Abstract
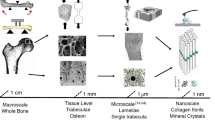
From a public health perspective, developing a detailed mechanistic understanding of the well-known increase with age in fracture risk of human bone is essential. This also represents a challenge from materials science and fracture mechanics viewpoints. Bone has a complex, hierarchical structure with characteristic features ranging from nanometer to macroscopic dimensions; it is therefore significantly more complex than most engineering materials. Nevertheless, by examining the micro-/nanostructural changes accompanying the process of aging using appropriate multiscale experimental methods and relating them to fracture mechanics data, it is possible to obtain a quantitative picture of how bone resists fracture. As human cortical bone exhibits rising ex vivo crack-growth resistance with crack extension, its fracture toughness must be evaluated in terms of resistance-curve (R-curve) behavior. While the crack initiation toughness declines with age, the more striking finding is that the crack-growth toughness declines even more significantly and is essentially absent in bone from donors exceeding 85 years in age. To explain such an age-induced deterioration in the toughness of bone, we evaluate its fracture properties at multiple length scales, specifically at the molecular and nano dimensions using vibrational spectroscopies, at the microscale using electron microscopy and hard/soft x-ray computed tomography, and at the macroscale using R-curve measurements. We show that the reduction in crack-growth toughness is associated primarily with a degradation in the degree of extrinsic toughening, in particular involving crack bridging, and that this occurs at relatively coarse size scales in the range of tens to hundreds of micrometers. Finally, we briefly describe how specific clinical treatments, e.g., with steroid hormones to treat various inflammatory conditions, can prematurely damage bone, thereby reducing its fracture resistance, whereas regulating the level of the cytokine Transforming Growth Factor-β can offer significant improvements in the stiffness, strength, and toughness of bone and as such may be considered a therapeutic target to treat increased bone fragility induced by aging, drugs, and disease.
Similar content being viewed by others
References
A.G. Jennings, P. de Boer: Should we operate on nonagenarians with hip fractures? Injury 30, 169 (1999).
R. Heaney: Is the paradigm shifting? Bone 33, 457 (2003).
P.D. Miller, M.C. Hochberg, L.E. Wehren, P.D. Ross, R.D. Wasnich: How useful are measures of BMD and bone turnover? Curr. Med. Res. Opin. 21, 545 (2005).
S.L. Hui, C.W. Slemenda, C.C. Johnston: Age and bone mass as predictors of fracture in a prospective study. J. Clin. Invest. 81, 1804 (1988).
T.J. Aspray, A. Prentice, T.J. Cole, Y. Sawo, J. Reeve, R.M. Francis: Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J. Bone Miner. Res. 11, 1019 (1996).
A. Burstein, D. Reilly, M. Martens: Aging of bone tissue: Mechanical properties. J. Bone Joint Surg. 58A, 82 (1976).
P. Zioupos, J.D. Currey: Changes in the stiffness, strength, and toughness of human cortical bone with age—An underexplored frontier. Bone 22, 57 (1998).
Y.N. Yeni, T.L. Norman: Fracture toughness of human femoral neck: Effect of microstructure, composition, and age. Bone 26, 499 (2000).
O. Akkus, F. Adar, M.B. Schaffler: Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 34, 443 (2004).
R.K. Nalla, J.J. Kruzic, J.H. Kinney, R.O. Ritchie: Effect of aging on the toughness of human cortical bone: Evaluation by R-curves. Bone 35, 1240 (2004).
J.Y. Rho, L. Kuhn-Spearing, P. Zioupos: Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 20, 92 (1998).
S. Weiner, H.D. Wagner: The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 28, 271 (1998).
D.R. Eyre, M.A. Paz, P.M. Gallop: Cross-linking in collagen and elastin. Annu. Rev. Biochem. 53, 717 (1984).
L. Knott, A.J. Bailey: Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone 22, 181 (1998).
A. Veis: Collagen fibrallar structure in mineralized and nonmineralized tissues. Curr. Opin. Solid State Mater. Sci. 2, 370 (1997).
J.D. Currey: Osteons in biomechanical literature. J. Biomech. 15, 717 (1982).
