Abstract
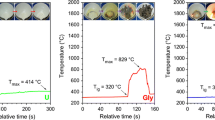
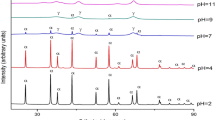
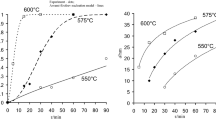
Nanocrystalline GdFeO3 powder was synthesized by a combustion technique, using glycine as the fuel and the corresponding metal nitrates as oxidants. Five different molar ratios of fuel-to-oxidant were chosen to study the effect of fuel content on the phase formation and the powder properties. The powders after calcination were characterized by x-ray diffraction (XRD) and crystallite sizes calculated by x-ray line broadening. The crystallite sizes for the phase pure products after calcination at 600 °C were in the range 40-65 nm. The transmission electron microscopy observations clearly highlight the pronounced crystallinity for the propellant chemistry samples. The nature of the agglomerates was investigated by light scattering studies. The lattice thermal expansion behavior was also studied by high-temperature XRD.
Similar content being viewed by others
References
L.L. Beecroft and C.K. Ober: Nanocomposite materials for optical applications. Chem. Mater. 9, 1302 (1997).
W.H. Rhodes: Agglomerate and particle-size effects on sintering yttria-stabilized zirconia, J. Am. Ceram. Soc. 64, 19 (1981).
H. Weller: Colloidal semiconductors Q-particles: Chemistry in the transition region between solid state and molecules. Angew. Chem. Int. Ed. Engl. 32, 41 (1993).
R.W. Siegel, S. Ramasamy, H. Hahn, Z. Li, T. Lu and R. Gronsky: Synthesis, characterization, and properties of nanophase TiO2. J. Mater. Res. 3, 1367 (1988).
H. Hahn, J. Logas and R.S. Averback: Sintering characteristics of nanocrystalline TiO2. J. Mater. Res. 5, 609 (1990).
G.L. Bauerle and K. Nobe: Ind. Eng. Chem. Prod. Rd. 13, 185 (1974).
A.H. Bobeck: Properties and device applications of magnetic domains in orthoferrites. Bell System Technol. J. 46, 1901 (1967).
S. Mathur, H. Shen, N. Lecerf, A. Kjekshus, H. Fjellvag and G. Goya: Nanocrystalline orthoferrite GdFeO3 from a novel heterobimetallic precursor. Adv. Mater. 14, 1405 (2002).
S. Mathur, M. Veith, R. Rapalavicuite, H. Shen, G. Goya, W. Filho and T. Berquo: Molecule derived synthesis of nanocrystalline YFeO3 and investigations on its weak ferromagnetic behavior. Chem. Mater. 16, 1906 (2004).
D.S. Schmool, N. Keller, M. Guyot, R. Krishnan and M. Tessier: Evidence of very high coercive fields in orthoferrite phases of PLD grown thin films. J. Magn. Magn. Mater. 195, 291 (1999).
J.J. Kingsley, K. Suresh and K.C. Patil: Combustion synthesis of fine-particle metal aluminates. J. Mater. Sci. 25, 1305 (1990).
R.L. Pederson, L.A. Chick, and G.J. Exarhos: Method of making metal oxide ceramic powders by using a combustible amino acid compound, U.S. Patent No. 5 114 702 (May 19, 1992).
S. Bhaduri, S.B. Bhaduri and E. Zhou: Auto ignition synthesis and consolidation of Al2O3-ZrO2 nano/nano composite powders. J. Mater. Res. 13, 156 (1998).
S.V. Chavan and A.K. Tyagi: Preparation and characterization of Sr0.09Ce0.91O1.91, SrCeO3, Sr2CeO4 by glycine-nitrate combustion: A crucial role of oxidant-to-fuel ratio. J. Mater. Res. 19, 3181 (2004).
S.S. Manoharan and K.C. Patil: Combustion synthesis of metal chromite powders. J. Am. Ceram. Soc. 75, 1012 (1992).
S.R. Jain, K.C. Adiga and V.R. Pai Verneker: A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame 40, 71 (1981).
R.D. Purohit, B.P. Sharma, K.T. Pillai and A.K. Tyagi: Ultrafine ceria powders via glycine-nitrate combustion. Mater. Res. Bull. 36, 2711 (2001).
S.V. Chavan, K.T. Pillai, and A.K. Tyagi: Combustion synthesis of nanocrystalline yttria: Tailoring of powder properties. (unpublished).
D. Grier and G. McCarthy: JCPDS-47-0067, North Dakota State University, Fargo, ND (1993).
L.A. Chick, L.R. Pederson, G.D. Maupin, J.L. Bates, L.E. Thomas and G.J. Exarhos: Glycine-nitrate combustion synthesis of oxide ceramic powders. Mater. Lett. 10, 6 (1990).
S.V. Chavan and A.K. Tyagi: Combustion synthesis of nanocrystalline yttria-doped ceria. J. Mater. Res. 19, 474 (2004).
R.D. Purohit, S. Saha, and A.K. Tyagi: Nanostructured ceria powders through citrate-nitrate combustion. (unpublished).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chavan, S.V., Tyagi, A.K. Nanocrystalline GdFeO3 via the gel-combustion process. Journal of Materials Research 20, 2654–2659 (2005). https://doi.org/10.1557/JMR.2005.0337
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2005.0337