to the so-called transition metals—metals found in the center groups of the periodic table— results in the formation of metal oxides. When these metal oxides are prepared as thin films, they can be reversibly electrochemically switched between near-colorless and highly colored states. In most cases, the reduced (following a gain of electrons) metal oxide is the highly colored state, which results from intense optical absorption in the visible spectral region due to the transfer of electrons between metal atoms with different valences (the outermost energy band in which they have electrons). This mechanism is the same one that causes sapphires to have their brilliant blue color.
A prominent example of this class of electrochromic material is tungsten oxide (WO3). The maximum oxidation state for tungsten is 6 (meaning six electrons have been removed), usually denoted in Roman numerals as WVI so as not to confuse it with formal charges. In this state, WO3 is pale yellow as a thin film, with its electronic absorption band in the ultraviolet region. On electrochemical reduction, WV sites are generated to give the electrochromic effect. Although there is still controversy about the detailed coloration mechanism, it is generally accepted that a key step is the injection and extraction of electrons and either protons or inert metal cations (positive ions). When the fraction of tungsten switched from the WVI to WV states is low, the films have a deep blue color, caused by charge transfer between the valence bands of adjacent WV and WVI sites. At higher switching fractions, however, insertion irreversibly forms a metallic “bronze” that is red or golden in color.
Viologens are salts of a carbon-based chemical called 4,4´-bipyridine, which consists of a bonded pair of carbon rings with a nitrogen atom substituted for one carbon in each ring (the numbers indicate the position of the nitrogen in the first and second rings, respectively, which in this case are the outside points). In addition to electrochromic properties, viologens have applications as herbicides, indicators of redox state in chemical reactions and mediators of electron transfer for substances bound to an electrode. The chemical 4,4´-bipyridine is readily available and is relatively easy to manipulate, which has allowed for intensive research into the electrochromic properties of viologens. The main prototype is called methyl viologen and its formula is N,N´-dimethyl-4,4´-bipyridylium (meaning that each nitrogen is cationic and is attached to a methyl group, CH3). Other simple symmetrical bipyridylium species are called “substituent” viologen. Of the three common viologen redox states, the dication is colorless when pure unless optical charge transfer with the counter-anion occurs. Reduction forms a radical cation, where unbound electrons usually make species highly chemically reactive. However, in this case the cation is quite stable: The overlapping electron orbitals of the central bipyridyl molecule allow the radical electron to delocalize and move through the framework, while the nitrogen atoms commonly bear some of the charge and thus keep the structure more electrically balanced.
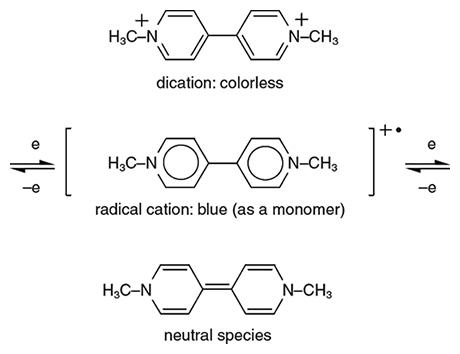
Illustration by Tom Dunne.
Viologen radical cations are vividly colored; they have what is called a high molar absorption coefficient (a measure of how intensely they absorb light of a certain wavelength) owing to charge transfer between the nitrogens. Suitable choice of nitrogen substituents in viologens to attain the appropriate molecular orbital energy levels can, in principle, allow color choice of the radical cation. For example, a methyl group or another alkyl group (methyls are the simplest form of alkyls; other alkyl groups include C2H5 (ethyl), C3H7 (propyl), and so forth) gives a blue-violet color, but using a cyanophenyl group (carbon triple-bonded to nitrogen, attached to a carbon ring) gives an intense green color. However, reactions must be controlled so as not to reduce viologens by more than one oxidation state. Otherwise, the intensity of the exhibited color will be low because no charge transfer or internal transition corresponding to visible wavelengths is available.
