Abstract
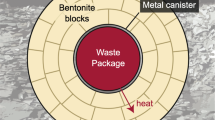
The knowledge of pore water chemistry in the clay barrier is essential for performance assessment purposes in a nuclear waste repository, since the pore water composition controls the processes involved in the release and transport of the radionuclides. The methodology followed to define the representative composition of the FEBEX bentonite pore water is presented in this paper.
A series of bentonite-water interaction tests have been performed with the aim of providing a database on the main chemical parameters of the bentonite. These tests were carried out both with high solid to liquid (s:l) ratios ( squeezing tests) and low s:l ratios ( aqueous extracts tests). The exchangeable cations have also been analyzed to determine the selectivity coefficient of the exchange reactions. To complete the data set, a physical and mineralogical characterization of the bentonite was made.
The most significant bentonite-water interaction processes controlling the chemistry of the system was identified. The ion concentrations basically depend on the s:l ratio of the system, and the pore water composition is controlled by the dissolution of chlorides, dissolution/precipitation of carbonates and sulphates and the cation exchange reactions in the smectite.
The bentonite/water system was modelled with the PHREEQC2 program to obtain the best possible estimation of the pore water composition for initial conditions of water content (≍14%), after checking the conceptual model with the experimental results. The model predictions fitted satisfactorily with the experimental data at low s:l ratios. At high s:l ratios, the modelled results agree adequately, except for the sulphate content, which could be affected by the effective porosity, anion exclusion or stagnant zones not taken into account in the model. According to the model, the FEBEX bentonite pore water at 14% moisture is a sodium-chloride type, with an ionic strength of 0.25 M and pH of 7.78.
Similar content being viewed by others
References
Bradbury M., and B. Baeyens A physicochemical characterization and geochemical modelling approach for determining pore water chemistries in argillaceous rocks. Geochimica et Cosmochimica Acta, 62, No.5: 783–795 (1998).
Curti E. Modelling bentonite pore waters for the Swiss High-level Radioactive Waste Repository. Nagra Technical Report NTB 93-45 (1993).
Wanner H., Wersin P., Sierro N. Thermodynamic modelling of bentonite-groundwater interaction and implications for near field chemistry in a repository for spent fuel. SKB 92-37, 28 pp. (1999).
Muurinen A. and Lehikoinen J. Pore water chemistry in compacted bentonite. Engineering Geology, 54: 207–214 (1999).
Pearson F.J., Waber H.N. and Scholtis A. Modelling the chemical evolution of pore water in the Palfris Marl, Wellenberg, Central Switzerland. Mat. Res. Soc. Symp. Proc., 506: 789–796 (1998).
Beaucaire C., Toulhoat P., and Pitsch H. Chemical characterization and modelling of the interstitial fluid in the Boom clay formation. In Water-Rock Interaction (ed. Y.K. Kharaka and O.V. Chudaev): 779–782. Balkema (1995).
Wieland E., Wanner H., Albinsson Y., Wersin P., Karnland O. A surface chemical model of the bentonite-water interface and its implications for modelling the near field chemistry in a repository for spent fuel. SKB 94-26 (1994).
Wanner H. Modelling interaction of deep groundwaters with bentonite and radionuclide speciation. Nagra Technical Report. NTB 86-21, 103 pp (1986).
Thomas G.W. Exchange cations. In: Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. Agronomy Monograph n° 9 (2nd Edition). ASA-SSSA, 677. WI 53711. USA (1982)
Rhoades J. D. Cation Exchange Capacity. In: Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. Agronomy Monograph n° 8 (2nd Edition). ASA-SSSA, 677. WI 53711. USA (1982).
Cuevas J., Villar M.V., Fernández A.M., Gómez P. and Martín P.L. Pore waters extracted from compacted bentonite subjected to simultaneous heating and hydration. Applied Geochemistry, 12: 473–481 (1997).
Peters C.A., Yang Y.C., Higgins J.D. and Burger P.A. (1992). A preliminary study of the chemistry of pore water extracted from tuff by one-dimensional compression. Water-Rock Interaction. Kharaka & Maest (eds), 741–745.
Entwisle D.C. and Reeder S. New apparatus for pore fluid extraction from mudrocks for geochemical analysis. In: Geochemistry of Clay-Pore Fluid Interactions. Chapter fifteen, 365-388. Manning D.A.C., Hall P.L. and Hughes C.R. (eds). Chapman & Hall. (1993).
Caballero E., E. Reyes, Linares J., and Huertas F. Hydrothermal solutions related to Bentonite genesis, Cabo de Gata region, Almería, SE Spain. Miner. Petrogr. Acta, 29–A: 187–196 (1985).
FEBEX working groups. Febex Project. Final report. ENRESA Technical Publication 1/2000 (2000).
Cuadros J. and Linares J. Experimental kinetic study of the smectite to illite transformation. Geochimica et Cosmochimica Acta, 60, No.3: 439–453 (1996).
Parkhurst D.L. and Appelo C.A.J. User’s guide to PHREEQC (Version 2)-a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 99-4259, 312 pp. (1999).
Appelo C.A.J. and Postma D. Geochemistry, groundwater and pollution. A.A. Balkema, Rotterdam. (1996).
Fernández A.M., Rivas P. and Cuevas J.. Estudio del agua intersticial de la arcilla FEBEX. FEBEX Interim Report 70-IMA-L-0-44 (1999); and In: Chapter 2 in [15].
Missana T., Turrero M.J. and Adell A. Surface charge and electrophoretic properties of colloids obtained from homoionic and natural bentonite. Mat. Res. Soc. Symp. Pro. XXIII, 608 (1999).
F. J. Pearson What is the porosity of a mudrock?. In: Muds and Mudstones: Physical and fluid flow properties (Aplin A.C., Fleet A.J. and Macquaker J.H.S., eds.) Special Publications, 158, pp. 9–21. Geological Society. London (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández, A.M., Cuevas, J. & Rivas, P. Pore Water Chemistry of the Febex Bentonite. MRS Online Proceedings Library 663, 573 (2000). https://doi.org/10.1557/PROC-663-573
Published:
DOI: https://doi.org/10.1557/PROC-663-573