R.K. Nalla, J.J. Kruzic, J.H. Kinney, R.O. Ritchie: Mechanistic aspects of fracture and R-curve behavior in human cortical bone. Biomaterials 26, 217 (2005).
T.C. Lee, A. Staines, D. Taylor: Bone adaptation to load: Microdamage as a stimulus for bone remodeling. J. Anat. 201, 437 (2002).
W. Bonfield: Advances in the fracture mechanics of cortical bone. J. Biomech. 20, 1071 (1987).
T.L. Norman, D. Vashishth, D.B. Burr: Fracture toughness of human bone under tension. J. Biomech. 28, 309 (1995).
X.D. Wang, N.S. Masilamani, J.D. Mabrey, M.E. Alder, C.M. Agrawal: Changes in the fracture toughness of bone may not be reflected in its mineral density, porosity, and tensile properties—Effects of sampling sites and crack orientations. Bone 23, 67 (1998).
C.U. Brown, Y.N. Yeni, T.L. Norman: Fracture toughness is dependent on bone location- A study of the femoral neck, femoral shaft, and the tibial shaft. J. Biomed. Mater. Res. 49, 380 (2000).
J.B. Phelps, G.B. Hubbard, X. Wang, C.M. Agrawal: Microstructural heterogeneity and the fracture toughness of bone. J. Biomed. Mater. Res. 51, 735 (2000).
P. Lucksanambool, W.A.J Higgs, R.J.E.D Higgs, M.W. Swain: Fracture toughness of bovine bone: Influence of orientation and storage media. Biomaterials 22, 3127 (2001).
J.C. Behiri, W. Bonfield: Fracture mechanics of bone—The effects of density, specimen thickness, and crack velocity on longitudinal fracture. J. Biomech. 22, 863 (1989).
R.O. Ritchie: Mechanisms of fatigue-crack propagation in metals, ceramics and composites: Role of crack tip shielding. Mater. Sci. Eng. 103, 15 (1988).
A.G. Evans: Perspective on the development of high-toughness ceramics. J. Am. Ceram. Soc. 73, 187 (1990).
R.O. Ritchie: Mechanisms of fatigue-crack propagation in ductile and brittle solids. Int. J. Fract. 100, 55 (1999).
R. Steinbrech, R. Knehans, W. Schaawächter: Increase of crack resistance during slow crack growth in Al2O3 bend specimens. J. Mater. Sci. 18, 265 (1983).
P.L. Swanson, C.J. Fairbanks, B.R. Lawn, Y.W. Mai, B.J. Hockey: Crack-interface grain bridging as a fracture resistance mechanism in ceramics: I. Experimental study on alumina. J. Am. Ceram. Soc. 70, 279 (1987).
Y.W. Mai, B.R. Lawn: Crack–interface grain bridging as a fracture resistance mechanism in ceramics: II. Theoretical fracture mechanics model. J. Am. Ceram. Soc. 70, 289 (1987).
D. Vashishth, J.C. Behiri, W. Bonfield: Crack growth resistance in cortical bone: Concept of microcrack toughening. J. Biomech. 30, 763 (1997).
P.C. Wu and D. Vashishth: Age-related changes in cortical bone toughness: Initiation vs. propagation, in Proceedings of the 2nd Joint EMBS/BMES Conference Vol. 1, edited by J.W. Clark and L.V. McIntire (IEEE, 2002), p. 425.
Q.D. Yang, B.N. Cox, R.K. Nalla, R.O. Ritchie: Fracture length scales in human cortical bone: The necessity of nonlinear fracture models. Biomaterials 27, 2095 (2006).
R.K. Nalla, J.H. Kinney, R.O. Ritchie: Mechanistic fracture criteria for the failure of human cortical bone. Nat. Mater. 2, 164 (2003).
B.A. Bilby, G.E. Cardew, I.C. Howard: Stress intensity factors at the tips of kinked and forked cracks, in Fracture 1977, Vol. 3, edited by D.M.R Taplin (Pergamon Press, Oxford, UK, 1978), pp. 197–200.
