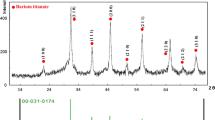
Abstract
Nanocrystalline BaTiO3 particle–polymer hybrid was synthesized by polymerization and hydrolysis of [2-(methacryloxy)ethoxy]triisopropoxytitanium (MEPT) and barium alkoxide. The precursor for hybrid was synthesized from prepolymerized MEPT and barium alkokide, which was then hydrolyzed to form BaTiO3 particle–polymer hybrids below 100 °C. BaTiO3 particles increased in crystallinity when the amount of water for hydrolysis increased. The nanocrystalline particles were identified to be BaTiO3 by electron diffraction. Nanometer-sized BaTiO3 particle–polymer hybrid was shaped to a film with a dielectric constant of 8.2 at 10 kHz. A suspension consisting of the hybrid and silicone oil responded to a direct-current field, exhibiting a typical electrorheological behavior.
Similar content being viewed by others
References
H. Schmidt: Organically modified silicates by the sol-gel process, in Better Ceramics Through Chemistry, edited by C.J. Brinker, D.E. Clark, and D.R. Ulrich (Mater. Res. Soc. Symp. Proc. 32, Elsevier Science Publishing, New York, NY, 1984), p. 327.
H. Schmidt: New type of non-crystalline solids between inorganic and organic materials. J. Non-Cryst. Solids 73, 681 (1985).
Organic/Inorganic Hybrid Materials-2002, edited by C. Sanchez, R.M. Laine, S. Yang, and C.J. Brinker (Mater. Res. Soc. Symp. Proc. 726, Warrendale, PA, 2002).
P.A. Alivisatos: Semiconductor clusters, nanocrystals and quantum dots. Science 271, 933 (1993).
S.W. Charles and J. Popplewell: In Ferromagnetic Materials, Vol. 2, edited by E.P. Wohlfarth (North-Holland, Amsterdam, The Netherlands, 1980), p. 509.
T. Yogo, T. Nakamura, K. Kikuta, W. Sakamoto, and S. Hirano: Synthesis of α-Fe2O3 particle/oligomer hybrid material. J. Mater. Res. 11, 475 (1996).
T. Yogo, T. Nakamura, W. Sakamoto, and S. Hirano: Synthesis of magnetic particle/organic hybrid from metalorganic compounds. J. Mater. Res. 14, 2855 (1999).
T. Yogo, T. Nakamura, W. Sakamoto, and S. Hirano: Synthesis of transparent magnetic particle/organic hybrid film using ironorganics. J. Mater. Res. 15, 2114 (2000).
T. Yogo, S. Yamada, K. Kikuta, and S. Hirano: Synthesis of barium titanate/polymer composites from metal alkoxide. J. Sol-Gel Sci. Technol. 2, 175 (1994).
S. Hirano, T. Yogo, K. Kikuta, and S. Yamada: Processing and properties of barium titanate/polymer hybrid materials by sol-gel method. Ceram. Trans. 68, 131 (1996).
T. Yogo, H. Ukai, W. Sakamoto, and S. Hirano: Synthesis of PbTiO3/organic hybrid from metalorganic compounds. J. Mater. Res. 14, 3275 (1999).
T. Yogo, K. Banno, W. Sakamoto, and S. Hirano: Synthesis of a KNbO3 particle–polymer hybrid from metalorganics. J. Mater. Res. 18, 1679 (2003).
K.S. Mazdiyasni, R.T. Dolloff, and J.S. Smith: Preparation of high-purity submicron barium titanate powders. J. Am. Ceram. Soc. 52, 523 (1969).
W.M. Winslow: Induced fibration of suspensions. J. Appl. Phys. 20, 1137 (1949).
T.C. Halsey: Electrorheological fluids. Science 258, 761 (1992).
G.I. Dzhardimalieva, A.D. Pomogailo, and A.N. Shupik: Interaction of titanium (IV) alkoxy derivatives with unsaturated alcohols. Izv. Akad. Nauk SSSR, Ser. Khim. (Engl. Trans., Bull. Acad. Sci. USSR, Div. Chem.) 34, 411 (1985).
B.D. Cullity: Elements of X-ray Diffraction, 2nd ed. (Addison- Wesley, Reading, MA, 1978), p. 284.
D.C. Bradley, R.C. Mehrotra, and D.P. Gaur: In Metal Alkoxide (Academic, New York, 1978), p. 118.
E. Breitmaier and W. Voelter: Carbon-13 NMR Spectroscopy, 3rd ed. (VCH, New York, 1987), pp.183–232.
G.C. Levy, R.C. Lichter, and G.L. Nelson: Carbon-13 Nuclear Magnetic Resonance Spectroscopy (Wiley Interscience, New York, 1980), p. 196.
J-F. Campion, D.A. Payne, H.K. Chae, J.K. Maurin, and S.R. Wilson: Synthesis of bimetallic barium titanium alkoxides as precursors for electrical ceramics. Inorg. Chem. 30, 3244 (1991).
M. Murata, K. Wakino, and S. Ikeda: X-ray photoelectron spectroscopic study of perovskite titanates and related compounds. J. Electron Spectrosc. Relat. Phenom. 6, 459 (1975).
T. Furukawa, K. Fujino, and E. Fukuda: Electromecanical properties in the composites of epoxy resin and PZT ceramics. Jpn. J. Appl. Phys. 15, 2119 (1976).
A.K. Goswami: Dielectric properties of unsintered barium titanate. J. Appl. Phys. 40, 619 (1969).
H. Block and H. Rattray: Recent developments in ER fluid. In Progress in Electrorheology, edited by K.O. Havelka and F.E. Filisko (Plenum, New York, 1995), p. 19.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yogo, T., Yamamoto, T., Sakamoto, W. et al. In situ synthesis of nanocrystalline BaTiO3 particle–polymer hybrid. Journal of Materials Research 19, 3290–3297 (2004). https://doi.org/10.1557/JMR.2004.0418
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/JMR.2004.0418