Molecules are said to be conjugated when they have alternating single and double bonds, and the electron orbitals of their constituent atoms are connected in such a way that they allow electrons to be delocalized, moving freely throughout the molecule. Certain aromatic molecules, which have an evenly distributed electron density, are resonance-stabilized from this electron delocalization, meaning they have a stability stronger than would be expected from conjugation alone. A class of these molecules is largely based on five-carbon rings with one substitution. Examples include thiophene (with a sulfur substitution), pyrrole (nitrogen substitution) and furan (oxygen substitution). Other chemicals (such as carbazole, azulene and indole) are based on six-carbon rings combined with five-carbon ones, with various substitutions. Another member of this class is aniline, a six-carbon ring joined to NH2. Chemical or electrochemical oxidation of these substances produces electroactive conjugated conducting polymers.
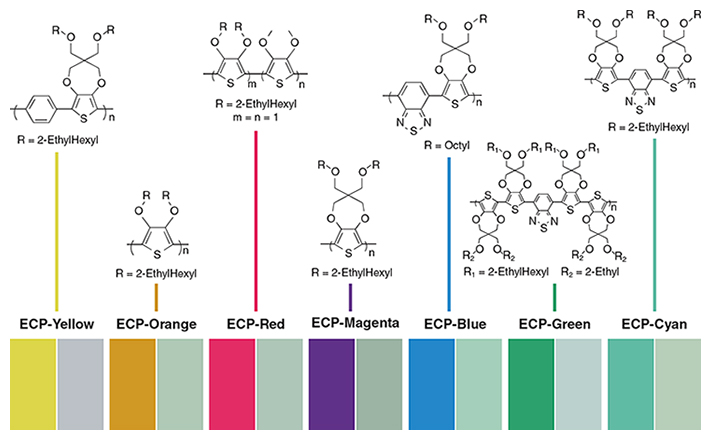
(Image reprinted from A. L. Dyer et al., ACS Applied Materials and Interfaces 3:1787, with permission of the American Chemical Society.)
Many of these materials are electrochromic as thin films, with polymers of thiophenes and pyrroles receiving the most attention in recent years. In the conducting oxidized state, conjugated conducting polymers have positive charge carriers, are charge balanced (or doped) with counter-anions (negative ions) and have delocalized electron band structures. When placed in an electrolytic solution, electrochemical reduction—with concurrent counter-anion egress to, or cation ingress from, the electrolyte—removes the electronic conjugation, resulting in the undoped, electrically neutral, insulating form.
For the undoped polymer, the energy difference (called the bandgap) between the highest occupied electron band (the valence band) and the lowest unoccupied band (the conduction band) determines the electrochromic properties. The color change or contrast between doped and undoped forms of a polymer depends on the magnitude of the bandgap. Thin films with a gap of about 400 nanometers (also given in terms of the energy gained by an electron moving across a potential, in this case about 3 electron volts) are colorless and transparent in the undoped form, whereas in the doped form they generally absorb visible radiation. Those with a bandgap of 650 to 730 nanometers (or 1.7 to 1.9 electron volts) are highly absorbing in the undoped form; after doping, the absorption of free charge carriers is relatively weak in the visible region because it is transferred to the near infrared. Polymers with intermediate bandgaps have distinct optical changes throughout the visible region and can exhibit several colors.
Like viologens, several examples of metal coordination complexes involve a form of pyridine, but in this case the type is 2,2´-bipyridine (the nitrogens are now at the lower inside corners of the carbon rings). The chemical takes on the role of a ligand, a molecule that binds to a central metal atom, and a number of ligand branches can join to one metal. The metal can be one of several that are in the II oxidation state, such as iron, ruthenium or osmium, and the resulting metal coordination complexes are, respectively, red, orange and green in color. The name of this compound refers to the type of bonding between ligand and metal, which is called a coordinate covalent bond: The ligand donates a lone pair of electrons into an empty orbital on the metal.
Metal coordination complexes show promise as electrochromic materials because of their intense coloration and redox reactivity. They have low energy barriers to moving around electrons by various mechanisms, including charge transfer from metal to ligand, from ligand to ligand, or between valence bands of adjacent ions, as well as other related visible-region electronic transitions. Because these transitions involve valence electrons, chromophoric characteristics are altered or eliminated on oxidation or reduction of a complex. Electrochromicity results from loss of the absorption band in metal to ligand charge transfer on switching the central metal from the II to the III redox state.