B. Cotterell, J.R. Rice: Slightly curved or kinked cracks. Int. J. Fract. 16, 155 (1980).
R. K. Nalla, J.J. Kruzic, J.H. Kinney, M. Balooch, J.W. Ager, III, and R.O. Ritchie: Role of microstructure in the aging-related deterioration of the toughness of human cortical bone. Mater. Sci. Eng., C 26 (2006, in press).
J.K. Shang, R.O. Ritchie: Crack bridging by uncracked ligaments during fatigue-crack growth in SiC-reinforced aluminum-alloy composites. Metall. Trans. A 20A, 897 (1989).
Y.N. Yeni, D.P. Fyhrie: Fatigue damage-fracture mechanics interaction in cortical bone. Bone 30, 509 (2002).
A.G. Evans, R.M. McMeeking: On the toughening of ceramics by strong reinforcements. Acta Metall. 34, 2435 (1986).
H. Peterlik, P. Roschger, K. Klaushofer, P. Fratzl: From brittle to ductile fracture of bone. Nat. Mater. 5, 53 (2006).
G.E. Fantner, T. Hassenkam, J.H. Kindt, J.C. Weaver, H. Birkedal, L. Pechenik, J.A. Cutroni, G.A.G Cidade, G.D. Stucky, D.E. Morse, P.K. Hansma: Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 4, 612 (2005).
N.D. Sahar, S.I. Hong, D.K. Koln: Micro- and nano-structural analysis of damage in bone. Micron 36, 617 (2005).
D. Vashishth, K.E. Tanner, W. Bonfield: Contribution, development and morphology of microcracking in cortical bone during crack propagation. J. Biomech. 33, 1169 (2000).
D. Vashishth, K.E. Tanner, W. Bonfield: Experimental validation of a microcracking-based toughening mechanism for cortical bone. J. Biomech. 36, 121 (2003).
A.G. Evans, K.T. Faber: Crack-growth resistance of microcracking brittle materials. J. Am. Ceram. Soc. 67, 255 (1984).
J.W. Hutchinson: Crack tip shielding by micro-cracking in brittle solids. Acta Metall. 35, 1605 (1987).
R.K. Nalla, J.J. Kruzic, R.O. Ritchie: On the origin of the toughness of mineralized tissue: Microcracking or crack bridging. Bone 34, 790 (2004).
C.U. Brown, Y.N. Yeni, T.L. Norman: Fracture toughness is dependent on bone location—A study of the femoral neck, femoral shaft, and the tibial shaft. J. Biomed. Mater. Res. 49, 380 (2000).
P. Zioupos, J.D. Currey, A.J. Hamer: The role of collagen in the declining mechanical properties of aging human cortical bone. J. Biomed. Mater. Res. 2, 108 (1999).
X. Wang, X. Shen, X. Li, C.M. Agrawal: Age-related changes in the collagen network and toughness of bone. Bone 31, 1 (2002).
J.D. Currey, K. Brear, P. Zioupos: The effects of ageing and changes in mineral content in degrading the toughness of human femora. J. Biomech. 29, 257 (1996).
V.A. Gibson, S.M. Stover, J.C. Gibeling, S.J. Hazelwood, R.B. Martin: Osteonal effects on elastic modulus and fatigue life in equine bone. J. Biomech. 39, 217 (2006).
E.P. Paschalis, E. Shane, G. Lyritis, G. Skarantavos, R. Mendelsohn, A.L. Boskey: Bone fragility and collagen cross-links. J. Bone Miner. Res. 19, 2000 (2004).
A. Boskey, R. Mendelsohn: Infrared analysis of bone in health and disease. J. Biomed. Opt. 10, 031102 (2005).
A. Carden, M.D. Morris: Application of vibrational spectroscopy to the study of mineralized tissues (review). J. Biomed. Opt. 5, 259 (2000).
J.J. Freeman, M.J. Silva: Separation of the Raman spectral signatures of bioapatite and collagen in compact mouse bone bleached with hydrogen peroxide. Appl. Spectrosc. 56, 770 (2002).
J.A. Timlin, A. Carden, M.D. Morris, J.F. Bonadio, II C.E. Hoffler, K.M. Kozloff, S.A. Goldstein: Spatial distribution of phosphate species in mature and newly generated mammalian bone by hyperspectral Raman imaging. J. Biomed. Opt. 4, 8 (1999).
