Abstract
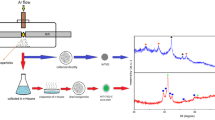
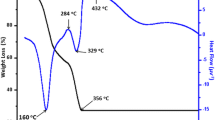
This study reports the synthesis of monosized Pr nanoparticles with a controllable size ranging from 5 to 20 nm. Pr agglomerates generated by a spark generator, first sizeselected by a differential mobility analyzer and subsequently sintered in-flight at different temperatures result in spherical and monocrystalline Pr nanoparticles. The dependence of size and size distribution of Pr nanoparticles has been studied as a function of deposition parameters related to spark generator, differential mobility analyzer, and sintering. Transmission electron microscopy, energy-dispersive x-ray analysis, glancing angle x-ray diffraction, and x-ray photoelectron spectroscopy studies confirm that initial Pr agglomerates and the resulting nanoparticles are metallic with d-hexagonal structure and remain stable in air during post-deposition exposure. Incomplete or partially sintered nanoparticles were found to be oxidized, resulting in the formation of amorphous oxide phase due to enhanced oxidation at grain boundaries.
Similar content being viewed by others
References
T. Uda, K.T. Jacob, and M. Hirasawa: Technique for enhanced rare earth separation. Science 289, 2326 (2000).
D.J. Fray: Separating rare earth elements. Science 289, 2295 (2000).
P.J. von Ranke, M.A. Mota, D.F. Grangeia, A. Magnus, G. Carvelho, F.C.G. Gandra, A.A. Coelho, A. Caldas, N.A. de Oliveira, and S. Gamaa: Magnetocaloric effect in the RNi5 (R= Pr, Nd, Gd, Tb, Dy, Ho, Er) series. Phys. Rev. B 70, 134428 (2004).
R. Rawat and I. Das: Magentic transitions in CeCu0.86Ge2 and PrCu0.76Ge2 as studied by magnetocaloric effect. Phys. Rev. B: Condens. Matter 64, 052407 (2001).
X. Song, J. Zhang, M. Yue, E. Li, H. Zeng, N. Lu, M. Zhou, and T. Zuo: Technique for preparing ultrafine nanocrystalline bulk material of pure rare-earth metals. Adv. Mater. 18, 1210 (2006).
I.R. Mitchell, P.M. Farrell, G.W. Baxter, S.F. Collins, K.T.V. Grattan, and T. Sun: Analysis of dopant concentration effects in praseodymium-based fluorescent fiber optic temperature sensors. Rev. Sci. Instrum. 71, 100 (2000).
J.N. Huiberts, R. Griessen, J.H. Rector, R.J. Wijngaarden, J.P. Dekker, D.G. de Groot, and N.J. Koeman: Yttrium and lanthanum hydride films with switchable optical properties. Nature 380, 231 (1996).
A. Züttel: Materials for hydrogen storage. Mater. Today 24, (September) (2003).
A. Amadeh, B. Pahlevani, and S. Heshmati-Manesh: Effects in rare earth metal addition on surface morphology and corrosion resistance of hot-dipped zinc coatings. Corros. Sci. 44, 2321 (2002).
S.K. Paul, A.K. Chakrabarty, and S. Basu: Effect of rare earth additions on the inclusions and properties of a Ca-Al deoxidized steel. Metall. Trans. B 13, 185 (1982).
B.M. Bist and O.N. Srivastava: A new f. c.c., gadolinium phase and its oxidation. J. Less-Common Met. 33, 99 (1973).
D. Weller and D.D. Sarma: Formation of a passive layer in surface oxidation of Gd: A LEED and AES study. Surf. Sci. 171, L425 (1986).
T. Arakawa, A. Kabumoto, and J. Shiokawa: Some electrical properties of praseodymium oxide films produced by oxidation of thin metal films. J. Less-Common Met. 115, 281 (1986).
M. Gasgnier, J. Ghys, G. Schiffmacher, Ch.H. La Blanchetais, P.E. Caro, C. Boulesteix, Ch. Loier, and B. Pardo: Rare-earthhydrides and rare earth oxides in and from thin films of rare-earthmetals. J. Less-Common Met. 34, 131 (1974).
P.Z. Si, I. Skorvánek, J. Kovàĉ, D.Y. Geng, X.G. Zhao, and Z.D. Zhang: Structure and magnetic properties of Gd nanoparticlesand carbon coated Gd/GdC2 nanocapsules. J. Appl.Phys. 94, 6779 (2003).
Y. Saito, M. Okuda, T. Yoshikawa, A. Kasuya, and Y. Nishina:Correlation between volatility of rare-earth metals and encapsulationof their carbides in carbon nanocapsules. J. Phys. Chem. 98, 6696 (1994).