An important class of industrial pigments, called phthalocyanines, also have been investigated as electrochromics. Phthalocyanines have at their core a nitrogen-based compound that looks a bit like a four-leaf clover with extra carbon rings attached to each leaf. They can bind an atom of a transition metal at the open center of the clover to form complexes called metallophthalocyanines. One such metal, lutetium, forms the complex lutetium bis(phthalocyanine), and thin films of this material have been discovered to be polyelectrochromic. The initially deposited films are vivid green, then are oxidized through a yellow-tan form to a red form. Upon reduction, the green state can be switched first to a blue redox form, and then to a violet-blue color.
Prussian blue was first created in the early 1700s. As the earliest modern synthetic pigment, it has an extensive history of use in the formulation of paints, lacquers, printing inks, typewriter ribbons and carbon paper. It has the molecular formula C18Fe7N18 and it forms crystals with a cubic lattice structure. Its synthesis is based on the hexacyanoferrate anion, which, like metal coordination complexes, has a central iron atom surrounded by six ligands of carbon triple-bonded to nitrogen.

Photograph courtesy of Gesimat GmbH.
The pigment is a mixed-valence compound, meaning it contains an element that is present in more than one oxidation state. That element is iron, which exists as FeIII and FeII, and charge transfer between the valence bands of these ions is what gives rise to the intense blue color. Oxidation or reduction generates green-yellow and bleached color states. Similar pigments, generally called metal cyanometallates, form an important class of mixed-valence compounds.
Color switching in materials by electron gain or loss has been known since the early 19th century. In 1815 Jöns Jacob Berzelius of the Karolinska Institute in Sweden reported that pure, pale-yellow tungsten oxide (WO3) changed color on reduction to a deep blue state when warmed under a flow of dry hydrogen gas. Today tungsten oxide remains the most intensely studied electrochromic material. In 1824 Friedrich Wöhler in Germany effected a similar chemical reduction of tungsten oxide with sodium metal. Wöhler also observed a metallic luster in lithium combined with tungsten oxide (LiWO3) and, thinking that this hue was due to the formation of metallic alloys, coined the phrase “tungsten bronzes.”

Images courtesy of Ynvisible.
In 1843, Scottish engineer Alexander Bain patented a primitive form of fax transmission that was based on the electrochemical generation of a Prussian blue compound. It involved a stylus of pure soft iron resting on damp paper preimpregnated with potassium ferrocyanide. In an electrical circuit, electro-oxidation of the (positive) iron tip forms ferric ions from the metal, which consumes the iron as it combines with ferrocyanide ions to produce Prussian blue pigment. Thus the iron electrode generates a track of dark-colored deposit wherever the positive stylus touches the paper.
This effect is analogous to the photochemical color change in an early form of photography devised in 1842 by Sir John Frederick William Hershel in England, who called his process “cyanotype.” By the 1880s, so-called blueprint paper was manufactured on a large scale, as engineers and architects required copies of architectural drawings and mechanical plans. This widespread availability revived cyanotype as a photographic process for large reproductions, a position it held until the late 20th century. The process became known by the common name “blueprint,” a word that became an English synonym for “plan.”
The year 1929 saw the first suggestion of an electrochromic device involving the electrochemical formation of color, presented in a London patent. The method involved the electrogeneration of molecular iodine from iodide ions. The molecular iodine then effects the chemical oxidation of a dye precursor, thus forming a bright color.
In 1930 the first recorded color change (colorless to blue) following electrochemical (rather than simply chemical) reduction of a solid, tungsten oxide, coated onto an electrode was reported. By 1942, there was a patent for electrochromic printing—“electrolytic writing paper”—in which paper was preimpregnated with particulate tungsten oxide and/or molybdenum oxide. A blue-gray image forms following an electron-transfer reaction: In effect, the electrode acts as a stylus, forming color wherever it transverses the paper. In 1951, Eugene O. Brimm and his colleagues at Union Carbide and Carbon Corporation in New York effected reversible color changes for sodium tungsten oxide immersed in aqueous acid. Next, in 1953, Thaddeus Kraus at Balzers AG in Lichtenstein advocated the reversible color-bleach behavior of tungsten oxide as the basis for a visual display device.