C.G. Kontoyannis, N.V. Vagenas: FT-Raman spectroscopy: A tool for monitoring the demineralization of bones. Appl. Spectrosc. 54, 1605 (2000).
R.J. Lakshmi, M. Alexander, J. Kurien, K.K. Mahato, V.B. Kartha: Osteoradionecrosis (ORN) of the mandible: A laser Raman spectroscopic study. Appl. Spectrosc. 57, 1100 (2003).
A. Carden, R.M. Rajachar, M.D. Morris, D.H. Kohn: Ultrastructural changes accompanying the mechanical deformation of bone tissue: A Raman imaging study. Calcif. Tissue Int. 72, 166 (2003).
J.W. Ager III, R.K. Nalla, K.L. Breeden, R.O. Ritchie: Deep-ultraviolet Raman spectroscopy study of the effect of aging on human cortical bone. J. Biomed. Opt. 10, 034012 (2005).
R.K. Nalla, M. Balooch, J.W. Ager III, J.J. Kruzic, J.H. Kinney, R.O. Ritchie: Effects of polar solvents on the fracture resistance of dentin: Role of water hydration. Acta Biomater. 1, 31 (2005).
G.M. Kiebzak: Age-related bone changes. Exp. Gerontol. 26, 171 (1991).
C. Cooper, C. Coupland, M. Mitchell: Rheumatoid arthritis, corticosteroid therapy, and hip fracture. Ann. Rheum. Dis. 54, 49 (1995).
N.E. Lane: An update on glucocorticoid-induced osteoporosis. Rheum. Disease Clin. N. Am. 27, 235 (2001).
K.G. Saag: Glucocorticoid-induced osteoporosis. Endocrinol. Metab. Clin. N. Am. 32, 135 (2003).
T.P. Van Staa, H.S. Leufkens, C. Cooper: The epidemiology of cortico-steroid osteoporosis. A meta-analysis. Osteoporos. Int. 13, 777 (2002).
T.P. Van Staa, R.F. Laan, I.P. Barton, S. Cohen, D.M. Reid, C. Cooper: Bone density threshold and other predictors of vertebral fractures in patients receiving oral Glucocorticoid therapy. Arthritis Rheum. 48, 3224 (2003).
N.E. Lane, W. Yao, M. Balooch, R.K. Nalla, G. Balooch, S. Habelitz, J.H. Kinney, L. Bonewald: Glucocorticoid treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo treated or estrogen deficient mice. J. Bone Miner. Res. 21, 466 (2006).
G. Balooch, M. Balooch, R.K. Nalla, S. Schilling, E.H. Filvaroff, G.W. Marshall, S.J. Marshall, R.O. Ritchie, R. Derynck, T. Alliston: TGF-β regulates the mechanical properties and composition of bone matrix. Proc. Natl. Acad. Sci. USA 102, 18813 (2005).
J.J. Kruzic, R.K. Nalla, J.H. Kinney, R.O. Ritchie: Crack blunting, crack bridging and resistance-curve fracture mechanics of dentin: Effect of hydration. Biomaterials 24, 5209 (2003).
J.H. Kinney, R.K. Nalla, J.A. Pople, T.M. Breunig, R.O. Ritchie: Age-related transparent root dentin: Mineral concentration, crystallite size, and mechanical properties. Biomaterials 26, 3363 (2005).
O. Akkus, A. Polyakova-Akkus, F. Adar, M.B. Schaffler: Aging of microstructural compartments in human compact bone. J. Bone Miner. Res. 18, 1012 (2003).
M.B. Schaffler, K. Choi, C. Milgrom: Aging and matrix microdamage accumulation in human compact bone. Bone 17, 521 (1995).
R.W. McCalden, J.A. McGeough, M.B. Barker, C.M. Court-Brown: Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J. Bone Joint Surg. Am. 75, 1193 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ager, J.W., Balooch, G. & Ritchie, R.O. Fracture, aging, and disease in bone. Journal of Materials Research 21, 1878–1892 (2006). https://doi.org/10.1557/jmr.2006.0242
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2006.0242