Abstract
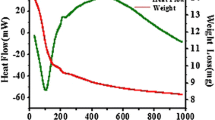
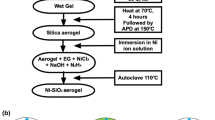
The sol-gel method was used to prepare nickel oxide–silica and nickel–silica nanocomposite materials and the corresponding silica matrices. Different drying conditions were used to obtain aerogel and xerogel materials. The samples were characterized by thermal analysis, x-ray diffraction, N2–physisorption, transmission electron microscopy techniques, and infrared spectroscopy. Aerogel samples had a much higher surface area than the xerogel samples; moreover, different supercritical drying conditions gave rise to a different porous structure, which influenced the size and distribution of the nanoparticles in the matrix.
Similar content being viewed by others
References
R. Birringer, Mater. Sci. Eng. A 117, 33 (1989).
H. Gleiter, J. Appl. Crystallogr. 24, 79 (1991).
R.W. Siegel, J. Phys. Chem. Solids 55, 1097 (1994).
S. Komarneni, J. Mater. Chem. 2, 1219 (1992).
R.E. Newnham, S.E. McKinstry, and H. Ikaua, in Multifunctional Ferroic Nanocomposites, edited by A. Buckley, G. Gallagher-Daggitt, F.E. Karasz, and D.R. Ulrich (Mater. Res. Soc. Symp. Proc. 175, Pittsburgh, PA, 1990), pp. 161–172.
C.J. Brinker and G.W. Scherer. Sol-gel Science (Academic Press, San Diego, CA, 1990).
Sol-Gel technology for Thin Films, Fibers, Preforms, Electronics and Specialty Shapes, edited by C.L. Klein (Noyes Publication, Park Ridge, NJ, 1988).
A. Corrias, G. Mountjoy, G. Piccaluga, and S. Solinas, J. Phys. Chem. B 103, 10081 (1999).
A. Corrias, G. Enans, G. Mountjoy, and G. Paschina, Phys. Chem. Chem. Phys. 5, 1045 (2000).
S. Bruni, F. Cariati, M. Casu, A. Lai, A. Musinu, and G. Piccaluga, Nanostruct. Mater. 11, 573 (1999).
M. Falconieri, G. Salvetti, E. Cattaruzza, F. Gonella, G. Mattei, P. Mazzoldi, M. Piovesan, and G. Battaglin, Appl. Phys. Lett. 73, 288 (1998).
A. Ueno, H. Suzuki, and Y. Kotera, J. Chem. Soc., Faraday Trans. I 79, 127 (1983).
A. Basumallick, K. Biswas, G.C. Das, and S. Mukherjee, J. Mater. Res. 10, 2938 (1995).
M. Keane, Langmuir 13, 41 (1997).
S. Roy, D. Chakravorty, and D.L. Agravol, J. Appl. Phys. 74, 4746 (1993).
G.M. Pajonk and S.J. Teichner, in Aerogels, edited by J. Fricke (Springer Proceedings in Physics, Berlin, Germany, 1986).
G. Ennas, G. Marongiu, G. Paschina, G. Piccaluga, and S. Solinas, Euromat 1999 Proceedings (2000, in press).
S. Brunauer, P.H. Emmet, and E. Teller, J. Am. Chem. Soc. 60, 309 (1938).
B.C. Lippens and J.H. De Boer, J. Catal. 4, 319 (1965).
A. Lecloux and J.P. Pirard, J. Colloid Interface Sci. 70, 265 (1979).
M.M. Dubinin, Q. Rev. Chem. Soc. 9, 101 (1955).
G. Horwarth and K. Kawazoe, J. Chem. Eng. Jpn. 16, 470 (1983).
S. Brunauer, L.S. Deming, W.S. Deming, and E. Teller, J. Am. Chem. Soc. 62, 1723 (1940).
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, and T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985).
E.P. Barret, L.G. Joyner, and P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951).
PDF-2 File, JCPDS International Centre for Diffraction Data, 1601 Park Lane, Swarthmore, PA, 1998.
H.P. Klug and L.E. Alexander, X-ray Diffraction Procedures (Wiley, New York, 1974).
K. Nakamoto, Infrared Spectroscopy of Inorganic and Co-ordination Compounds (Wiley, New York, 1970).
D. Weigel, B. Imelik, and P. Lafitte, Bull. Soc. Chim. Fr. 345 (1962).
O. Clause, M. Kermarec, L. Bonneviot, F. Villain, and M. Che, J. Am. Chem. Soc. 114, 4709 (1992).
M. Prassas, J. Phalippou, and J. Zarzycki, J. Mater. Sci. 19, 1656 (1984).
A.H. Boonstra and J.M.E. Baken, J. Non-Cryst. Solids 109, 1 (1989).
G. Ennas (private communication).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casula, M.F., Corrias, A. & Paschina, G. Nickel oxide–silica and nickel–silica aerogel and xerogel nanocomposite materials. Journal of Materials Research 15, 2187–2194 (2000). https://doi.org/10.1557/JMR.2000.0315
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2000.0315