John R. Platt of the University of Chicago coined the term electrochromism in 1961, based on work he had done at Bell Telephone Laboratories in New Jersey. However, he used the word here to denote a different color-switching effect—color generated via a molecular Stark effect, which shifts and splits the spectral absorption lines of molecules on application of a strong electric field, applied to polarizable dye molecules.
In 1962, Solomon Zaromb of Philco Corporation in Pennsylvania published studies on the electrodeposition of silver ions from aqueous solutions onto a transparent conducting glass surface. When the silver ions in solution are reduced to silver metal, they form a film on the glass. The film is then stripped off by oxidation, returning the silver ions to solution. These films would be expected to reflect incident light if continuous, or to become optically absorbing if the silver is particulate. Zaromb called his system an “electroplating light modulator” and explicitly said it represented a “viable basis for a display.” Although the deposition of reflective metals seems like an attractive idea for energy management in architecture, the work has only been followed by studies on bismuth, bismuth-copper codeposits and lead.
Probably the first company to seek commercial exploitation of an electrochromic product was the Dutch division of Philips; their interest began in the early 1960s, and their first patent was awarded in 1973. Their prototype image-display device used an aqueous organic viologen, heptyl viologen (1,1’-n-heptyl-4,4’-bipyridylium, where the heptyl (C7H15) group is a long, wavy chain of carbons and hydrogens). In parallel, Imperial Chemical Industries in England developed similar viologen-based devices. Other devices based on heptyl viologen were being investigated by Donald J. Barclay’s group at IBM and by Texas Instruments in Dallas in the 1970s, although the latter work was not published until after their program was discontinued.
As none of these studies attracted much attention, most researchers now probably attribute the first widely accepted suggestion of an electrochromic device to Satyen K. Deb, then at American Cyanamid Company in Stamford. In 1969, Deb formed electrochromic color by applying an electric field of 1,000 volts per centimeter across a thin film of tungsten oxide that had been vacuum deposited on quartz; he called the effect “electrophotography.”
Deb’s film of tungsten oxide was open to the air rather than immersed in an ion-containing electrolyte solution, suggesting that the mobile counter-cations necessary for the reaction might have come from water either adsorbed onto the film or incorporated within it, which was ionized simultaneously with the tungsten oxide. At the time, Deb suggested the color arose from F-centers, defects in a crystal that are filled by electrons, which tend to absorb color in the visible spectrum. Crystals of metal halides (compounds formed by binding a metal with one of the elements in the group called halogens—fluorine, chlorine, bromide, iodine or astatine) are known to form colors by this mechanism when they are heated or irradiated in an electric field.
These days most workers cite Deb’s later paper, published in 1973, as the true birth of electrochromic technology, because in it the electrochromic device, the film of tungsten oxide, is immersed in an ion-containing electrolyte. Since the 1970s, there has been intense interest in tungsten oxide–based electrochromic systems, particularly with the development of many prototype “smart” windows. Along with Deb, Claes G. Granqvist of Uppsala University in Sweden is an international authority on metal oxide electrochromics.
Following a 1978 report by Vernon D. Neff of Kent State University on the preparation of Prussian blue as a thin film and its color changes on electrochemical switching, such cyanometallates have been intensively studied for their electrochromic properties.
Meanwhile, since the late 1970s, the electrochromism of organic materials has developed momentum, especially in the field of conjugated conducting polymers. In 1979 came the first account of an electrochromic conjugated conducting polymer, when G. Brian Street and his coworkers at IBM in San Jose announced the electrosynthesis of thin-film poly(pyrrole). Thin films of these polymers may now be prepared both by electrochemical oxidation from monomer solution, and from soluble polymer solutions, the latter of which is more amenable to large-scale production.