A.R. Wildes, R.C.C. Ward, M.R. Wells, and B. Hjörvarsson: The formation of epitaxial YH 2 in MBE grown yttrium thinfilms with a thin gold capping layer. J. Alloys Compd. 242, 49(1996).
H. Wan, A. Tsoukatos, Y.J. Zhang, G.C. Hadjipanayis, and S.I. Shah: Magnetic properties of Er-Ta granular thin films.Nanostruct. Mater. 1, 505 (1992).
Y. Zhang, C.E. Nelson, Z.C. Yan, V. Skumryev, and G.C. Hadjipanayis: Application of energy-filtered imaging andHREM hrem in the study of terbium nanoparticles. Microsc. Microanal. 8, 1360 (2002).
C.E. Krill, F. Merzoug, W. Krauss, and R. Birringer: Magneticproperties of nanocrystalline Gd and W/Gd. Nanostruct. Mater. 9, 455 (1997).
N.B. Shevcenko, A.S. Murthy, and G.C. Hadjipanayis: Microstructuraland magnetic studies of granular Gd-W films. Mater.Sci. Eng., A 204, 39 (1995).
D. Johnson, P. Perera, and M.J. O’Shea: Finite size effect innanoscale Tb nanoparticles. J. Appl. Phys. 79, 5299 (1996).
N.B. Shevcenko, A.S. Murthy, and G.C. Hadjipanayis: Preparationand characterization of Dy nanoparticles. Appl. Phys. Lett. 74, 1478 (1999).
D. Weller, S.F. Alvarado, M. Campagna, W. Gudat, and D.D. Sarma: Structure, magnetism and electronic excitations of epitaxial gadolinium (0001) on tungsten (110). Less-Common Metals 111, 277(1985).
I. Aruna, B.R. Mehta, L.K. Malhotra, and S.M. Shivaprasad: Stabilityand hydrogenation of bare gadolinium nanoparticles. Adv.Mater. 16, 169 (2004).
J.A. Nelson, L.H. Bennett, and M.J. Wagner: Solution synthesis of gadolinium nanoparticles. J. Am. Chem. Soc. 124, 2979 (2002).
J.A. Nelson, L.H. Bennett, and M.J. Wagner: Dysprosium nanoparticles synthesized by alkalide reduction. J. Mater. Chem. 13, 857 (2003).
J.A. Ascencio, G. Canizal, A. Medina-Flores, L. Bejar, L. Tavera, H. Matamoros, and H. Liu: Neodymium nanoparticles: Biosíntesis and structural análisis. J. Nanosci. Nanotechnol. 6, 1044 (2006).
D. Michels, C.E. Krill III and R. Birringer: Grain-size-dependent Curie transition in nanocrystalline Gd: The influence of interface stress. J. Magn. Magn. Mater. 250, 203 (2002).
C.H. Shek and Y.Z. Shao: Characteristics of growth fractal of nanosized gadolinium powder and its abnormality in magnetic susceptibility. Scr. Mater. 44, 959 (2001).
J. Dixkens and H. Fissan: Development of an electrostatic precipitator for off-line particle analysis. Aerosol Sci. Technol. 30, 438 (1999).
M.N.A. Karlsson, K. Deppert, L.S. Karlsson, M.H. Magnusson, J-O. Malm, and N.S. Srinivassan: Compaction of agglomerates of aerosol nanoparticles: A compilation of experimental data. J. Nanopart. Res. 7, 43 (2005).
J.J. Hanak and A.H. Daane: High temperature allotropy and thermal expansion of the rare-earth metals. J. Less-Common Met. 3, 110 (1961).
G. Crecelius, G.K. Wertheim, and D.N.E. Buchanan: Core-hole screening in lanthanide metals. Phys. Rev. B: Condens. Matter 18, 6519 (1978).
Y. Uwamino, T. Ishizuka, and H. Yamatera: X-ray photoelectron spectroscopy of rare-earth compounds. J. Electron Spectrosc. Relat. Phenom. 34, 67 (1984).
M.G. Mason: Electronic structure of supported small metal clusters. Phys. Rev. B: Condens. Matter 27, 748 (1983).
G.K. Wertheim, S.B. DiCenzo, and D.N.E. Buchanan: Noble- and transition-metal clusters: The d bands of silver and palladium. Phys. Rev. B: Condens. Matter 33, 5384 (1986).
D.W. Fischer and W.L. Baun: Self-absorption effects in the soft x-ray Ma and Mb emission spectra of the rare earth elements. J. Appl. Phys. 38, 4830 (1967).
G.K. Wertheim and M. Champagna: Screening of 3d holes in the rare earths. Solid State Commun. 26, 553 (1978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kala, S., Raj Mehta, B., Kruis, F.E. et al. Synthesis and oxidation stability of monosized and monocrystalline Pr nanoparticles. Journal of Materials Research 24, 2276–2285 (2009). https://doi.org/10.1557/jmr.2009.0281
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2009.0281