From the 1980s to now, John Reynolds, now at the Georgia Institute of Technology, and his coworkers have been especially influential in the field of conjugated conducting polymers. Through their systematic development of material structure and properties, Reynolds’s group has manipulated the composition of thiophene-based electrochemical polymers to create the first set of soluble materials that exhibit the whole gamut of possible colors. This achievement in color-changing polymers is a scientific milestone that is expected to initiate development of a greater variety of low-cost organic electronic displays and tinted window applications.
Electrochromic devices (ECDs) operate as rechargeable electrochemical cells, with each containing a minimum of two electrodes separated by a layer of ion-containing electrolyte in liquid, gel or solid form. The electrochromic material can be either in solution or a solid film. Color switching takes place on charge or discharge by application of an appropriate electrical potential. ECDs with liquid electrochromics operate by electrolysis of the soluble electrochromic materials.
ECDs are designed to operate in either absorptive/transmissive or reflective modes. All ECDs require at least one optically transparent electrode. Devices operating in an absorptive/transmissive mode—such as spectacles, goggles, visors or smart windows—require a second optically transparent electrode as the rear electrode. Devices operating in a reflective mode—such as information displays and antiglare mirrors—employ a polished metal (or a reflective coating) in place of or behind the rear electrode.
For absorptive/transmissive devices, the major color change occurs at one of the electrodes (called the primary electrode). The redox reaction at the opposite (or secondary) electrode is chosen either to be one where there is imperceptible visible color change, or to use an additional electrochromic material where the change in color is complementary to that at the primary electrochromic electrode, thus providing enhancement of the colored/bleached contrast. Complementary-coloring ECDs that have been investigated include, for example, those with thin films of Prussian blue (colorless to blue on oxidation) and tungsten oxide (colorless to blue on reduction). Another combination is the conjugated conducting polymer polyethylenedioxythiophene (also called PEDOT) (light blue to deep blue on reduction) with poly(butyl viologen) (colorless to purple on reduction).
In a recent advance, reflective-type ECDs on metallized substrates have been investigated, where patterned electrodes are prepared using line patterning, screen printing and metal vapor deposition techniques. For these devices, the secondary electrode color change is hidden.
Electrode substrates consist of an optically transparent, electrically conducting film coated onto glass or the flexible polymer polyethylene terephthalate (PET). The film is usually a transparent conducting oxide such as indium oxide, which has been doped with tin, fluorine or antimony.
The layer of electrolyte between the two electrodes in an ECD must be ionically conductive but an electronic insulator. Additional requirements are transparency in the wavelength range used, a wide electrochemical window and a low volatility. In ECDs that use thin-film electrochromic materials, the electrolyte also supplies the mobile counter-ions that enter and leave the facing solid-electrochromic material layers during coloration and bleaching.
An example of a device with this type of structure is the smart window that has been developed for architectural applications by Gesimat GmbH in Germany. In these windows, the electrochromic layers are solids of tungsten oxide and Prussian blue, separated by ion-conducting poly(vinylbutyral)—a standard material for glass lamination—as a solid polymer electrolyte. The choice of tungsten oxide with Prussian blue provides a complementary-coloring mechanism. When the Prussian blue–coated electrode is the anode (where current flows into the cell), and the tungsten oxide-coated electrode is the cathode (where current flows out), then both materials are in their blue state. On switching polarity, both electrodes decolorize. Gesimat has recently started a pilot production line to manufacture electrochromic glass laminates with sizes up to 2,400 millimeters by 1,000 millimeters.

Photograph courtesy of Gentex Corporation.
But the stand-out commercial success of electrochromic devices is Gentex Corporation’s automatically dimming ”Night Vision Safety” (NVS) mirrors for glare elimination. In the Gentex system, a glass surface coated with tin-doped indium oxide (with its conductive side facing inward) and a reflective metallic surface, spaced a fraction of a millimeter apart, form the two electrodes of the cell. The thin space between the electrodes is filled with a gel formulation containing two electrochromic materials, without additional supporting electrolyte. The exact composition of the mirror is obscured within patents, but it is possible to infer that the two electroactive chemical species are a cathodic-coloring substitutent (cationic) viologen, and an anodic-coloring negatively charged thiazine dye (based on a six-carbon ring with a nitrogen and sulfur substitution) or a phenylenediamine species (a carbon ring attached to two NH2 groups, and a common ingredient in hair dyes).
After switching the mirror on, the species migrate to their respective electrodes, and an intense blue-green color— a combination of the two colored states of the electrochromic materials—is generated, which reduces glare by both removing some of the light intensity and absorbing some of the wavelengths. The migration transport process takes place because no additional inert supporting electrolyte is present. Once the dual electrochromic coloration process has begun, the products diffuse away from their respective electrodes and meet in the intervening solution, where a mutual reaction regenerating the original uncolored species takes place. This type of ECD therefore requires a continuous small current for replenishing the colored electroactive species lost by their mutual self-erasing redox reaction. The regeneration reaction obviates any need to electro-bleach the mirror, because color fades spontaneously on switch-off. For this reason, the Gentex NVS is sometimes called a self-erasing mirror. United States regulations also require the “failure mode,” on loss of current, to be the clear condition.
Although not part of the electrochromic phenomenon, the ingenious control system in the Gentex NVS mirror is worth noting. A photosensitive detector is placed facing rearward to monitor any dazzling incident light. However, in daylight it would also be triggered, resulting in inappropriate darkening of the mirror. This outcome is avoided by the use of a second forward-looking detector, which on seeing daylight, is programmed to cancel any operation of the controlling detector, and thus it responds only in the dark of night.

Photograph courtesy of Gentex Corporation.
In combination with PPG Aerospace, Gentex has now expanded its product line with interactive windows for use in aircraft. Designed to replace conventional plastic pull-down shades, these dimmable window systems afford operating efficiencies for airlines while allowing flight crews and passengers enhanced control of their environment. Marketed by PPG Aerospace under the name Alteos Interactive Window Systems, the world’s first electrochromic window shades switch from a bright, clear state to an extremely dark state, or to a comfortable intermediate level in between, at the touch of a button. In 2011 such smart windows premiered in commercial aircraft on the Boeing 787 Dreamliner and in business aircraft on the Hawker Beechcraft King Air 350i.
Research and development in electrochromic materials continues to expand both in terms of the range of electrochromic materials and device structures that are being reported and in the novel applications being proposed.
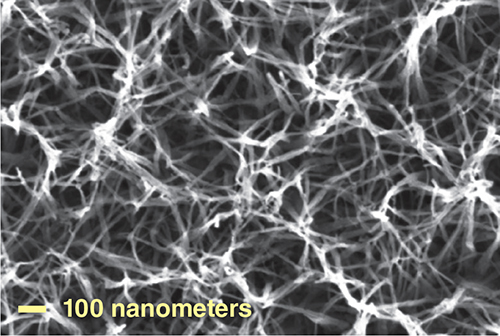
Image reprinted from J. Chen et al., ACS Nano 8:6633, with permission of the American Chemical Society.
A major long-term goal of many contemporary electrochromism studies is the provision of large-scale electrochromic windows for light attenuation in buildings at modest expenditure which if applied widely, would save extensive air-conditioning energy costs and improve office comfort. Several groups have been working on improving the transparency of electrochromic windows in their uncolored state so they are not too dark. For instance, Kuan-Jiuh Lin and his colleagues at National Chung Hsing University in Taiwan have developed a way to grow nanowires of titanium oxide on glass in a single processing step. The porosity of the nanowires allows the windows to maintain a lower refractive index, and they color to a pale gray when sun blocking is desired.
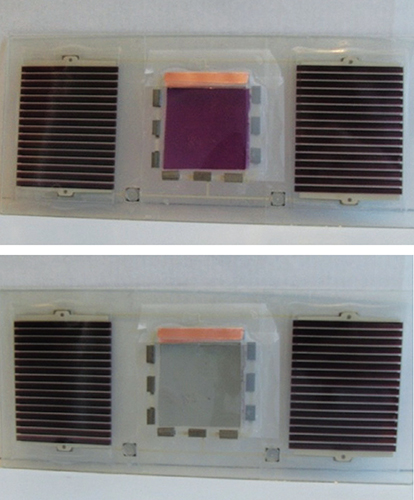
Photographs reprinted from J. Jensen et al., Journal of Polymer Science Part B: Polymer Physics 50:536, with permission of the American Chemical Society.
Early electrochromics research was directed to information-display applications, but such efforts became of less interest as liquid crystal displays began to dominate the market. It seems unlikely that electrochromics will be used in large-screen televisions, but they may be useful in low-cost multicolored displays for other applications, such as electronic paper, signs or reusable price labels. Using their full color range of soluble electrochromic polymers, Reynolds’s group has already been able to use airbrush paint sprayers or ink-jet printing to quickly create prototype ECDs. By spraying solutions through masks, they have been able to pattern a substrate with multicolored layered films that display a variety of shades. In 2012, Reynolds and his colleagues used roll-to-roll printing methods to coat flexible PET substrates, and they incorporated a printed photovoltaic device to create a self-powered ECD.
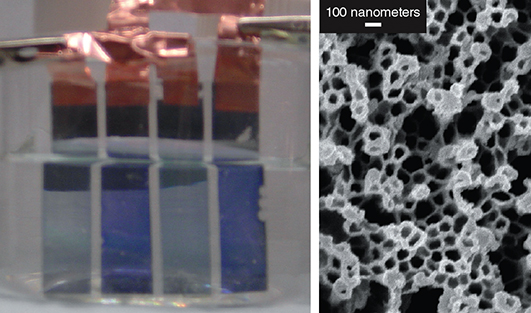
Images reprinted from S. I. Cho et al., Nanotechnology 18:405705, with permission of the Institute of Physics.
One chronic limitation with ECDs has been their relatively long switching times, which is limited by the diffusion rate of counter-ions during the redox process. Sang Bok Lee and his colleagues at the University of Maryland have been exploring nanotubes as a way to increase the switching speeds. If the thickness of electrochromic films is reduced, the diffusion distance of the ions is decreased—but thinner films alone often don’t have enough contrast to show good coloration. Lee and his colleagues instead grow hollow tubes of electrochromic polymers with walls only 10 to 20 nanometers thick but several hundred nanometers in length. The ions only have to diffuse through the wall thickness to create the redox reaction, with resulting switching times on the order of 10 milliseconds. The nanotube length allows for good color contrast, and in 2012 Lee’s group figured out methods for growing hybrid nanotubes of several polymers to further increase coloring. They have been able to demonstrate films of nanotubes that work in both reflective and transmissive modes, potentially making the arrangement useful for both displays and windows.

Images reprinted from M. A. Invernale et al., ACS Applied Materials and Interfaces 2:296, with permission of the American Chemical Society
Color-changing fabrics are another goal for electrochromics. Chameleonic materials could be used for adaptive camouflage or wearable displays, or simply for fashion. Because electrochromic polymers can be put into solution, they can be stenciled onto flexible fabric substrates, and low power requirements mean that flexible electrodes could be used to switch the colors. Gregory A. Sotzing and his group at the University of Connecticut have been working on an electrochromic spandex where color intensity is modulated by the stretch of the fabric. The spandex itself becomes the electrode when it is soaked in a conductive polymer solution; two layers of spandex were sandwiched together over a film of gel electrolyte. A coating of electrochromic polymers on both surfaces made for a reversible fabric that could have a different color on each side. Sotzing’s group is also working on electrochromic threads and fibers for direct weaving into fabrics.
Despite their long history, the past few years seem to have shown an exponential leap in the potential for electrochromic materials. An increase in their color range, switching speeds and ease of processing seems to be catching up with their ubiquity, low power use and low cost. Their future, it seems, is not only bright but also high-contrast and colorful.
Click "American Scientist" to access home page
American Scientist Comments and Discussion
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
If we re-share your post, we will moderate comments/discussion following our comments policy.